Abstract
Background
Hyperosmolar therapy is the cornerstone of medical management of sustained elevated intracranial pressure from cerebral edema. Acute intracranial hypertension and herniation is a medical emergency that requires rapid treatment and stabilization to prevent secondary brain injury or death. Intravenous hypertonic sodium chloride (NaCl) 23.4% is an effective treatment modality commonly used in this setting. Because of its high osmolarity, use has historically been limited primarily to central venous line administration as an intermittent infusion due to concerns about thrombophlebitis, injection site pain, and tissue necrosis or injury with extravasation. The objective of this analysis was to prospectively evaluate the safety of administration of 23.4% NaCl as a rapid intravenous push over 2–5 min.
Methods
A prospective analysis of patients admitted between April 2021 and December 2021 who received 23.4% NaCl intravenous push over 2–5 min in a central or peripheral line was performed. Safety end points included incidence of new onset hypotension [defined as systolic blood pressure (SBP) < 90 mm Hg or SBP decrease of at least 20 mm Hg], bradycardia (defined as heart rate < 50 beats per minute), and infusion site reactions documented within 1 h of administration. For secondary safety outcomes, highest and lowest SBP and lowest heart rates documented within 1 h before 23.4% NaCl administration were compared with values collected within 1 h post administration and evaluated by mixed-design analysis of variance test with adjustment for peripheral versus central line administration.
Results
We identified 32 patients who received 79 administrations of 23.4% NaCl through a central line or peripheral line during the study period. An SBP decrease of at least 20 mm Hg was observed in 13% of patients, an SBP < 90 mm Hg occurred in 16% of patients, and bradycardia occurred in 3% of patients who received 23.4% NaCl. Injection site pain was reported by one patient without documented thrombophlebitis, cellulitis, or tissue damage. Pain was not reported during two subsequent administrations in the same patient. There was no documented occurrence of soft tissue injury or necrosis in any patient. Compared with baseline vital signs before 23.4% NaCl administration, no difference in vital signs post administration was observed.
Conclusions
Central and peripheral administration of 23.4% NaCl over 2–5 min was well tolerated, and incidence of hypotension, bradycardia, or infusion site–related adverse events was rare.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In patients with acute brain injury, sustained intracranial hypertension > 20 mm Hg is considered a medical emergency that requires immediate recognition and treatment to prevent progression to cerebral ischemia, brain herniation, and death [1]. Hyperosmolar agents, such as mannitol and hypertonic saline [sodium chloride (NaCl)] are considered first line therapies for the treatment of intracranial hypertension in current guidelines [2]. These agents may rapidly reduce brain volume and intracranial pressure (ICP) in patients with vasogenic cerebral edema by creating an osmolar gradient facilitating fluid shifts out of the intracranial space [3]. Furthermore, mannitol exerts a rheological effect, reducing blood viscosity and promoting plasma expansion and cerebral oxygen delivery. In response, cerebral vasoconstriction occurs due to autoregulation, and cerebral blood volume is decreased [4]. Further mechanisms of hypertonic saline include direct vasodilation, increased cardiac output, and potential neurochemical and immune-modulating effects [5]. Mannitol and hypertonic saline were compared in at least eight randomized trials of patients with elevated ICP from a variety of causes, such as stroke, traumatic brain injury, and tumors [6]. Meta-analyses of these trials found that hypertonic saline may have greater efficacy in managing elevated ICP and is effective for the reversal of transtentorial herniation without the unwarranted side effects of mannitol, such as its potent diuretic effect, rebound intracranial hypertension, and the risk of crystallization on administration requiring use of an in-line filter [7]. Hypertonic saline is available in concentrations ranging from 3 to 23.4%, administered intravenously (IV) by central or peripheral lines and by intraosseous injection [8, 9].
Highly concentrated 23.4% NaCl is operationally advantageous, given its standardized dosing and availability in compact 30-mL containers allowing for easier transportation or inclusion in “brain code kits” [3]. However, because of its high osmolarity (8008 mOsm/l), there are concerns about thrombophlebitis, injection site pain, and tissue necrosis or injury with extravasation when administered rapidly via IV push (IVP), which has limited the use of 23.4% NaCl in such a setting [10]. Additionally, because of its direct vasodilatory effects, there are concerns that rapid administration may result in hypotension, bradycardia, or hemodynamic collapse [5]. Recently, the safety of 23.4% NaCl administered through peripheral IV lines as IVP over 10 min was established in a single-center retrospective study [11].
In the emergency department and critical care settings, increased nursing workloads, higher patient volumes, and ongoing staff shortages have placed increased strain on demands for nursing time. Use of IVP medications can reduce time spent preparing for medication administration to allow performance of other critical tasks. It can further reduce operational barriers, such as acquisition of an in-line filter or IV infusion pump [11]. In 2021, our institution revised its guidance to allow for rapid administration of 23.4% NaCl as IVP over 2–5 min. The objective of this study was to prospectively evaluate the safety of administration of 23.4% NaCl as rapid IVP over 2–5 min.
Methods
A single-center prospective, observational analysis of patients with at least one documented administration of 23.4% NaCl over 2–5 min through a peripheral or central line in the emergency department or inpatient setting between April 2021 and December 2021 at Massachusetts General Hospital in Boston, Massachusetts, was performed. Patients were excluded if they were less than 18 years of age, if safety data were not collected following administration, or if the 23.4% NaCl administration was not administered over 2–5 min. Information on patient demographics and physiological variables, including blood pressure, heart rate, and infusion site reaction grading scale, were collected for analysis by using a secure, Web-based application (Epic Systems Corporation, Verona, WI).
Institutional Protocol
Prior to April 2021, the institutional protocol recommended administration of 23.4% NaCl as an intermittent infusion over 10 min by using an IV syringe pump in adult and pediatric patients. However, health-system wide standardization of IV pump technology resulted in the retirement of IV syringe pump use in adult patients, and all adult patients were transitioned to receive intermittent infusions through a large volume IV infusion pump. The lack of syringe pumps introduced barriers to administration of 23.4% NaCl at our institution. Therefore, our hospital followed the Institute for Safe Medication Practices (ISMP) strategies regarding prefilled and/or ready-to-administer syringes with the rate of administration on the pharmacy label [12]. Standardized doses of 23.4% NaCl 30 mL were compounded by the pharmacy department in 60-mL leur-lock syringes as ready-to-administer packaging that was stored in the automated dispensing cabinets in the emergency department and intensive care units, requiring dual authorization for removal.
Through process examination with nursing, physician, and pharmacists, the institutional protocol maintained current packaging of this ready-to-use, high-risk medication and revised recommendations for administration by IVP to streamline hospital-wide implementation, storage, and nursing education. Safe administration as quick as possible is necessary to ensure adequate nursing support to stabilize the patient beyond just treatment with 23.4% NaCl. Previous small case series reported protocols that administered 23.4% NaCl over shorter time periods, ranging from 10 to 20 min [13, 14]. Therefore, in April 2021 our institutional protocol was adjusted to administer 23.4% NaCl as IVP over 2–5 min with close monitoring for adverse events. Our hospital policy allows for peripheral administration of hypertonic saline via 16–20-gauge catheters; however central line is preferred.
Outcomes
The primary safety outcome was the composite incidence of hypotension, bradycardia, and infusion site reactions (infiltration, phlebitis) within 60 min of 23.4% NaCl administration. Hypotension was defined as a systolic blood pressure (SBP) < 90 mm Hg or a reduction in SBP by 20 mm Hg within 60 min of drug administration. This difference in SBP was calculated by using the lowest SBP values within the 60 min preadministration and post administration. Bradycardia was defined as a heart rate ≤ 50 beats per minute within 60 min of drug administration. Vital signs in the emergency department and inpatient setting were cycled and documented at variable intervals. Blood pressure cycling approximately every 5 min in the emergency department or during acute neurologic declines, whereas patients admitted to the intensive care unit with arterial line placement undergo continuous vital sign monitoring. Because of the variability in documentation and observation periods, we collected the lowest and highest blood pressure within the 60-min time period. Infusion site reaction outcomes assessed included the incidence of infusion site reactions defined as any documented infiltration or phlebitis noted in a peripheral, central, or intraosseous line. Signs of infiltration or phlebitis were prospectively assessed and documented by the bedside nurse who delivered the 23.4% NaCl (Table 1). Assessment of infusion site reactions occurred after each administration of 30 mL of 23.4% NaCl IVP over 2–5 min. Secondary safety end points included comparison of highest and lowest SBP values and lowest heart rate documented within 60 min pre-23.4% NaCl administration by IVP to values documented 60 min post 23.4% NaCl administration by IVP. Clinical pharmacists verify all medication orders for 23.4% NaCl and are able to prospectively evaluate patients for inclusion. On receipt of a 23.4% NaCl medication order, the clinical pharmacist communicates with the bedside nurse to assess for signs of infiltration or phlebitis and documents the responses along with the site of administration in the patient’s medical record. To ensure maximum documentation of 23.4% NaCl administrations, a report was generated each morning, identifying all 23.4% NaCl orders, so that the pharmacist could communicate with the bedside nurse to assess for safety outcomes within 60 min post administration. This project was undertaken as a Quality Improvement Initiative at our hospital, and as such was not formally supervised by the institutional review board and exempt from review per their policies, with a waiver for informed consent.
Statistical Analysis
The primary safety outcome was described by using descriptive statistics. For the secondary safety outcomes comparing within-patient postadministration vital signs to baseline, a mixed-design analysis of variance with a two-level repeated patients factor for preadministration and postadministration data, and a two-level between-patients factor for IV line type (central line or peripheral line) was used on each of the following outcomes: highest and lowest SBP values 60 min before and 60 min after 23.4% NaCl administration and lowest heart rate 60 min before and 60 min after 23.4% NaCl administration. The threshold for statistical significance was a two-sided p value less than 0.05. Statistical analyses were performed using the Statistical Package for the Social Sciences, version 27.0 (IBM Corp, Armonk, NY).
Results
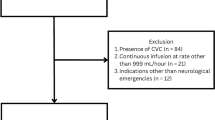
During the study period, 32 patients representing 79 distinct administrations of 30 mL of 23.4% NaCl. Fourteen (44%) patients received at least one dose through a peripheral venous line, and 22 (69%) patients received at least one dose through a central venous line. Central line administrations occurred in 78% (62/79) and peripheral line administration occurred in 22% (17/79) of total administrations. Fourteen administrations were excluded from the analysis; 13 administrations in nine patients lacked safety data assessment, and 1 administration in one patient was excluded for deviancy from protocol administration rate recommendations (Fig. 1). The median age was 55 years (interquartile range 37–67), and 11 (34%) patients were women (Table 2).
Safety
Overall, the primary composite safety outcome was reported in 10 (31%) unique patients following 12 of 79 (15%) administrations of 23.4% IVP. Safety outcomes stratified by peripheral and central line administration are further described in Table 3. A decrease in SBP of at least 20 mm Hg after administration of 23.4% NaCl IVP was noted following 4 of 32 (13%) of patients and 6% (5/79) total administrations.
However, all instances of fall in SBP by at least 20 mm Hg were associated with potential alternative or exacerbating factors, such as up-titrations of infusions of propofol, dexmedetomidine, and/or nicardipine (Table 4). An SBP of < 90 mm Hg was observed in 5 of 32 (16%) patients and after 8% (6/79) of 23.4% NaCl IVP administrations. Three events occurred after peripheral line administration, and three events occurred after central line administration, with one patient experiencing this event after both central and peripheral line administration. However, all these patients were on vasopressor support prior to the first administration of 23.4% NaCl. Bradycardia was seen in one patient who received 23.4% NaCl IVP via central line, requiring no medical interventions. Infusion sites reactions were uncommon in our analysis, occurring in one patient. This patient received 23.4% NaCl via peripheral line, with a complaint of pain at the injection site. However, there was no documented occurrence of thrombophlebitis, cellulitis, or tissue damage at the time of pain report. In a separate incident, a patient’s stay was complicated by cardiac arrest shortly after IVP administration of 23.4% NaCl over less than 1 min via peripheral line, incongruent with the administration time being evaluated, and was excluded from the analysis. However, this event is reported for awareness. On further investigation, the neurocritical care team attributed the cardiac arrest to acute subfalcine and transtentorial uncal herniation syndrome in the setting of lying flat for neuroimaging. Of note, the patient’s blood pressure prior to administration of 23.4% NaCl was 220/137 mm Hg, a heart rate of 59 beats per minute, and a respiratory rate of 8 breaths per minute.
The before and after magnitude of change in physiological variables per 30-mL of 23.4% NaCl administered and the analysis of statistical significance are shown in Table 5. There were no significant changes in the highest SBP value, lowest SBP value, and the lowest heart rate before administration versus post administration was observed.
Discussion
In this prospective analysis of patients receiving 23.4% hypertonic saline at our institution, we found administration through IVP over 2–5 min to be safe and associated with a low incidence of drug-related adverse events among total administrations. The high osmolarity of 23.4% NaCl (8008 mOsm/l) has brought forth concerns about thrombophlebitis, injection site pain, and tissue necrosis or injury with extravasation when administered rapidly through IVP. However, infusion sites reactions were uncommon in our analysis, occurring in 3% of patients who received 23.4% NaCl IVP. This is consistent with the retrospective study performed by Faiver et al. [11] evaluating the safety of 299 administrations of 23.4% NaCl 30 mL infused over 10 min in 141 patients. The authors found no documented occurrence of soft tissue injury or necrosis in any patient, and only one patient developed hypotension following central administration (defined as a mean arterial pressure (MAP) 65 mm Hg or lower) [11].
Our study revealed a SBP decrease by at least 20 mm Hg in 16% of patients and 8% of total administrations who received 23.4% NaCl IVP over 2–5 min. This is consistent with the retrospective study performed by Faiver et al. [11], who reported 17% of patients and 11% of administrations had a SBP decrease by ≥ 20 mm Hg following 23.4% administration. Furthermore, our low rate of bradycardia, demonstrated in only one patient, is also consistent with Faiver et al. [11], who found no documented occurrence of bradycardia.
In a separate incident, a patient’s stay was complicated by cardiac arrest shortly after IVP administration of 23.4% NaCl over less than 1 min via peripheral line. Although the cardiac arrest was attributed to acute herniation syndrome and lying flat for neuroimaging, we cannot rule out that overly rapid administration contributed to the severity of this event. Hemodynamic collapse has been reported in the literature with inadvertent rapid IVP of < 1 min with NaCl [15]. Administration over 2–5 min may be safe, but a straight push over < 1 min may cause harm.
Lack of or institutional changes in equipment, such as IV syringe pumps or IV smart infusion pumps, introduce barriers to clinical care associated with limitation of resources. The ISMP proposes several different planning strategies for anticipated shortages of smart infusion pumps, one of which is to use IVP instead of infusions. To support IVP administration, prefilled and/or ready-to-administer syringes of medications should be dispensed whenever possible. Furthermore, ISMP encourages pharmacy staff to indicate how fast to administer the IVP medication on the pharmacy label. Therefore, decreased access to syringe or infusion pumps may force institutions to evaluate ISMP optimization strategies for administration of medications. On evaluation of the limited literature, 23.4% NaCl was identified as a candidate for IVP administration over 2–5 min [12]. The transition to 23.4% NaCl IVP was potentially advantageous for several reasons. First, material and labor costs could be reduced by administering 23.4% NaCl without a syringe pump and by reducing the time needed to establish an IV infusion by nursing staff. Second, administering 23.4% NaCl IVP via a peripheral line permits expedient treatment of patients with elevated ICP, reducing the time and expertise required to obtain central or intraosseous access. Of note, our hospital policy allows for peripheral administration of hypertonic saline via 16–20-gauge catheters, and the majority of the patients in our study received 23.4% NaCl peripherally by the antecubital site. Finally, the availability of 23.4% NaCl in compact 30-mL vials can result in a shorter time to first dose in a variety of settings. This is particularly advantageous for military medical personnel deployed in austere environments, with limited equipment and resources [16]. Although 23.4% NaCl is administered via peripheral venous access at other institutions, our analysis is the first, to our knowledge, to prospectively demonstrate that such administration over 2–5 min is safe and well tolerated. As such, our analysis benefits from several strengths. First, all infusion site reactions were evaluated prospectively during 23.4% NaCl administration. Second, the administration time of 2–5 min is notably shorter than 10 min, which is the amount of time used in the most recent study [11]. The time period of risk of extravasation-related injury is, therefore, much lower compared with that for continuous infusion of 3% NaCl over hours or days and allows for rapid administration during emergent situations. Our findings suggest the risks of peripheral administration of 23.4% NaCl are low.
There are some potential limitations to our analysis. First, in patients with low levels of arousal secondary to acute brain injury or sedation, injection site pain may not have been able to be communicated, and/or extravasation and vessel injury may not have been physically apparent. Second, although a pharmacist communicated with the bedside nurse to assess for and document infusion site reactions prospectively, it is possible that delayed mild events were not adequately documented in the medical record given the fluid acuity in the department if not documented by the inpatient floor team once the patient is admitted. Furthermore, there may have been adverse reactions that were under documented in the electronic medical record. Because this was a prospective analysis of a hospital-wide medication administration policy change, this study lacked a comparator arm. Fourth, our study has a relatively small sample size. Finally, because of limitations in staffing and centralized clinical pharmacy model on overnight shifts (22:00–07:00), 13 administrations in four unique patients were not able to be prospectively evaluated for safety.
Conclusions and relevance
This study represents the largest prospective analysis, to date, to examine the safety of 23.4% NaCl IVP over 2–5 min. IVP administration of 23.4% NaCl was associated with comparable rates of hypotension, bradycardia, and infusion site–related adverse events. IVP administration of 23.4% NaCl may be considered a safe, alternative method of administration.
References
Cook AM, Morgan Jones G, Hawryluk GWJ, et al. Guidelines for the acute treatment of cerebral edema in neurocritical care patients. Neurocrit Care. 2020;32(3):647–66. https://doi.org/10.1007/s12028-020-00959-7.
Carney N, Totten AM, O’Reilly C, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. 2017;80(1):6–15. https://doi.org/10.1227/NEU.0000000000001432.
Koenig MA, Bryan M, Lewin JL, Mirski MA, Geocadin RG, Stevens RD. Reversal of transtentorial herniation with hypertonic saline. Neurology. 2008;70(13):1023–9. https://doi.org/10.1212/01.wnl.0000304042.05557.60.
Messeter K, Nordström CH, Sundbärg G, Algotsson L, Ryding E. Cerebral hemodynamics in patients with acute severe head trauma. J Neurosurg. 1986;64(2):231–7. https://doi.org/10.3171/jns.1986.64.2.0231.
Strandvik GF. Hypertonic saline in critical care: a review of the literature and guidelines for use in hypotensive states and raised intracranial pressure. Anaesthesia. 2009;64(9):990–1003. https://doi.org/10.1111/j.1365-2044.2009.05986.x.
Alnemari AM, Krafcik BM, Mansour TR, Gaudin D. A comparison of pharmacologic therapeutic agents used for the reduction of intracranial pressure after traumatic brain injury. World Neurosurg. 2017;106:509–28. https://doi.org/10.1016/j.wneu.2017.07.009.
Rockswold GL, Solid CA, Paredes-Andrade E, Rockswold SB, Jancik JT, Quickel RR. Hypertonic saline and its effect on intracranial pressure, cerebral perfusion pressure, and brain tissue oxygen. Neurosurgery. 2009;65(6):1035–41. https://doi.org/10.1227/01.NEU.0000359533.16214.04 (discussion 1041–2).
Jones GM, Bode L, Riha H, Erdman MJ. Safety of continuous peripheral infusion of 3% sodium chloride solution in neurocritical care patients. Am J Crit Care Off Publ Am Assoc Crit-Care Nurses. 2016;26(1):37–42. https://doi.org/10.4037/ajcc2017439.
Ware ML, Nemani VM, Meeker M, Lee C, Morabito DJ, Manley GT. Effects of 23.4% sodium chloride solution in reducing intracranial pressure in patients with traumatic brain injury: a preliminary study. Neurosurgery. 2005;57(4):727–36 (discussion 727–36).
Al-Benna S, O’Boyle C, Holley J. Extravasation injuries in adults. ISRN Dermatol. 2013;2013:856541. https://doi.org/10.1155/2013/856541.
Faiver L, Hensler D, Rush SC, Kashlan O, Williamson CA, Rajajee V. Safety and efficacy of 23.4% sodium chloride administered via peripheral venous access for the treatment of cerebral herniation and intracranial pressure elevation. Neurocrit Care. 2021;35(3):845–52. https://doi.org/10.1007/s12028-021-01248-7.
Planning for Anticipated Shortage of Smart Infusion Pumps and Dedicated Administration Sets. Institute For Safe Medication Practices. https://www.ismp.org/resources/planning-anticipated-shortage-smart-infusion-pumps-and-dedicated-administration-sets. Accessed Jan 22, 2022.
Valentino AK, Nau KM, Miller DA, Hanel RA, Freeman W. Repeated dosing of 23.4% hypertonic saline for refractory intracranial hypertension. a case report. J Vasc Interv Neurol. 2008;1(4):113–7.
Wang J, Fang Y, Ramesh S, et al. Intraosseous administration of 23.4% NaCl for treatment of intracranial hypertension. Neurocrit Care. 2019;30(2):364–71. https://doi.org/10.1007/s12028-018-0637-2.
de Oliveira MF, Pinto FCG. Hypertonic saline: a brief overview of hemodynamic response and anti-inflammatory properties in head injury. Neural Regen Res. 2015;10(12):1938–9. https://doi.org/10.4103/1673-5374.169620.
Pancaro C, Shah N, Pasma W, et al. Risk of major complications after perioperative norepinephrine infusion through peripheral intravenous lines in a multicenter study. Anesth Analg. 2020;131(4):1060–5. https://doi.org/10.1213/ANE.0000000000004445.
Funding
Author Bryan Hayes has received consulting fees for reviewing database medication monographs for Wolters Kluwer—Lexicomp and has received payment for expert testimony from Law Feehan Adams, Goodell, Devries, Leech & Dann, LLP, and Chimpoulis, Hunter & Lynn, P.A. law firms. Author Megan Barra has received salary support from Marinus Pharmaceuticals, Inc, although support was obtained after completion of data collection and analysis of this article, and owns stocks in Marinus Pharmaceuticals, Inc. Author Lindsay Demers has received payment for an educational event from the University of Massachusetts-Amherst Center for Research on Families.
Author information
Authors and Affiliations
Contributions
SO’B made substantial contribution to acquisition of data, analysis and interpretation of data, and drafting the article. JK made substantial contributions to conception and design and critical revision for important intellectual content. LD made substantial contributions to analysis and interpretation of data and critical revision for important intellectual content. BH made substantial contributions to analysis and interpretation of data and critical revision for important intellectual content. MB made substantial contributions to conception and design, analysis and interpretation of data, and drafting the article. The final manuscript was approved by all authors.
Corresponding author
Ethics declarations
Conflict of interest
Author Sean O’Brien is employed by the United States Army. The views expressed are those of the author and do not reflect the official policy or position of the US Army, Department of Defense or the US Government. Author Jennifer Koehl has received for an emergency medicine certification course honorarium from American Society of Health-System Pharmacists and is a member of the Emergency Medicine Section Advisory Group of the American Society of Health-System Pharmacists.
Ethical Approval/Informed Consent
We confirm adherence to ethical guidelines, and the institutional review board determined that this prospective study, with data obtained exclusively from chart review, was exempt from the need for approval. For this type of study, formal consent was not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
O’Brien, S.K., Koehl, J.L., Demers, L.B. et al. Safety and Tolerability of 23.4% Hypertonic Saline Administered Over 2 to 5 Minutes for the Treatment of Cerebral Herniation and Intracranial Pressure Elevation. Neurocrit Care 38, 312–319 (2023). https://doi.org/10.1007/s12028-022-01604-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-022-01604-1