Abstract
Purpose
The neutrophil-to-lymphocyte ratio (NLR) is associated with decreased overall survival in patients with pancreatic adenocarcinoma (PAC) in studies including few minority patients. We investigated the association between NLR and survival in patients with advanced PAC in an ethnically diverse population.
Methods
We retrospectively evaluated 226 patients with advanced PAC treated at Montefiore Medical Center between 2006 and 2015. Adjusted Cox proportion hazard regression models were utilized to derive effect estimates for survival duration.
Results
Patients with a NLR ≤ 5 (126 patients, median age 66 years) were more likely to be non-Hispanic Black (30.8% vs. 20%), while patients with a NLR > 5 (70 patients, median age 66 years) were more likely to be non-Hispanic White (21.4% vs. 12.2%) or Hispanic (44.3% vs. 34%). A NLR > 5 compared with a NLR ≤ 5 was significantly associated with a worse overall survival when adjusted for a priori and exploratory variables from the univariate analysis (median survival 7.4 vs. 12 months, HR 1.650, 95% CI 1.139, 2.390).
Conclusions
In an ethnically diverse population, elevated NLR is an independent marker of poor prognosis and a potentially valuable factor in driving therapeutic decisions and defining prognosis for patients in the locally advanced or metastatic for PAC setting, meriting investigation in prospective clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer is the fourth leading cause of cancer-related deaths and 11th most common malignancy in the USA [1]. Whereas the overall incidence and mortality of cancer is declining, pancreatic cancer incidence and mortality continue to increase worldwide [1, 2]. Despite advances in therapy over the last 40 years, disease-specific 5-year survival rates remain below 10% [3]. For non-resectable advanced pancreatic cancer, the development of combination cytotoxic regimens has resulted in modest survival gains, albeit with significant associated toxicities [4,5,6]. Limited data exists to guide patient risk stratification and treatment selection to optimize responses and minimize complications of therapy [7]. Studies in patient cohorts treated with gemcitabine-based regimens have previously correlated several biomarkers including lactate dehydrogenase, carcinoembryonic antigen, and carbohydrate antigen (CA) 19-9 with improved survival [8, 9].
Emerging evidence suggests that systemic and local inflammation may be critical determinants in carcinogenesis, progression of disease, and chemotherapeutic response [10, 11]. Prognostic biomarkers and scores utilizing surrogate markers for systemic inflammation including the modified Glasgow Prognostic Score and the neutrophil-to-lymphocyte ratio (NLR) have been associated with survival differences in patients with various malignancies [12, 13]. The NLR, in particular, has been noted to be an easily obtainable prognostic factor for multiple malignancies including colorectal, breast, and non-small-cell lung cancer [14]. Retrospective studies have elucidated that an elevated baseline NLR is associated with a worse overall survival in patients with resectable and advanced pancreatic adenocarcinoma [15,16,17]. Significantly, post hoc analyses of the prospective MPACT and NAPOLI-1 trials have demonstrated that an NLR > 5 is associated with a significantly worse overall survival compared with an NLR ≤ 5 in both pooled cohort and treatment-specific analyses [18, 19].
However, these prior studies of the NLR in pancreatic adenocarcinoma have included homogeneous populations with a few minority, i.e., non-Hispanic Black (NHB) or Hispanic patients [15,16,17,18,19]. Prospective clinical trials predominantly enroll non-Hispanic White (NHW) patients, and a disproportionally lower number of NHB and Hispanic patients [20]. The average baseline NLR for NHB patients may also be lower than that of NHW and Hispanic patients [21]. Additionally, in the USA, NHB patients have a higher overall incidence and age-adjusted death rate for pancreatic adenocarcinoma compared with NHW patients [22]. Differences in baseline comorbid characteristics including diabetes mellitus, body mass index, and tobacco exposure along with socioeconomic factors may further influence disparities in pancreatic adenocarcinoma survival and reflect putative changes in the systemic inflammatory milieu [21, 23].
Therefore, we investigated the association between an elevated NLR in patients with incurable pancreatic adenocarcinoma and survival in an ethnically diverse population. We hypothesized that an elevated NLR was independently associated with shortened survival in this population.
Methods
Study Population
We performed a retrospective chart review of patients treated at the Montefiore Medical Center (MMC), an urban tertiary care center that provides primary and specialty care to a predominantly minority population in Bronx, NY. We reviewed the MMC electronic medical record system using “Clinical Looking Glass,” a software application developed at MMC that allows clinicians and researchers to identify populations of interest from the medical center database to gather information on laboratory data, medications, demographics, and mortality [24].
Study Design
Patients over the age of 18 between January 1, 2006, and December 31, 2015 diagnosed with pathologically confirmed locally advanced or metastatic pancreatic ductal adenocarcinoma were included in the study. Patients with prior surgical resection were only included if progression to metastatic disease was noted on follow-up imaging and no cytotoxic chemotherapy had been administered in the 6 months prior to inclusion. Patients must have received chemotherapy to be included in the analysis and patients who had received chemotherapy prior to treatment at MMC were excluded from this study. All patient information was de-identified and stored as a Microsoft 2010 Excel file that was encrypted and password protected. The MMC institutional review board approved the study.
We collected the following baseline demographic and clinical data from medical record review: age, gender, ethnicity and race, date of diagnosis of locally advanced or metastatic cancer, primary tumor site (pancreas head/neck or body/tail), tumor surgical resection status, Eastern Cooperative Oncology Group (ECOG) performance status (PS), presence of liver metastases, body mass index prior to administration of chemotherapy, diagnosis of hypertension and/or type 2 diabetes mellitus, category of first-line chemotherapy administered (gemcitabine monotherapy, FOLFIRINOX, or other), any administration of radiation therapy, adjusted Charlson comorbidity score at diagnosis, and date of last follow-up or date of death. In calculation of the adjusted Charlson comorbidity score, diagnosis of metastatic or locally advanced disease was not included to evaluate only the contribution of additional baseline comorbidities. Laboratory values including serum CA 19-9 level, white blood cell count (WBC), hemoglobin, platelet count, neutrophil count, lymphocyte count, and albumin, within 48 h of chemotherapy initiation were collected. C-reactive protein level was available for fewer than 10% of patients and was therefore not included in the analysis. Chemotherapeutic agent doses and schedules were allocated per the discretion of the treating physician at the time of therapy. The NLR was calculated using the baseline neutrophil and lymphocyte count obtained on the day of or within 48 h preceding chemotherapy administration. A NLR cutoff of ≤ 5 or > 5 was selected based upon previously reported parameters [18, 19].
Statistical Analysis
Baseline characteristics were compared between NLR ≤ 5 and NLR > 5 patient subgroups. Pearson’s chi-square tests, or Fisher’s exact test in the case of limited data, were used to compare categorical/nominal variables between patient subgroups as defined above based on NLR. Survival time was defined as time from diagnosis of locally advanced or metastatic disease to date of death or last follow-up. Patients were followed through December 31, 2016. All patients alive at the time of last follow-up were censored at that time. Dates of death were verified via the MMC death registry, and when not available via the MMC database, the United States National Death Index [25]. Survival durations of patient groups of interest were summarized using a Kaplan-Meier plot. A Cox proportion hazard regression model was utilized in deriving effect estimates and associated 95% confidence intervals (CI). In the multivariable analysis, change in the effect estimate of NLR (≥ 10%) was employed as a criterion for adjustment for potential confounding, except for selected demographic and clinical variables, as well as comorbidity as assessed by the Charlson comorbidity index, which were kept in the model regardless as they were a priori identified as clinically important prognostic factors. Effect estimates are presented as hazard ratios (HRs) and the associated 95% CI. Model assumptions of proportional hazards were assessed with plots of Schoenfeld residuals vs. time. A computed p value < 0.05 (two-sided) was considered statistically significant. Statistical analysis was performed using the SAS 9.4 software package (SAS Institute Inc., Cary, NC, USA).
Results
A total of 567 patients diagnosed with pancreatic adenocarcinoma from 2006 to 2015 at MMC were identified (Fig. 1). Of these, 341 patients were excluded from the final analysis (70 referred directly to hospice care, 216 patients with stage I or II disease, and 55 with incomplete treatment data). The remaining 226 patients with locally advanced or metastatic disease underwent analysis for the study cohort, of whom 70 had a NLR > 5. Demographic and clinical characteristics between the NLR ≤ 5 and NLR > 5 patient subgroups were similar, with some exceptions (Table 1).
There were no significant differences in age, gender, body mass index, tobacco use, and ECOG PS between patients with a NLR ≤ 5 or NLR > 5 (Table 1). The median age of patients with a NLR ≤ 5 was 66 (range 44, 90) and for NLR > 5 was 65 (range 42, 88). Patients with a NLR ≤ 5 were more likely to be of NHB (30.8% vs. 20%) or other ethnicity (23.1% vs. 14.3%), whereas patients with a NLR > 5 were more likely to be NHW (21.4% vs. 12.2%) or Hispanic (44.3% vs. 34%). Patients with an NLR ≤ 5 were also more likely to have locally advanced disease than metastatic disease (19.2% vs. 5.7%) and a primary tumor located in the head or neck of the pancreas (68.6% vs. 51.4%). Patients with NLR > 5 were more likely to have liver metastases (58.6% vs. 40.4%) and albumin < 3.4 g/dL (38.6% vs. 15.4%). Baseline comorbidity measures, CA 19-9 levels, hemoglobin, platelet counts, chemotherapy regimen types, prior surgical resection, and palliative radiation were balanced between the groups.
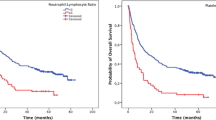
At the time of last follow-up, there were 181 deaths in the entire cohort, with a median follow-up of 8.8 months (interquartile range 5.1 to 15.8) and median survival of 10.7 months (95% CI 8.8, 12.7). Patients with a NLR ≤ 5 had a significantly greater survival time from diagnosis of locally advanced or metastatic disease than patients with a NLR > 5 (Fig. 2, median survival 12.0 vs. 7.4 months, HR 1.9, 95% CI 1.384, 2.596, p < 0.0001). Exploratory univariate survival estimates, summarized in Table 2, revealed as expected that chemotherapy with FOLFIRINOX (HR 0.434, 95% CI 0.285, 0.661) or other combination regimens (HR 0.634, 95% CI 0.441, 0.912) improved survival relative to gemcitabine monotherapy. Radiotherapy exposure was associated with improved survival in exposed patients (HR 0.517, 95% CI 0.374, 0.714). Univariate analysis revealed that a NLR > 5 (HR 1.9, 95% CI 1.384, 2.596), albumin of < 3.4 g/dL (HR 3.622, 95% CI 2.380, 5.512), anemia with Hgb ≤ 12 (HR 1.505, 95% CI 1.100, 2.060), presence of liver metastases (HR 1.951, 95% CI 1.427, 2.668), and ever-smoker status (HR 1.397, 95% CI 1.040, 1.876) were associated with worse survival in the cohort.
In the multivariable survival models, we incorporated chemotherapy, radiation therapy, and stage (locally advanced or metastatic disease) as stratification variables and demonstrated that a NLR > 5 compared with a NLR ≤ 5 remained significantly associated with a worse overall survival when adjusted for multiple a priori factors and exploratory variables from the univariate analysis (Table 3, HR 1.650, 95% CI 1.139, 2.390). Ever-smoker status (HR 1.691, 95% CI 1.188, 2.409), presence of liver metastases (HR 1.547, 95% CI 1.063, 2.251), and albumin of < 3.4 g/dL (HR 2.8, 95% CI 1.784, 4.395) continued to be significantly associated with worse survival, as previously reflected in the univariate analyses. The variable of race did not remain significant in the multivariable-adjusted analysis of NLR as a prognostic factor for survival (Table 3).
Discussion
Risk stratification models comprised of biomarkers that can be applied in routine clinical practice for prognostication and prediction in advanced pancreatic adenocarcinoma remain an area of active investigation. In this single-center study, we demonstrated that in an ethnically diverse population of patients with locally advanced or metastatic pancreatic adenocarcinoma, survival from time of diagnosis differed by NLR, such that after adjusting for clinically relevant variables, a NLR > 5 remained significantly associated with a decreased survival time when compared with a NLR ≤ 5.
Prior prognostic models have attempted to incorporate the NLR as a stratification variable in survival analysis in advanced pancreatic adenocarcinoma to characterize survival and chemotherapy risks [17, 26]. However, these models have employed disparate designs and heterogeneous inclusion criteria limiting their utility in clinical practice. The majority of prior models have also included predominantly Caucasian or Asian populations. To our knowledge, this study is the first to analyze the role of the NLR in an ethnically diverse population including a significant NHW and NHB population. Analysis of data from the National Health and Nutrition Examination Survey has suggested that the baseline NLR may be significantly lower for NHB and Hispanic patients compared with NHW’s reflecting a unique inflammatory milieu and potential differential responses to inflammation between ethnic groups [21]. Genetic differences may account in part for this variation as NHB patients have lower mean leukocyte counts compared with NHW and Hispanic patients, potentially due to the Duffy receptor antigen [27, 28]. Our study population reflected some of these prior findings, with a greater number of NHW and Hispanic patient’s having a NLR > 5 compared with non-Hispanic black patients. However, ethnic differences in NLR did not remain significant after multivariate adjustment and further studies will be necessary to ascertain prospectively whether these initially noted differences may be significant.
The underlying mechanism for the association between systemic inflammation, reflected through surrogate indices including the NLR, and clinical outcomes in malignancy remains elusive [29]. Complex tumor microenvironment interactions between repertoires of immune infiltrating cells engaged in subtle or overt communication with tumor cells allows for tumor proliferation, resistance of senescence, metabolic modulation, angiogenic growth, and metastatic spread [30]. Neutrophils play a multifaceted context-specific role in tumor development and have been implicated in various steps of oncogenic progression [31]. In pancreatic cancer, neutrophils may have a role in promoting tumor angiogenesis by secreting numerous cytokines including vascular endothelial growth factor and matrix metalloproteinase 9 [32]. Neutrophils may also foster the epithelial-mesenchymal transition and as a consequence stimulate metastasis in pancreatic cancer by secreting elastase [33]. The role of lymphocytes in the tumor microenvironment remains less certain, with studies indicating that pancreatic adenocarcinoma cells enhance the natural inflammatory cascade by secreting TGFβ and IL-10 resulting in differentiation of CD4+ T cells towards the TH2 lineage which in turn leads to enhanced tumor growth [34]. The production of TGFβ can stimulate neutrophil release of chemicals including nitric oxide synthase and arginase 1, which can concurrently attenuate the immune system by inhibiting cytotoxic CD8+ T lymphocyte antitumor responses and nurture a tumor microenvironment hospitable to neoplastic growth and metastatic spread [35, 36].
There are several limitations to our study related with its retrospective design. As a moderately sized single-center study, there was heterogeneity in the study population over the analyzed time period and the chemotherapeutic treatment regimens involved. By including both patients with locally advanced and metastatic disease, one would expect better survival outcomes associated with the cohort with locally advanced disease. Furthermore, this group is offered radiation therapy more often. Patients offered triplet combination chemotherapy typically have a superior baseline performance status compared with those who are offered alternate regimens. Tumor burden may also be associated with a higher NLR and worse outcomes and we adjusted for this by evaluating for the presence of liver metastases. Yet, after accounting for these variables in multivariate analysis, NLR remained a significant predictor of prognosis. However, we lacked complete data on ECOG PS, which may influence survival outcomes, and we attempted to account for this through an adjusted Charlson comorbidity score. As such, analysis of the potential role of performance status and comorbidity profiles is warranted in future cohorts.
Pre-treatment albumin represents another marker of nutritional status and systemic inflammation and has been associated with survival outcomes multiple malignancies [37]. Emerging recent data suggests that the neutrophil-to-lymphocyte ratio (NLR) and albumin are bidirectionally linked surrogate markers reflecting a complex interaction between inflammation, body composition, and cancer progression [38]. In our multivariate model, the hazard ratios for survival for albumin and NLR were attenuated suggesting a biologically plausible interaction. Yet, our retrospective analysis is unable to unravel the precise mechanisms and relationships involved in this series of interactions. Future prospective studies in an ethnically diverse patient population of advanced pancreatic cancer patients should consider correlations of body composition, CRP, NLR, and albumin to develop a comprehensive inflammation-based prognostic index.
The current study has several important strengths including a large ethnically diverse patient cohort reflective of clinical practice in an urban setting, a priori selection of clinically relevant variables based on prior studies, and a conservative cutoff value for NLR. No current standard cutoff value exists for NLR and there is marked variability in the index value selected across prior NLR studies in pancreatic adenocarcinoma [39]. Although a lower cutoff may have increased sensitivity, in the absence of prospective criteria, we selected a conservative value of 5 for the NLR based on the MPACT and NAPOLI-1 analyses [18]. We also restricted our analysis of the NLR to the peri-chemotherapy period to reduce the putative influence of systemic factors, infections, and medications (including prophylactic steroids) on neutrophil counts and therefore could not analyze the effect of longitudinal trends in NLR and survival. Similarly, the heterogeneity of chemotherapy regimens used over the studied time period limited predictive modeling of the NLR. Further studies are warranted including defined treatment groups to discern the potential predictive effect of NLR.
Our findings support data reported by other retrospective series which conclude that an elevated NLR is an independent marker of poor prognosis in advanced pancreatic adenocarcinoma within an ethnically diverse patient population. Therefore, NLR may be a valuable factor in driving therapeutic decisions for patients and merits further investigation in prospective clinical trials.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016 (in eng). CA: Cancer J Clin. 2016;66(1):7–30. https://doi.org/10.3322/caac.21332.
Malvezzi M, Bertuccio P, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2014. Ann Oncol. 2014;25(8):1650–6. https://doi.org/10.1093/annonc/mdu138.
C. S. Yabar and J. M. Winter, “Pancreatic cancer: a review,” Gastroenterology Clinics of North America, vol. 45, no. 3, pp. 429-445, 9// 2016, https://doi.org/10.1016/j.gtc.2016.04.003.
Burris HA 3rd, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial in eng. J Clin Oncol : Off J Am Soc Clin Oncol. 1997;15(6):2403–13. https://doi.org/10.1200/jco.1997.15.6.2403.
D. D. Von Hoff et al., Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine(in eng) N Engl J Med, vol. 369, no. 18, pp. 1691-703, Oct 31 2013, 10.1056/NEJMoa1304369.
T. Conroy et al., “FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer,” (in eng), The New England journal of medicine, vol. 364, no. 19, pp. 1817-25, May 12 2011, 10.1056/NEJMoa1011923.
Le N, Sund M, Vinci A. Prognostic and predictive markers in pancreatic adenocarcinoma (in eng). Digest Liver Dis: Off J Italian Soc Gastroenterol Italian Assoc Stud Liver. 2016;48(3):223–30. https://doi.org/10.1016/j.dld.2015.11.001.
T. M. Bauer et al., Carbohydrate antigen 19-9 is a prognostic and predictive biomarker in patients with advanced pancreatic cancer who receive gemcitabine-containing chemotherapy: a pooled analysis of 6 prospective trials in eng, Cancer, vol. 119, no. 2, pp. 285-92, Jan 15 2013, 10.1002/cncr.27734.
M. Haas et al., “Prognostic value of CA 19-9, CEA, CRP, LDH and bilirubin levels in locally advanced and metastatic pancreatic cancer: results from a multicenter, pooled analysis of patients receiving palliative chemotherapy,” (in eng), J Cancer Res Clin Oncol, vol. 139, no. 4, pp. 681-689, 2013, https://doi.org/10.1007/s00432-012-1371-3.
Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms (in eng). Nat Rev Cancer. 2013;13(11):759–71. https://doi.org/10.1038/nrc3611.
Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer (in eng). Cell. 140(6):883–99, Mar 19 2010. https://doi.org/10.1016/j.cell.2010.01.025.
Proctor MJ, McMillan DC, Morrison DS, Fletcher CD, Horgan PG, Clarke SJ. A derived neutrophil to lymphocyte ratio predicts survival in patients with cancer (in eng). Br J Cancer. 2012;107(4):695–9. https://doi.org/10.1038/bjc.2012.292.
Proctor MJ, et al. An inflammation-based prognostic score (mGPS) predicts cancer survival independent of tumour site: a Glasgow Inflammation Outcome Study (in eng). Br J Cancer. 2011;104(4):726–34. https://doi.org/10.1038/sj.bjc.6606087.
Templeton AJ, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis in eng. J Natl Cancer Inst. 2014;106(6):dju124. https://doi.org/10.1093/jnci/dju124.
Stotz M, et al. “Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer,” (in eng). Br J Cancer. 2013;109(2):416–21. https://doi.org/10.1038/bjc.2013.332.
Xue P, Kanai M, Mori Y, Nishimura T, Uza N, Kodama Y, et al. Neutrophil-to-lymphocyte ratio for predicting palliative chemotherapy outcomes in advanced pancreatic cancer patients (in eng). Cancer Med. 2014;3(2):406–15. https://doi.org/10.1002/cam4.204.
Kou T, Kanai M, Yamamoto M, Xue P, Mori Y, Kudo Y, et al. Prognostic model for survival based on readily available pretreatment factors in patients with advanced pancreatic cancer receiving palliative chemotherapy. Int J Clin Oncol. 2016;21(1):118–25. https://doi.org/10.1007/s10147-015-0864-x.
Goldstein D, et al. nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III trial(in eng). J Natl Cancer Inst. 2015;107(2). https://doi.org/10.1093/jnci/dju413.
Wang-Gillam A, Hubner RA, Siveke JT, von Hoff D, Belanger B, de Jong FA, et al. NAPOLI-1 phase 3 study of liposomal irinotecan in metastatic pancreatic cancer: final overall survival analysis and characteristics of long-term survivors (in eng). Eur J Cancer. 2019;108:78–87. https://doi.org/10.1016/j.ejca.2018.12.007.
Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities(in eng). Jama. 2004;291(22):2720–6. https://doi.org/10.1001/jama.291.22.2720.
Azab B, Camacho-Rivera M, Taioli E. Average values and racial differences of neutrophil lymphocyte ratio among a nationally representative sample of United States subjects (in eng). PLoS One. 2014;9(11):e112361. https://doi.org/10.1371/journal.pone.0112361.
QuickStats. age-adjusted death rates* for top five causes of cancer death,(dagger) by race/Hispanic ethnicity - United States, 2014 (in eng). MMWR Morb Mortal Wkly Rep. 2016;65(37):989. https://doi.org/10.15585/mmwr.mm6536a10.
Silverman DT, et al. Why do Black Americans have a higher risk of pancreatic cancer than White Americans? Epidemiology Cambridge, Mass. 2003;14(1):45–54. https://doi.org/10.1097/01.ede.0000034393.39604.ff.
Bellin E, Fletcher DD, Geberer N, Islam S, Srivastava N. Democratizing information creation from health care data for quality improvement, research, and education-the Montefiore Medical Center Experience. Acad Med : J Assoc Am Med Coll. 2010;85(8):1362–8. https://doi.org/10.1097/ACM.0b013e3181df0f3b.
Lash TL, Silliman RA. A comparison of the National Death Index and Social Security Administration databases to ascertain vital status. Epidemiology Cambridge, Mass. 2001;12(2):259–61.
Park HS, Lee HS, Park JS, Park JS, Lee DK, Lee SJ, et al. Prognostic scoring index for patients with metastatic pancreatic adenocarcinoma. Cancer Res Treat. 2016;48(4):1253–63. https://doi.org/10.4143/crt.2015.400.
Hsieh MM, Everhart JE, Byrd-Holt DD, Tisdale JF, Rodgers GP. Prevalence of neutropenia in the U.S. population: age, sex, smoking status, and ethnic differences(in eng). Ann Intern Med. 2007;146(7):486–92.
D. Reich et al., Reduced neutrophil count in people of African descent is due to a regulatory variant in the Duffy antigen receptor for chemokines gene(in eng) PLoS Genet, vol. 5, no. 1, p. e1000360, 2009, 10.1371/journal.pgen.1000360.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation,(in eng). Cell. 2011;144(5):646–74. https://doi.org/10.1016/j.cell.2011.02.013.
Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment(in eng). Cancer Cell. 2012;21(3):309–22. https://doi.org/10.1016/j.ccr.2012.02.022.
Swierczak A, Mouchemore KA, Hamilton JA, Anderson RL. Neutrophils: important contributors to tumor progression and metastasis (in eng). Cancer Metastasis Rev. 2015;34(4):735–51. https://doi.org/10.1007/s10555-015-9594-9.
Bausch D, Pausch T, Krauss T, Hopt UT, Fernandez-del-Castillo C, Warshaw AL, et al. Neutrophil granulocyte derived MMP-9 is a VEGF independent functional component of the angiogenic switch in pancreatic ductal adenocarcinoma (in eng). Angiogenesis. 2011;14(3):235–43. https://doi.org/10.1007/s10456-011-9207-3.
Grosse-Steffen T, Giese T, Giese N, Longerich T, Schirmacher P, Hänsch GM, et al. Epithelial-to-mesenchymal transition in pancreatic ductal adenocarcinoma and pancreatic tumor cell lines: the role of neutrophils and neutrophil-derived elastase in eng. Clin Dev Immunol. 2012;2012:720768. https://doi.org/10.1155/2012/720768.
Bellone G, et al. Tumor-associated transforming growth factor-beta and interleukin-10 contribute to a systemic Th2 immune phenotype in pancreatic carcinoma patients (in eng). Am J Pathol. 1999;155(2):537–47.
Coffelt SB, et al. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis(in eng). Nature. 2015;522(7556):345–8. https://doi.org/10.1038/nature14282.
Ocana A, Nieto-Jimenez C, Pandiella A, Templeton AJ. Neutrophils in cancer: prognostic role and therapeutic strategies(in eng). Mol Cancer. 2017;16(1):137. https://doi.org/10.1186/s12943-017-0707-7.
Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9:69. https://doi.org/10.1186/1475-2891-9-69.
Feliciano EMC, et al. Association of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: results from the C SCANS study, in eng. JAMA Oncol. 2017;3(12):e172319. https://doi.org/10.1001/jamaoncol.2017.2319.
Vano YA, et al. Optimal cut-off for neutrophil-to-lymphocyte ratio: fact or fantasy? A prospective cohort study in metastatic cancer patients(in eng). PLoS One. 2018;13(4):e0195042. https://doi.org/10.1371/journal.pone.0195042.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Preliminary analyses and portions of this study were presented as part of an electronic abstract at the 53rd Annual Meeting of the American Society of Clinical Oncology, 2017, Chicago, USA.
Rights and permissions
About this article
Cite this article
Shusterman, M., Jou, E., Kaubisch, A. et al. The Neutrophil-to-Lymphocyte Ratio is a Prognostic Biomarker in An Ethnically Diverse Patient Population with Advanced Pancreatic Cancer. J Gastrointest Canc 51, 868–876 (2020). https://doi.org/10.1007/s12029-019-00316-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-019-00316-8