Abstract
Background
The health benefits of probiotics and synbiotics in healthy adults are well established, but their role in preventing infectious complications after surgery for colorectal cancer remains controversial. The aim of this meta-analysis was to assess the impact of probiotics/synbiotics on the incidence of infectious complications in patients who had surgery for colorectal cancer.
Methods
A comprehensive literature search of all randomized control trials (RCTs) was conducted using PubMed, Embase, World Health Organization (WHO) Global Index Medicus, WHO clinical trial registry, and Clinicaltrials.gov. Inclusion criteria included RCTs comparing the use of any strain or dose of a specified probiotic/synbiotic with placebo or a “standard care” control group. The incidence of postoperative infectious complications was analyzed.
Results
Fourteen RCTs involving 1566 patients (502 receiving probiotics, 273 receiving synbiotics, and 791 receiving placebo) were analyzed. Overall, probiotic or synbiotic administration significantly reduced the risk of developing postoperative infectious complications by 37% (relative risk (RR) = 0.63, 95% confidence interval (CI) 0.54–0.74, p < 0.001). Furthermore, when considering the six different types of postoperative infectious complications (septicemia, incision infection, central line infection, pneumonia infection, urinary infection, and incidence of diarrhea), probiotic or synbiotic administration was beneficial in reducing the incidence of each one of them. The quality of evidence was listed below: incidence of diarrhea (high), septicemia (moderate), incision infection (moderate), pneumonia infection (moderate), urinary infection (moderate), and central line infection (low). However, for the main outcome of infectious complications, we found evidence of possible publication bias, although estimates still showed a reduction following trim-and-fill analysis (RR = 0.72, 95% CI 0.62–0.84, p < 0.001).
Conclusions
The use of probiotic/synbiotic supplementation is associated with a significant reduction in the risk of developing postoperative infectious complications in patients who had surgery for colorectal cancer. Additional studies are needed to confirm the findings due to publication bias and low quality of evidence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introductions
Colorectal cancer (CRC) is one of the most common cancers. It ranks third in terms of incidence and second in mortality worldwide, accounting for 1.8 million new cases and almost 900,000 deaths [1]. In the adults over 65 years of age, CRC incidence and mortality have decreased steadily in recent decades. However, in adults under 50 years of age, the incidence of CRC presents an obvious rising trend [2]. The disease burden of CRC on the whole population continues to increase.
The etiology of CRC is complex, Surgery is the main treatment option at present [3]. Although surgical management has significantly improved, postoperatively a considerable number of patients still develop infectious complications, which may cause sepsis, multiple organ dysfunction, and even lead to death if not diagnosed in time [4, 5]. Postoperative infection is closely related to disorders of gut microbiota, and the occurrence and development of postoperative infection can be effectively prevented by regulating gut microbiota [6].
Probiotics are active microorganisms that are beneficial to the host by regulating the immune function of the host mucosa and the system, or by regulating the balance of intestinal flora [7]. Prebiotics are organic substances that are not digested or absorbed by the host but selectively promote the metabolism and proliferation of beneficial bacteria in the body, thus improving the health of the host [8]. When prebiotics are used in combination with probiotics, they are known as synbiotics [9]. Probiotics have been shown to play a multifaceted role in preventing gastrointestinal infections; they can promote the digestion and absorption of nutrients, improve the body’s immunity, maintain the structural balance of intestinal flora, improve the body’s antioxidant level, and protect the intestinal mucosal barrier [10,11,12]. These nutritional adjuncts have potential benefits of reducing the incidence of postoperative infection.
Many randomized controlled trials (RCTs) have reported the effect of probiotics [13,14,15,16,17,18,19,20,21] or synbiotics [22,23,24,25] on reducing postoperative infection complications with inconsistent results, most likely due to variations in experiment design and methodological measurements. Some recent studies have been published to demonstrate the benefits of probiotics or synbiotics; however, they did not evaluate the publication bias, risk of bias, and rate the quality of evidence [26, 27]. Therefore, an explicit systematic review and meta-analysis were needed to evaluate the effect of perioperative or postoperative probiotics/synbiotics on postoperative infectious complications in adult patients undergoing colorectal resections.
Materials and methods
Study selection
All RCTs evaluating the effect of probiotics or synbiotics on preventing postoperative infection complications were searched using PubMed (1966–2021), Embase (1980–2021), and World Health Organization (WHO) Global Index Medicus. Unpublished or ongoing studies were identified by checking clinical trials registers through Clinicaltrials.gov and WHO clinical trial registry. Literature in all languages was included in the search. Meta-analyses, and systematic reviews were also hand-searched to find relevant literature that might have been missed by the initial search. Logical combinations of “probiotics,” “synbiotics,” “Lactobacillus”, “Bifidobacterium”, “infection complications”, and “colorectal cancer” were used as keywords to search for relevant literatures. RCTs of any route of administration and dose were accepted, either preoperatively, postoperatively, or both. Control groups should be those that did not receive any probiotics or synbiotics. Only CRC surgery in patients > 18 years of age was included.
Data extraction
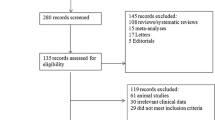
Articles retrieved from the searches were evaluated independently by two reviewers (Yuhui Chen and Aiying Qi) using predefined standardized data extraction forms, and then, data were evaluated by a third reviewer (Xiaohui Du) independently based on the United States National Institute of Health National Heart, Lung, and Blood Institute (NHLBI) study quality assessment tool for controlled intervention studies [28]. Clinical outcome of interest was incidence of postoperative infectious complications as defined by the trial authors. Data pertaining to patients, the kinds of probiotics or synbiotics, control groups, and methodology were abstracted (Fig. 1).
Meta-analysis
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement methodology [29] was adhered to. Relative risks (RRs) with a 95% CI for postoperative infectious complications of each trial were calculated to estimate treatment effects. Meta-analysis of the pooled data was performed using the fixed-effect model or random-effects model, depending on the heterogeneity of the included studies. If clinical heterogeneity was observed, data were analyzed using a random-effect model. Heterogeneity was quantified using the Cochrane’s Q statistic and \({I}^{2}\) statistic, with the values of 25%, 50%, and 75% signifying the limits of low, moderate, and high statistical heterogeneity, respectively [30]. A funnel plot was used to explore publication bias for the studies and was further evaluated using Egger’s test [31]. A two-tailed p value of < 0.05 was considered statistically significant. All statistical analyses were performed using R package meta (R version 4.0.1).
The risk of bias was evaluated using the Cochrane risk of bias tool. It was used to evaluate the selection bias, performance bias, detection bias, attrition bias, reporting bias, and other bias. The evidence quality was evaluated using the GRADEPro based on the results of systematic evaluation. To achieve transparency and implicity, the GRADE system classifies the certainty of evidence in one of four grades: high: further research is very unlikely to change our confidence in the estimate of effect; moderate: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; very low: any estimate of effect is very uncertain.
When publication bias exists in the results, the trim-and-fill method was used to test whether this publication bias would affect the results of the comprehensive effect size [32]. The basic idea is to cut out the asymmetric part of the funnel plot after initial estimation, the center value of the funnel plot was estimated using the remaining symmetric part, then the cut part and corresponding missing part were patched along both sides of the center, and finally, the value of the combined effect size was estimated based on the patched funnel plot.
Results
Demographic characteristics of the studies
The literature search process, shown in Fig. 1, identified 221 potential studies for fully analyses. Following application of exclusion criteria, 14 studies were identified for further quantitative meta-analyses [13,14,15,16,17,18,19,20,21,22,23,24,25, 33] (Fig. 1), involving a total of 1566 patients. Of 14 studies included in the final analysis, 5 studies used probiotics or synbiotics preoperatively, 8 studies used probiotics or synbiotics both preoperatively and postoperatively, and only 1 study used them postoperatively (Table 1). The probiotic strains used were Lactobacillus acidophillus, L. casei, L. paracasei, L. bulgaricus, L. paracasei, L. plantarum, Bifidobacterium lactis, Bifidobacterium breve, Bifidobacterium longus, Streptococcus thermophilus, Pediacoccus pentosaceus, and Leuconostoc mesenteroides. The prebiotics used were oligofructose powder, oat fiber, beta-glucan, inulin, pectin, and resistant starch. Nine studies used probiotics as the sole intervention with the remaining four studies using synbiotics instead.
Effects of probiotics or synbiotics on postoperative infectious complications
Overall, the probiotics or synbiotics can prevent developing postoperative infectious complications as compared to the control group who received placebo or standard care (RR = 0.63, 95% CI 0.54–0.74, p < 0.001; Fig. 2). There was no significant heterogeneity between trials (I2 = 7%). Of 14 studies included in the final analysis, 10 studies used probiotics as the sole intervention with the remaining 4 studies using synbiotics only. In the probiotic group, the incidence of postoperative infectious complications showed a significantly lower risk compared to the control group (RR = 0.65, 95% CI 0.54–0.78, p < 0.001; Fig. 2). No between-study heterogeneity was observed (I2 = 0%). In the synbiotics group, meta-analysis showed no significantly lower risk of developing postoperative infectious complications compared to the control group (RR = 0.57, 95% CI 0.39–0.84, p < 0.001; Fig. 2). There is moderate heterogeneity between studies (I2 = 55%). The subgroup difference between probiotics and synbiotics was not significant (p = 0.54).
Effects of preoperative or postoperative administration of probiotics/synbiotics on postoperative infectious complications
Of the14 studies included in the final analysis, 5 studies used probiotics or synbiotics preoperatively, 8 studies used probiotics or synbiotics both preoperatively and postoperatively, and only 1 study used them postoperatively. Overall, preoperative or postoperative administration of probiotics/synbiotics was beneficial in reducing the risk of postoperative infectious complications (RR = 0.63, 95% CI 0.54–0.74, p < 0.001; Fig. 3). Subgroup analysis showed that preoperative administration was beneficial in reducing the risk of developing postoperative infectious complications (RR = 0.33, 95% CI 0.17–0.61, p < 0.001; Fig. 3). However, the benefit of postoperative administration was not discovered (RR = 0.66, 95% CI 0.38–1.16, p = 0.15; Fig. 3). When probiotics or synbiotics were administrated both preoperatively and postoperatively, the incidence of postoperative infectious complications in the treatment group was reduced significantly (RR = 0.68, 95% CI 0.57–0.81, p < 0.001; Fig. 3). No between-study heterogeneity was observed in any subgroups (I2 = 0%; I2 = 11%; Fig. 3), and the subgroup difference was not significant (p = 0.09).
Effects of probiotics or synbiotics on different types of postoperative infectious complications
The effects of probiotics or synbiotics on different types of postoperative infectious complications were also explored (Fig. 4). Six types of postoperative infectious complications were reported in the meta-analyses: septicemia, incision infection, central line infection, pneumonia infection, urinary infection, and incidence of diarrhea. Results showed that the use of probiotics/synbiotics was associated with a significant decrease in the 6 types of postoperative infectious complications (septicemia: RR = 0.65, 95% CI 0.55–0.78, p < 0.001; incision infection: RR = 0.60, 95% CI 0.44–0.81, p < 0.01; central line infection: RR = 0.51, 95% CI 0.27–0.96, p = 0.04; pneumonia infection: RR = 0.52, 95% CI 0.29–0.95, p = 0.03; urinary infection: RR = 0.35, 95% CI 0.19–0.67, p < 0.001; incidence of diarrhea: RR = 0.52, 95% CI 0.38–0.70, p < 0.001). Only low heterogeneity was detected in the meta-analysis for the central line infection (I2 = 39%; Fig. 4C). Furthermore, the GRADEpro was applied to rate the quality of evidence (Table 2). Risk of bias, inconsistency, and imprecision of the interval estimation are the main uncertainties of the evidence. High certainty can be drawn that probiotics/synbiotics could reduce the incidence of diarrhea. Moderate certainty could be expected that probiotics/synbiotics were beneficial to protect colorectal cancer patients from developing septicemia, incision infection, pneumonia infection, and urinary infection. The correlation between probiotics/synbiotics and central line infection has low certainty. Additional studies are needed to confirm the findings due to low quality of evidence.
Publication bias
A funnel plot was used to visually assess for publication bias (Fig. 5). There was some asymmetry on the funnel plot, suggesting that studies are more likely to be published if positive outcomes are demonstrated. Egger’s test also indicates that there exists publication bias (p < 0.001). The trim-and-fill method was used to test whether this publication bias would affect the results of the comprehensive effect size [32], and the hollow circles represent the effect size of the fill. After automatic completion by the algorithm, a new comprehensive RR was obtained (RR = 0.72, 95% CI 0.62–0.84, p < 0.001), and its significance was unchanged from that before the trim-and-fill method. Therefore, to some extent, it can be shown that the result is not affected by publication bias.
Risk of bias analysis
The risk of bias of the studies included is summarized in Fig. 6. The Cochrane risk of bias tool was used to evaluate the selection bias, performance bias, detection bias, attrition bias, and other bias.
Discussion
Postoperative infections were significantly correlated to recurrence and poor survival in CRC patients. To gain a better surgical outcome and long-term oncological outcome, postoperative infection should be minimized as much as possible [34]. Many RCTs have demonstrated that preoperative or postoperative administration of probiotics/synbiotics is beneficial to prevent postoperative infection [13,14,15,16,17,18,19,20,21,22,23,24,25]. In the present study, we evaluated the effect of perioperative or postoperative probiotics/synbiotics on postoperative infectious complications in adult patients undergoing colorectal resections systematically.
In summary, the probiotics or synbiotics can prevent developing postoperative infectious complications as compared to the control group who received placebo or standard care (RR = 0.63, 95% CI 0.54–0.74, p < 0.001; Fig. 2). The subgroup difference between probiotics and synbiotics was not significant (p = 0.54), and the subgroup difference between preoperative and postoperative administrations of probiotics/synbiotics was also not significant (p = 0.09). Furthermore, when considering the six types of postoperative infectious complications (septicemia, incision infection, central line infection, pneumonia infection, urinary infection, and incidence of diarrhea), probiotic or synbiotic administration significantly reduced the incidence of each of them, but more studies need to be carried out to confirm this conclusion due to the low quality of evidence. However, for the main outcome of infectious complications, we found evidence of possible publication bias, although estimates still showed a reduction following trim-and-fill analysis (RR = 0.72, 95% CI 0.62–0.84, p < 0.001).
Although probiotic use has been greatly popularized among the general public, there are conflicting clinical results for many probiotic strains and formulations [35]. Theoretical risks have been described in case reports, clinical trial results and experimental models, include systemic infections, deleterious metabolic activities, excessive immune stimulation in susceptible individuals, gene transfer, and gastrointestinal side effects [36, 37]. The included studies did not report the incidence of side effects and mortality after administration of probiotics or synbiotics. Therefore, probiotics and synbiotics should be used in consideration of their possible side effects, which need to be confirmed by more studies [38].
This study has some limitations. First, beneficial outcomes of probiotic/synbiotics are more likely to be published, but the publication bias would not affect the results demonstrated by the trim-and-fill method. Second, the composition of probiotics/synbiotics and antibiotics used in each study varied, and different probiotic strains or antibiotics may have different capacities to prevent postoperative infection [39]. Most of the studies employed a Lactobacillus probiotic, while a few studies incorporated a Bifidobacteria species along with some prebiotics. Third, definitions of infectious complications were not specified among these studies, and differences in the definition of postoperative infectious complications can affect estimation of effect size. Fourth, the timing and duration of administration were different among these studies, and this factor should be important for the outcomes.
Conclusions
In summary, the pre- or postoperative use of probiotics and synbiotics can prevent the development of all types of postoperative infectious complications as compared to the control group. There was a large variety of proposed probiotics/synbiotics; therefore, the effect on postoperative complications may not be the same for all. Finally, the results of our meta-analysis must be interpreted with caution and more studies need to be carried out to confirm our conclusions due to the low quality of evidence of the trials included.
References
Bray F et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin 68:394–424
Siegel RL et al (2017) Colorectal cancer statistics, 2017. CA: Cancer J Clin 67:177–193
Song M, Chan AT, Sun J (2020) Influence of the gut microbiome, diet, and environment on risk of colorectal cancer. Gastroenterology 158:322–340
Xu Z et al (2020) Update on risk factors of surgical site infection in colorectal cancer: a systematic review and meta-analysis. Int J Colorectal Dis 35:2147–2156
MacFie J (2004) Current status of bacterial translocation as a cause of surgical sepsis. Br Med Bull 71:1–11
Bartolini I et al (2020) Role of gut microbiota-immunity axis in patients undergoing surgery for colorectal cancer: focus on short and long-term outcomes. World J Gastroenterol 26:2498–2513
Wieërs G et al (2019) How probiotics affect the microbiota. Front Cell Infect Microbiol 9:454
Holscher HD (2017) Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut microbes 8:172–184
Markowiak P, Śliżewska K (2017) Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 9:1021
Ng SC, Hart AL, Kamm MA, Stagg AJ, Knight SC (2009) Mechanisms of action of probiotics: recent advances. Inflamm Bowel Dis 15:300–310
Morrow LE, Kollef MH (2008) Kollef Probiotics in the intensive care unit: why controversies and confusion abound. Crit Care 12:160
Walker WA (2008) Mechanisms of action of probiotics. Clin Infect Dis: Off Publ Infect Dis Soc Am 46(Suppl 2):S87-91 (discussion S144–51)
Liu Z et al (2011) Randomised clinical trial: the effects of perioperative probiotic treatment on barrier function and post-operative infectious complications in colorectal cancer surgery—a double-blind study. Aliment Pharmacol Ther 33:50–63
Mangell P et al (2012) Lactobacillus plantarum 299v does not reduce enteric bacteria or bacterial translocation in patients undergoing colon resection. Dig Dis Sci 57:1915–1924
Zhang J et al (2012) Preoperative probiotics decrease postoperative infectious complications of colorectal cancer. Am J Med Sci 343:199–205
Liu Z et al (2013) The effects of perioperative probiotic treatment on serum zonulin concentration and subsequent postoperative infectious complications after colorectal cancer surgery: a double-center and double-blind randomized clinical trial. Am J Clin Nutr 97:117–126
Sadahiro S et al (2014) Comparison between oral antibiotics and probiotics as bowel preparation for elective colon cancer surgery to prevent infection: prospective randomized trial. Surgery 155:493–503
Kotzampassi K et al (2015) A four-probiotics regimen reduces postoperative complications after colorectal surgery: a randomized, double-blind, placebo-controlled study. World J Surg 39:2776–2783
Liu Z et al (2015) Positive regulatory effects of perioperative probiotic treatment on postoperative liver complications after colorectal liver metastases surgery: a double-center and double-blind randomized clinical trial. BMC Gastroenterol 15:34
Consoli MLD et al (2016) Randomized clinical trial: impact of oral administration of saccharomyces boulardii on gene expression of intestinal cytokines in patients undergoing colon resection. JPEN J Parenter Enter Nutr 40:1114–1121
Tan CK et al (2016) Pre-surgical administration of microbial cell preparation in colorectal cancer patients: a randomized controlled trial. World J Surg 40:1985–1992
Horvat M, Krebs B, Potrč S, Ivanecz A, Kompan L (2010) Preoperative synbiotic bowel conditioning for elective colorectal surgery. Wien Klin Wochenschr 122:26–30
Komatsu S et al (2016) Efficacy of perioperative synbiotics treatment for the prevention of surgical site infection after laparoscopic colorectal surgery: a randomized controlled trial. Surg Today 46:479–490
Flesch AT, Tonial ST, Contu PDC, Damin DC (2017) Perioperative synbiotics administration decreases postoperative infections in patients with colorectal cancer: a randomized, double-blind clinical trial. Rev Colegio Bras Cir 44:567–573
Polakowski CB, Kato M, Preti VB, Schieferdecker MEM, Ligocki Campos AC (2019) Impact of the preoperative use of synbiotics in colorectal cancer patients: a prospective, randomized, double-blind, placebo-controlled study. Nutrition (Burbank Los Angeles County, Calif) 58:40–46
Amitay EL, Carr PR, Gies A, Laetsch DC, Brenner H (2020) Probiotic/synbiotic treatment and postoperative complications in colorectal cancer patients: systematic review and meta-analysis of randomized controlled trials. Clin Transl Gastroenterol 11:e00268
Wu X et al (2018) Efficacy of prophylactic probiotics in combination with antibiotics versus antibiotics alone for colorectal surgery: a meta-analysis of randomized controlled trials. J Surg Oncol 117:1394–1404
Qumseya BJ (2021) Quality assessment for systematic reviews and meta-analyses of cohort studies. Gastrointest Endosc 93:486e1–494e1
Liberati A et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 151:W65–W94
Higgins JPT, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ (Clin Res ed) 315:629–634
Duval S, Tweedie R (2000) Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56:455–463
Yang Y et al (2016) The effect of perioperative probiotics treatment for colorectal cancer: short-term outcomes of a randomized controlled trial. Oncotarget 7:8432–8440
Chen G et al (2021) Relationship between postoperative complications and the prognosis of gastric carcinoma patients who underwent surgical resection: a systematic review and meta-analysis. Cancer Control : J Moffitt Cancer Center 28:10732748211011956–10732748211011956
Suez J, Zmora N, Segal E, Elinav E (2019) The pros, cons, and many unknowns of probiotics. Nat Med 25:716–729
Doron S, Snydman DR (2015) Risk and safety of probiotics. Clin Infect Dis: Off Publ Infect Dis Soc Am 60(Suppl 2):S129–S134
Zawistowska-Rojek A, Tyski S (2018) Are probiotic really safe for humans? Pol J Microbiol 67:251–258
Didari T, Solki S, Mozaffari S, Nikfar S, Abdollahi M (2014) A systematic review of the safety of probiotics. Expert Opin Drug Saf 13:227–239
Jäger R et al (2019) International society of sports nutrition position stand: probiotics. J Int Soc Sports Nutr 16:62
Funding
This study was supported by the National Natural Science Foundation of China (81871317) and Military medical innovation project (18CXZ025).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Ethics Statement
The study protocol was consistent with the Declaration of Helsinki (as revised in 2013). The approval from ethics board was not required since our study was a meta study.
Informed Consent statements
Individual consent for this meta analysis was waived.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, Y., Qi, A., Teng, D. et al. Probiotics and synbiotics for preventing postoperative infectious complications in colorectal cancer patients: a systematic review and meta-analysis. Tech Coloproctol 26, 425–436 (2022). https://doi.org/10.1007/s10151-022-02585-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10151-022-02585-1