Abstract
Objective
Total neoadjuvant therapy (TNT) combining chemoradiotherapy (CRT) with chemotherapy (CT) was a novel pre-surgical approach to cancer treatment. This meta-analysis aimed to compare the clinical outcomes between neoadjuvant CRT (nCRT) with induction CT and nCRT with consolidated CT in locally advanced rectal cancer (LARC) patients.
Method
In July 2022, a literature search was conducted using the following public databases: PubMed, MEDLINE, Embase, the Cochrane Library, and Web of Science, retrieved all relevant articles comparing nCRT-combining induction CT with nCRT-combining-consolidated CT treatments for LARC patients.
Results
Four eligible studies were identified, including a total of 995 LARC patients: 473 in the nCRT with consolidated CT group and 522 in the nCRT with induction CT group. The organ preservation (OP) rate of the nCRT with consolidated CT group was higher than that of the nCRT with induction CT group (RR [relative risk]: 1.53; 95% CI (confidence interval): 1.09–2.14). The pathological complete response (PCR, RR: 1.22; 95% CI 0.37–2.17), the 3-year disease-free survival (DFS, RR 1.02; 95% CI 0.71–1.46), the local recurrence (LR, RR 0.98; 95% CI 0.52–1.85), rates of R0 resection (RR 0.74; 95% CI 0.55–1.10), compliance (RR 0.52; 95% CI 0.12–2.26), and grade 3-–4 toxicities (RR 0.78; 95% CI 0.57–1.06) were all similar between the two groups.
Conclusion
In this meta-analysis of TNT regimens for rectal cancer, consolidative CT following nCRT was associated with similar PCR, 3-year DFS, LR, R0 resection, compliance, and grade 3–4 toxicities compared to induction CT prior to nCRT but a higher rate of organ preservation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) represents the second most fatal and third most common malignancy worldwide. In 2020, about 147,950 people were diagnosed with CRC, and 53,200 people died of CRC. A considerable proportion of new CRC cases (17,930/147,950) were diagnosed in younger adults (ages < 50 years), with a reported 3640 death toll [1].
Locally advanced rectal cancer (LARC) is a subtype of CRC characterized by tumors invading or extending in close proximity to the mesorectal fascia. Treatment of LARC often involves administration of nCRT, conservative surgery for total mesorectum resection, and adjunctive chemotherapy (ACT) to remove any residual cancerous cells or lesions [2].
TNT for CRC refers to a combinatorial therapy of CRT and CT before surgical intervention. Recent clinical investigations have been focusing on improving PCR and DFS of CRC using TNT approaches [3]. TNT, as a prospective therapy for LARC patients, has been previously studied in single-arm clinical trials with small sample sizes. However, the findings revealed large PCR variations (20–40%) in LARC patients post-TNT treatment [4, 5]. In a retrospective study, LARC patients subjected to TNT with an additional induction chemotherapeutic dose on top of the standard CRT prior to surgery showed an improved PCR rate in comparison to the ACT-treated patients (36% vs. 21%) [6].
Recently, several clinical trials have started to explore the possibility of separately incorporating CT and CRT into the neoadjuvant LARC regimens. However, it is not clear which combination of TNT imparts the highest therapeutic potential of significantly improving the PCR [7]. Nonetheless, TNT interventions exhibit a high level of safety and efficacy in LARC patients and are likely becoming a standard remedy for LARC in the near future [8].
Given that TNT shows higher PCR than conventional nCT, it should be routinely practiced in LARC patients. There are two distinct TNT treatment modalities: CRT with induction CT and CRT with consolidated CT. The main difference between the two modalities lies in the timing of CT induction with respect to CRT initiation. Currently, there are no studies available to compare which of the two modalities would give superior clinical outcomes. In this study, we aim to conduct a meta-analysis to comparatively evaluate rates of OP, PCR, 3-year DFS, LR, R0 resection, compliance, and grade 3–4 toxicities between the nCRT combined with induction CT and nCRT combined with consolidated CT in LARC patients.
Materials and Methods
Literature Search Strategy
We retrieved relevant research articles published on PubMed, MEDLINE, Embase, Cochrane, and Web of Science between the study inception and July 2022. We primarily identified clinical trial-based articles comparing TNT treatment modalities in cohorts of CRC patients using the following MeSH terms: “rectum tumor OR rectal cancer,” “neoplasm,” “chemoradiotherapy,” “induction chemotherapy,” “consolidation chemotherapy,” “neoadjuvant therapy,” and “preoperative.”
Selection Criteria
For this meta-analysis, eligible studies were screened based on the following inclusion/exclusion criteria.
A study was included if it was (1) a randomized, controlled trial (RCT); (2) mainly focusing on nCRT with induction CT and/or nCRT with consolidated CT for treating CRC; (3) published in English; and (4) involving human subjects only.
On the other hand, a study was excluded if it was (1) not an RCT; (2) performed in a cohort of < 30 participants; (3) a duplicate study; (4) not relevant; and (5) not published in English.
Data Extraction and Quality Control
Two investigators independently searched, screened, reviewed, and extracted data from the eligible studies. Any discrepancy in the opinion of the two investigators was further reviewed and resolved by a third investigator.
The following data were extracted from our screened, eligible studies: (1) general information, including authors’ names, publication time, country, cohort size, radio- and chemotherapeutic regimens and doses, and sequence of treatment (simultaneous or sequential); (2) OP rate; (3) PCR; (4) 3-year DFS; (5) LR; (6) R0 resection rate; (7) compliance rate; and (8) grade 3–4 toxicities.
The primary endpoint is an OP that is defined as TME (total mesorectal excision)-free survival measured in the intention-to-treat population. The secondary endpoints are PCR and DFS. PCR is defined as the disappearance of all invasive cancer in the rectum upon completion of TNT, although some authors require clearance of residual tumors in axillary nodes as well. DFS, frequently used under adjuvant settings, is defined as the time from randomization to recurrence of tumor or death. It is a direct measure of clinical benefits when the efficacy of an experimental therapy outweighs its toxicity. Other secondary outcomes included LR, R0 resection, compliance, and grade 3–4 toxicities.
The Jadad scale (JS) was utilized by two investigators to evaluate the quality of pre-screened articles. Only the RCTs with JS scores > 3 passed the QC check for the final analysis.
Statistical Analysis
This meta-analysis was registered under PROSPERO (Registration # CRD42022300059) and conducted by Stata 16.0/MP. The relative risk with a 95% confidence interval was generated for binary variants. Q tests were applied to heterogeneous results. If I2 was less than 50% and p was more than 0.01, a fixed-effect (FE) model was implemented; otherwise, a random-effect (RE) model was used. If heterogeneity was observed, sensitivity and subgroup analyses were carried out. Funnel plots were generated to evaluate the risk of publication bias (PB), and PB was subsequently quantified by Egger’s regression. All results with p < 0.05 were regarded as statistically significant.
Results
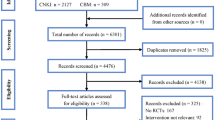
Our retrieval strategy initially obtained 128 articles. After applying the pre-determined inclusion/exclusion criteria, the 128 articles were condensed to only 4 that reported in-depth descriptions of clinical trials and were selected for our meta-analysis (Fig. 1). Tables 1, 2, 3, listed the characteristics and details of the above four clinical trials.
Two of the four articles analyzed the effects of TNT treatment modalities on the OP rate. No statistical heterogeneity was reported between the groups (p = 0.37), when an FE model was employed. The OP rate of the nCRT with consolidated CT group was significantly higher than that of the nCRT with induction CT group (RR 1.53; 95% CI 1.09–2.14) (Fig. 2A).
Three of the four articles analyzed PCR rates. An RE model was applied to these articles, since there was significant statistical heterogeneity between the two groups (p = 0.000). After thoroughly scrutinizing the results, we did not find any therapeutic difference in subjects treated with nCRT and induction CT or nCRT and consolidation CT (RR 0.74; 95% CI 0.15–3.54) (Fig. 2B).
Two of the four articles were analyzed for the influence of TNT treatment modalities on the 3-year DFS rate. No statistical heterogeneity was observed between these two groups (p = 0.186); hence, an FE model was utilized. The 3-year DFS outcome of the nCRT with consolidated CT group was comparable to that of the nCRT with induction CT group (RR 1.02; 95% CI 0.71–1.46) (Fig. 2C).
The LR effect was assessed in 3 articles and revealed no statistical heterogeneity in results between these groups (p = 0.778). Hence, an FE model was applied. The outcome analysis indicated comparable LR effects of both therapeutic regimens on the LR (RR 0.98; 95% CI 0.52–1.85) (Fig. 2D).
Two of the four articles compared the R0 resection rates between the two TNT modality groups. An FE model was employed due to an absence of statistical heterogeneity (p = 0.190). Comparable R0 resection outcomes were observed in both treatment modalities (RR 0.74; 95% CI 0.55–1.10) (Fig. 3A).
The compliance rate was measured in 3 articles and showed significant statistical heterogeneity (p = 0.007); hence, anRE model was used. The compliance rate did not vary between the two groups (RR 0.52; 95% CI 0.12–2.26) (Fig. 3B).
Three of the four articles analyzed the grade 3–4 toxicities. No statistical heterogeneity was observed between the groups (p = 0.800). Therefore, a FE model was employed and showed no remarkable diversities in outcomes between the two groups (RR 0.78; 95% CI 0.57–1.06) (Fig. 3C).
Egger’s regression tests were conducted to evaluate the PB and symmetry of the funnel plot. There was no detectable PB in our study (p > 0.05) (Fig. 4). Consistently, the funnel plot was symmetric.
Discussion
To the best of our knowledge, our study represents the first meta-analysis comparing the clinical outcomes between preoperative nCRT with induction CT and that with consolidated CT in CRC. The pooled analysis demonstrated a significantly higher organ preservation rate in patients treated with nCRT and consolidated CT than that with nCRT and induction CT. No significant differences were observed between the two treatment modality groups in terms of PCR, 3-year DFS, LR, R0 resection, compliance, and grade 3–4 toxicities.
The National Comprehensive Cancer Network (NCCN) guidelines suggest that multiple small trials should be performed so that the efficacy of respective CT courses can be determined before initiating CRT and surgical excision to avoid postoperative CT [9,10,11,12,13,14]. In the Spanish GCR-3 phase II RCT, patients received a combined regimen of oxaliplatin and capecitabine (CapeOX) either before the CRT or postoperatively [11, 15]. The results showed that induction CT elicited reduced toxicity and better tolerance in CRC patients, thus improving the PCR rate. In another stage II trial, patients were randomized to CRT and surgery groups in the presence or absence of FOLFOX [intravenous (IV) oxaliplatin and leucovorin calcium for > 2 h on day 1, and IV 5-fluorouracil continuously for > 44 h on days 1–3] induction CT [16]. No significant differences were observed in treatment outcomes between these two clinical trial arms. Notably, patients receiving induction CT encountered greater toxicities. In the stage II CRC trial AVACROSS, the safety and potency of bevacizumab supplemented to induction CT with CapeOX before the capecitabine/bevacizumab-CRT and surgical interventions, were evaluated [14]. The treatment plan was well tolerated, with a PCR rate of 36%. A meta-analysis of two phase II clinical trials, EXPERT and EXPERT-C, also evaluated the efficacy and safety of neoadjuvant CT before CRT and surgery [17]. Of the 269 patients tested, 91.1% finished the induction CT, 88.1% completed CRT, and 89.2% received therapeutic surgeries. The 5-year PFS and OS rates were 66.4% and 73.3%, respectively.
TNT is increasingly adopted for CRC and can be administered exclusively as an induction CT before CRT or in a consolidated manner after CRT. Thus, the optimal sequence of TNT needs to be established. The prior CAO/Ark/AIO-12 and the OPRA (organ preservation of rectal adenocarcinoma) trials both investigated the TNT sequence, with different duration and clinical approaches [18,19,20,21]. At a median follow-up of 2 years, no significant differences were reported in 3-year DFS, while the OP was significantly elevated in the consolidation arm, which is highly consistent with our meta-analysis results. Overall, the prior studies and our data indicate that although the sequence of treatment does not significantly affect clinical outcomes, consolidation CR after long-course chemoradiotherapy (LCRT) is consistently associated with better tolerance and organ preservation. However, the optimal drug regimen remains a matter of debate since the CAO/Ark/AIO-12 and OPRA studies used FOLFOX (a doublet), while another clinical study (PRODIGE-23) that also reported high PCR rates used FOLFIRINOX (a triplet). This debate may be addressed by ongoing clinical trials such as GRECCAR12 with a FOLFIRINOX regimen.
The randomized phase II KIR clinical study was conducted to optimize the treatment sequence of chemotherapy for CRC patients. In this study, 180 patients were randomized (2:1) to either Arm A (AA) with 6 cycles of FOLFOX prior to high-dose rate brachytherapy (HDRBT) and surgery plus adjuvant 6 cycles of FOLFOX or Arm B (AB) with neoadjuvant HDRBT with surgery and adjuvant 12 cycles of FOLFOX. No statistical differences were found in PCR rate, LR, and DFS between the two arms [22], which is in line with our meta-analysis results. Interesting, the “pick-the-winner” CAO/Ark/AIO-12 trial compared induction versus consolidation FOLFOX and found that the consolidation arm was better at achieving a higher PCR rate and lower grade 3–4 toxicities. In contrast, our meta-analysis showed no such differences in the above clinical outcomes. More randomized controlled trials with different samples sizes and comparable parameters may be needed to explain the discrepancies between the two studies.
To assess whether adding cycles of FOLFOX between CRT and surgery could increase PCR, a phase II trial was conducted to include groups of LARC patients who received different cycles of consolidation chemotherapy (FOLFOX) [18]. The PCR rates from these groups were 18%, 25%, 30%, and 38%, respectively, suggesting that longer consolidation CT leads to better PCR rates. Furthermore, increasing consolidation CT also led to improved 5-year DFS in a different study) [23]. In contrast to the above data, a study comparing FOLFOX with fluorouracil/(oxaliplatin)-based CRT after short-course chemoradiotherapy (SCRT) found that consolidation CT provided better 3-year overall survival than CRT (73% vs. 65%) but had no effects on PCR, DFS, or R0 resection [24]. Results of the similarly designed RAPIDO trial are pending [25].
In a phase II trial assessing induction therapy before CRT [11, 15], 108 patients received capecitabine/oxaliplatin either before CRT or as an adjuvant after. Compared to the adjuvant group, induction CT yielded lower grade 3–4 toxicities and better compliance, whereas the 5-year DFS and PCR showed no differences [11, 26]. Similarly, patients treated with FOLFOX induction before CRT demonstrated significantly higher complete response than those treated with adjuvant CT in a retrospective cohort [18].
In a pioneer study carried out by Van Zoggel et al. [27], the 3-year OS, local recurrent-free survival, and distant metastasis-free survival all increased for patients with a PCR compared to those without a PCR. In a recently published phase III clinical trial, Conroy et al. [28] compared neoadjuvant chemotherapy (induction FOLFIRINOX, CRT, and adjuvant CT/capecitabine) with standard-of-care (CRT and adjuvant CT) and found that the PCR rate was significantly higher in patients who received induction (27.5% vs. 11.7%). The disease-free survival was also improved (75.7%vs. 68.5%), although the three-year overall survival data have not yet matured.
Limitations
Our meta-analysis has several limitations. For instance, the sample size was relatively small and the follow-up time was short for the evaluation of long-term survival outcomes. Second, some of the outcome measures were incomplete; we did not have sufficient data to calculate a pooled overall survival. Third, we did not directly compare induction and consolidation programs in this work; these data will be collected from ongoing trials and analyzed. More randomized controlled trials will be carried out for best beneficiaries and choice of indications in both nCRT with induction CT, and nCRT with consolidated CT groups. Up to now, the optimal TNT in CRC patients is still under debate. Rather than discussing the merits of TNTs, our primary aim is to demonstrate which patient group can benefit most from TNT, and further extrapolations can be made with our stratified analysis in the future. Despite these limitations, we believe that this study made a valuable contribution and treatment reference to TNT.
Conclusion
This meta-analysis compared the clinical outcomes between two TNT treatment modalities: nCRT with induction CT and nCRT with consolidated CT in CRC patients. Our results showed that the consolidation arm significantly improved organ preservation but had no impacts on PCR, 3-year DFS, LR, R0 resection, compliance, and grade 3–4 toxicities.
Data Availability
All the data for this article can be found on PubMed, MEDLINE, Embase, the Cochrane Library, and Web of Science.
References
Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics. CA Cancer J Clin. 2020;70:145–64.
De Felice F, et al. Total neoadjuvant treatment in locally advanced rectal cancer, Transl Oncol. 2021.
Kong JC, et al. Ann Surg Oncol. Total neoadjuvant therapy in locally advanced rectal cancer: a systematic review and meta analysis of oncological and operative outcomes. 2021;28:7476–86.
Chua YJ, Barbachano Y, Cunningham D, et al. Neoadjuvant capecitabine and oxaliplatin before chemoradiotherapy and total mesorectal excision in MRI-defined poor-risk rectal cancer: a phase 2 trial. Lancet Oncol. 2010;11:241–8.
Gao YH, Lin JZ, An X, et al. Neoadjuvant sandwich treatment with oxaliplatin and capecitabine administered prior to, concurrently with, and following radiation therapy in locally advanced rectal cancer: a prospective phase 2 trial. Int J Radiat Oncol Biol Phys. 2014;90:1153–60.
Cercek A, Roxburgh CSD, Strombom P, et al. Adoption of total neoadjuvant therapy for locally advanced rectal cancer. JAMA Oncol. 2018;4: e180071.
Ludmir EB, et al. Total neoadjuvant therapy for rectal cancer: an emerging option. Cancer. 2017;123:1497–506.
Gilshtein H, Ghuman A, Dawoud M, Yellinek S, Kent I, Sharp SP, et al. Total Neoadjuvant Treatment for Rectal Cancer: Preliminary Experience. Ann Surg. 2021;87:708–13.
Cercek A, Goodman KA, Hajj C, et al. Neoadjuvant chemotherapy first, followed by chemoradiation and then surgery, in the management of locally advanced rectal cancer. J Natl Compr Canc Netw. 2014;12:513–9.
Chau I, Brown G, Cunningham D, et al. Neoadjuvant capecitabine and oxaliplatin followed by synchronous chemoradiation and total mesorectal excision in magnetic resonance imaging-defined poor-risk rectal cancer. J Clin Oncol. 2006;24:668–74.
Fernandez-Martos C, Pericay C, Aparicio J, et al. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo Cancer de Recto 3 study. J Clin Oncol. 2010;28:859–65.
Perez K, Safran H, Sikov W, et al. Complete neoadjuvant treatment of rectal cancer: the Brown University Oncology Group CONTRE study. Am J Clin Oncol. 2017;40:283–7.
Kim SY, Joo J, Kim TW, et al. A randomized phase 2 trial of consolidation chemotherapy after preoperative chemoradiation therapy versus chemoradiation therapy alone for locally advanced rectal cancer: KCSG CO 14–03. Int J Radiat Oncol Biol Phys. 2018;101:889–99.
Nogue M, Salud A, Vicente P, et al. Addition of bevacizumab to XELOX induction therapy plus concomitant capecitabine-based chemoradiotherapy in magnetic resonance imaging-defined poor-prognosis locally advanced rectal cancer: the AVACROSS study. Oncologist. 2011;16:614–20.
Fernandez-Martos C, Garcia-Albeniz X, Pericay C, et al. Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: long-term results of the Spanish GCR-3 phase II randomised trial. Ann Oncol. 2015;26:1722–8.
Marechal R, Vos B, Polus M, et al. Short course chemotherapy followed by concomitant chemoradiotherapy and surgery in locally advanced rectal cancer: a randomised multicentric phase II study. Ann Oncol. 2012;23:1525–30.
Sclafani F, Brown G, Cunningham D, et al. PAN-EX: a pooled analysis of two trials of neoadjuvant chemotherapy followed by chemoradiotherapy in MRI-defined, locally advanced rectal cancer. Ann Oncol. 2016;27:1557–65.
Garcia-Aguilar J, Patil S, Gollub MJ. Organ preservation in patients with rectal adenocarcinoma treated with total neoadjuvant therapy. J Clin Oncol. 2022;2546–56.
Bahadoer RR, Dijkstra EA, van Etten B, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:29–42.
Conroy T, Bosset JF, Etienne PL, et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:702–15.
Fokas E, Allga¨uer M, Polat B, et al. Randomized phase II trial of chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for locally advanced rectal cancer: CAO/ARO/AIO-12. J Clin Oncol. 2019;37:3712–3222.
Garant A, Kavan P, Martin AG, et al. Optimizing treatment sequencing of chemotherapy for patients with rectal cancer: the KIR randomized phase II trial. Radiother Oncol. 2021;155:237–45.
Marco MR, Zhou L, Patil S, et al. Consolidation mFOLFOX6 chemotherapy after chemoradiotherapy improves survival in patients with locally advanced rectal cancer: final results of a multicenter phase II trial. Dis Colon Rectum. 2018;61:1146–55.
Bujko K, Wyrwicz L, Rutkowski A, et al. Long-course oxaliplatin-based preoperative chemoradiation versus 535 Gy and consolidation chemotherapy for cT4 or fixed cT3 rectal cancer: results of a randomized phase III study. Ann Oncol. 2016;27:834–42.
Nilsson PJ, van Etten B, Hospers GA, et al. Short-course radiotherapy followed by neo-adjuvant chemotherapy in locally advanced rectal cancer--the RAPIDO trial. BMC Cancer 201313:279.
Golo D, But-Hadzic J, Anderluh F, Brecelj E, Edhemovic I, Jeromen A, Omejc M, Oblak I, Secerov-Ermenc A, Velenik V. Induction chemotherapy, chemoradiotherapy and consolidation chemotherapy in preoperative treatment of rectal cancer - long-term results of phase II OIGIT-01 Trial. Radiol Oncol. 2018;52(3):267–74.
van Zoggel DMGI, Bosman SJ, Kusters M, et al. Preliminary results of a cohort study of induction chemotherapy-based treatment for locally recurrent rectal cancer. Br J Surg. 2018;105(4):447–52.
Conroy T, Lamfichekh N, Etienne P-L, et al. Total neoadjuvant therapy with mFOLFIRINOX versus preoperative chemoradiation in patients with locally advanced rectal cancer: final results of PRODIGE 23 phase III trial, a UNICANCER GI trial. J Clin Oncol. 2020;38(suppl 15):4007.
Author information
Authors and Affiliations
Contributions
Pengkhun Nov acquisition of data, analyzing, interpretation of data, and drafting the article; Kunpeng Du and Jiqiang Li designing, revising, and guiding the study. The authors read and approved.
Corresponding author
Ethics declarations
Ethics approval
Not applicable
Consent for publication
All the authors of the article agreed to be published in the journal.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nov, P., Du, K., Huang, Z. et al. A Meta-analysis of Total Neoadjuvant Therapies Combining Chemoradiotherapy with Induction or Consolidated Chemotherapy for Locally Advanced Rectal Cancer. J Gastrointest Canc 54, 693–702 (2023). https://doi.org/10.1007/s12029-022-00864-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-022-00864-6