Abstract
Background
Although levetiracetam has been increasingly used as an alternative to phenytoin for early posttraumatic seizure prophylaxis following traumatic brain injury (TBI), an optimal dosing strategy has not been elucidated. The objective of this study is to determine whether different dosing strategies of levetiracetam are associated with the incidence of early posttraumatic seizures when used as prophylaxis following TBI.
Methods
This retrospective single-center cohort study included admitted patients ≥ 18 years of age with a diagnosis of TBI and receiving levetiracetam for early posttraumatic seizure prophylaxis between July 1, 2013, and September 1, 2019. The primary outcome of this study was to evaluate three different dosing strategies of levetiracetam (≤ 1000 mg/day, 1500 mg/day, and ≥ 2000 mg/day) and associated rates of early posttraumatic seizures. Secondary outcomes were to summarize absolute total daily maintenance doses of levetiracetam among patients who experienced early posttraumatic seizures compared with those who did not, to determine the impact of three different dosing strategies on hospital length of stay and in-hospital mortality, and to assess patient-specific variables on the occurrence of posttraumatic seizures. Overlap propensity score weighting was used to address the potential for confounding.
Results
Of the 1287 patients who received levetiracetam for early posttraumatic seizure prophylaxis during the study time frame, 866 patients met eligibility criteria and were included in the study cohort (289 patients in the ≤ 1000 mg/day group, 137 patients in the 1500 mg/day group, and 440 patients in the ≥ 2000 mg/day group). After weighting, the cumulative incidence of early posttraumatic seizure was 2.9% in the ≤ 1000 mg/day group, 8.8% in the 1500 mg/day group, and 9% in the ≥ 2000 mg/day group. The 1500 mg/day and ≥ 2000 mg/day levetiracetam groups had a 209% and 216% increase in the subdistribution hazard of early posttraumatic seizures compared with the ≤ 1000 mg/day levetiracetam group, respectively, but these differences were not statistically significant.
Conclusions
In conclusion, the results of this study demonstrate no statistically significant difference in the cumulative incidence of early posttraumatic seizures within 7 days of TBI between three different levetiracetam dosing strategies. After weighting, the ≤ 1000 mg/day levetiracetam group had the lowest rates of early posttraumatic seizures, death without seizure, and in-hospital mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Posttraumatic seizures are a potential sequela of traumatic brain injury (TBI) that contributes to significant morbidity for affected patients [1, 2]. The landmark randomized controlled trial by Temkin and colleagues [3] demonstrated a significant decrease in early posttraumatic seizures following severe TBI in patients who were given phenytoin prophylaxis compared with those who were not (3.6% vs. 14.2%, p < 0.01). On the basis of the results of this trial and subsequent studies, the Brain Trauma Foundation Guidelines recommend phenytoin to decrease the incidence of early posttraumatic seizures within 7 days of severe TBI [1].
In recent years, levetiracetam has been increasingly used as an alternative agent to phenytoin because of fewer adverse effects, fewer drug interactions, ease of administration, and absence of pharmacokinetic variability requiring therapeutic drug monitoring [4,5,6,7,8,9,10]. In 2013, Inaba and colleagues [9] conducted a prospective observational multicenter study (n = 813) comparing levetiracetam with phenytoin for the prevention of early posttraumatic seizures. No significant differences were identified in the incidence of early posttraumatic seizures, adverse drug reactions, or mortality. Although the results of this study suggest levetiracetam is as efficacious as phenytoin, the study was limited by selection bias, lack of routine electroencephalogram (EEG) monitoring, and inclusion of only patients with blunt trauma. Aside from this study, the majority of available literature comparing phenytoin with levetiracetam for prevention of posttraumatic seizures consists of single-center data with small sample sizes [4,5,6,7,8,9,10,11,12]. Because of this limited evidence, the Brain Trauma Foundation Guidelines do not currently recommend levetiracetam over phenytoin for the prevention of early posttraumatic seizures [1].
In addition to limited comparative data, levetiracetam dosing strategies for posttraumatic seizure prophylaxis are highly variable, ranging from 500 to 1500 mg twice daily, and may be dependent on institution-specific practices [4,5,6,7,8,9,10,11]. Presently, there is a paucity of available literature regarding the effectiveness of various levetiracetam dosing strategies when used for this indication. Additionally, the effect of patient-specific factors, including age, weight, renal function, and severity of illness, has not yet been evaluated [13]. The aim of this study is to determine whether different dosing strategies of levetiracetam are associated with the incidence of early posttraumatic seizures when used as prophylaxis following TBI.
Methods
Design and Setting
This retrospective single-center cohort study conducted at a level I trauma center included admitted patients ≥ 18 years of age with a diagnosis of TBI and receiving levetiracetam for early posttraumatic seizure prophylaxis between July 1, 2013, and September 1, 2019. Patients were excluded if they had a past medical history of seizure disorder prior to TBI and were receiving an antiseizure drug at the time of TBI, if they had a documented seizure prior to levetiracetam administration during the index hospital admission for TBI, if they died within 24 h of hospital admission, if there had been > 24 h between hospital admission and initiation of levetiracetam, if they received < 2 consecutive doses of levetiracetam, or if they had a past medical history of end-stage renal disease or no serum creatinine measurements available to calculate creatinine clearance (CrCl). CrCl was calculated using the Cockcroft–Gault formula [14]. Patients with end-stage renal disease were excluded because levetiracetam is cleared with hemodialysis, requiring supplemental dosing after dialysis sessions. Poor medication adherence after hemodialysis could result in fluctuating plasma drug levels [15]. If a patient was admitted with TBI more than once during the study period, only the first admission was included. At the study institution, indication for seizure prophylaxis was based on a protocolized approach. On the basis of institutional protocol, seizure prophylaxis was indicated for patients who experienced head trauma and had a Glasgow Coma Scale (GCS) score of ≤ 10 with or without head computed tomography (CT) scan findings or any head CT scan findings in the setting of head trauma (regardless of GCS score). Head CT scan findings included, but were not limited to, subdural or epidural hematoma; subarachnoid, intraparenchymal, or intraventricular hemorrhage; linear or depressed skull fracture; penetrating head injury; and cortical contusion. Levetiracetam dosing for early posttraumatic seizure prophylaxis and EEG monitoring were guided by the treating clinician. During the study time frame, an institutional guideline recommended levetiracetam at 1000 mg every 12 h for patients with a CrCl ≥ 30 mL/min or levetiracetam at 250–500 mg every 12 h for patients with CrCl < 30 mL/min. There were additional dosing recommendations for patients who received intermittent hemodialysis or continuous renal replacement therapy; however, these patients were not included in the study. This study was approved by the institutional review board with a waiver of informed consent.
Measures
The primary objective of this study was to evaluate three different dosing strategies of levetiracetam (≤ 1000 mg/day, 1500 mg/day, and ≥ 2000 mg/day) and associated rates of early posttraumatic seizures. The secondary objective was to summarize absolute total daily maintenance doses of levetiracetam among patients who experienced early posttraumatic seizures compared with those who did not. Additional objectives included assessment of EEG confirmed seizure, hospital length of stay (LOS), and in-hospital mortality among three different dosing strategies of levetiracetam. The final objective of the study was to assess the association of patient-specific variables with the occurrence of posttraumatic seizures. The specific stratification of levetiracetam dosing strategies (≤ 1000 mg/day, 1500 mg/day, ≥ 2000 mg/day) was selected on the basis of the range of observed dosing strategies in the current literature and at our institution as well as for the treatment of adult and pediatric epilepsy [4,5,6,7,8,9,10,11, 15].
Patients were categorized into three levetiracetam dosing strategies: ≤ 1000 mg/day, 1500 mg/day, and ≥ 2000 mg/day. The primary outcome was a time-to-event outcome consisting of a binary event indicator of whether the patient experienced early posttraumatic seizure and a continuous variable of the number of days from TBI to the occurrence of early posttraumatic seizure. Posttraumatic seizures were diagnosed by a confirmatory EEG or clinical diagnosis per the treating clinician. Patients who survived or died after the occurrence of posttraumatic seizure within 7 days of TBI were included in the seizure group. Death was considered a competing risk to TBI. Patients who survived or were discharged within 7 days of TBI without seizures while receiving levetiracetam were included in the no seizure group. Patients were reviewed for readmission with early posttraumatic seizure if discharged within 7 days. Because only early posttraumatic seizures were being evaluated, patients were administratively censored on day 7 after TBI. Late posttraumatic seizures (> 7 days after index TBI) were not evaluated.
Data Source and Data Collection
Individual patient data were collected through retrospective review of the institutional trauma registry and electronic health record, including demographics and clinical details (e.g., mechanism of injury, Injury Severity Score, Trauma and Injury Severity Score, Abbreviated Injury Scale [AIS] score, GCS score, CT scan findings, and operative interventions). Operative interventions included the burr hole procedure, decompressive craniectomy/hemicraniectomy, or other hematoma evacuation. Additionally, intensive care unit and hospital LOS, in-hospital mortality, levetiracetam dosing and frequency, and documentation of seizure occurrence were collected. EEG data were collected when available. If confirmatory EEG data were not available, clinical characteristics of early posttraumatic seizures were collected as documented by the diagnosing clinician. The levetiracetam maintenance dose and frequency were collected for all included patients. The levetiracetam maintenance dose was collected as the first maintenance dose ordered for seizure prophylaxis following admission for TBI. Dose adjustments, including those following early posttraumatic seizure occurrence, were not collected.
Statistical Analysis
Descriptive statistics were used to summarize variables. Overlap propensity score weighting was used to address potential confounding when examining the association between dosing strategy and posttraumatic seizures. Propensity score weighting is a common approach to balancing the distribution of these prognostic factors between treatment groups so that one can directly estimate the association between the treatment and the outcome after weighting. In this study, because selection of the levetiracetam dosing strategy could have depended on various patient characteristics, injury severity, and interventions that may have influenced outcomes, we first built a propensity score model to obtain the probability of being assigned to each treatment group for each patient based on the prognostic factors. Because there were three different common dosing strategies, a generalized propensity score model was adopted for multiple treatments [16]. The generalized propensity score model was a multivariable multinomial logistic regression with the dosing group as the outcome variable and adjustment for the following variables: age, head AIS score (≤ 3 or > 3), prehospital GCS score (≤ 10 or > 10), operative intervention, cortical contusion, depressed skull fracture, intracranial hemorrhage, penetrating head injury, and CrCl. Intracranial hemorrhage included subdural hematoma, epidural hematoma, subarachnoid hemorrhage, intraparenchymal hemorrhage, and/or intraventricular hemorrhage. To improve balance in covariates, age and CrCl were represented as categorical variables in the propensity score model (age group: 18–30, 31–50, 51–70, > 70; CrCl group: ≥ 80 mL/min, 50–80 mL/min, 30–49 mL/min, < 30 mL/min). The study cohort was then weighted to estimate the average treatment effect for the population using the generalized overlap weights (OW) derived from the propensity scores so that patients who were likely to receive all three treatments were upweighted, whereas patients who were unlikely to receive one of the treatments were downweighted. In other words, the OW-weighted cohort emphasizes the portion of the study cohort with the most overlap in prognostic factors among treatment groups. To assess balance in covariates, standardized differences of covariates between pairs of dosing strategies were used after overlap propensity score weighting [17]. An absolute standardized mean difference of less than 0.1 was considered a negligible imbalance [18]. We selected OW instead of inverse probability of treatment weighting to avoid removing patients with extreme propensity scores and because OW achieved better covariate balance than inverse probability of treatment weighting in our study. Effective sample sizes for the weighted cohort were calculated for each dose group [19]. Time to posttraumatic seizure and death were modeled using the OW-weighted Fine–Gray model for competing risk analysis. Subdistribution hazard ratios of early posttraumatic seizures comparing different dosing groups were reported with 95% confidence intervals. Subdistribution hazard ratios of posttraumatic seizures can be interpreted as the relative change in the instantaneous rate of the occurrence of early posttraumatic seizures in those patients who were event free or who had experienced a competing event, death. The estimated cumulative incidences of early posttraumatic seizures and death were estimated and plotted for each dosing group. Generalized propensity scores and balance diagnostics after overlap weighting are further described in the Supplementary Appendix.
Secondary outcomes were summarized for each dosing group using both the original cohort and the OW-weighted cohort. To assess the association of patient-specific variables with the occurrence of posttraumatic seizures, an unweighted Fine–Gray model was used using the original cohort to model time to posttraumatic seizures as a function of the dosing group and the same set of variables included in the generalized propensity score model with age and CrCl modeled as continuous variables. A sensitivity analysis was conducted by additionally adjusting for dosing weight, and the results were similar (data not shown). The significance of tests was assessed at α = 0.05 without accounting for multiplicity owing to the exploratory nature of this study. All statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC) and R Statistical Software (version 4.1.1; R Foundation for Statistical Computing, Vienna, Austria). Cumulative incidence plots were created using the ggplot2 package [20]. Maximum pairwise standardized differences were plotted using functions modified on the basis of the PSweight package [21].
Results
Patient Demographics
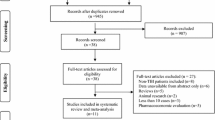
Of the 1287 patients who received levetiracetam for early posttraumatic seizure prophylaxis during the study time frame, 866 patients met eligibility criteria and were included in the study cohort (Fig. 1). Patient characteristics are reported in Table 1. In the original cohort, there were 289 patients in the ≤ 1000 mg/day group, 137 patients in the 1500 mg/day group, and 440 patients in the ≥ 2000 mg/day group. Prior to weighting, patients in the ≥ 2000 mg/day group were younger and had higher injury severity compared with patients in the other levetiracetam dosing groups. The majority of patients were male and had a primary mechanism of injury of fall or motor vehicle collision. Penetrating head injury was uncommon, representing 4.4% of the total cohort. Prehospital GCS scores were ≤ 10 in 251 patients (29%) and head AIS scores were > 3 in 475 patients (54.8%). The most common head CT scan finding was intracranial hemorrhage, and operative intervention was needed in 188 patients (21.7%). The majority of patients required intensive care unit admission (68.8%). After weighting, patient demographics and injury severity were similar among all three dosing groups.
Primary Outcome
In the original cohort, 51 (5.9%) patients experienced an early posttraumatic seizure, 54 (6.2%) patients died without seizures, and 761 (87.9%) did not encounter these events in 7 days. Of the patients who experienced early posttraumatic seizures, 27 (51.9%) experienced convulsive seizures and 25 (48.1%) experienced nonconvulsive seizures. The cumulative incidence of early posttraumatic seizure was 3.9% in the ≤ 1000 mg/day group, 7.8% in the 1500 mg/day group, and 8.4% in the ≥ 2000 mg/day group. After weighting, the cumulative incidence of early posttraumatic seizure was 2.9% in the ≤ 1000 mg/day group, 8.8% in the 1500 mg/day group, and 9% in the ≥ 2000 mg/day group (Table 2, Fig. 2). After weighting, the 1500 mg/day and ≥ 2000 mg/day levetiracetam groups had a 209% and 216% increase in the subdistribution hazard of early posttraumatic seizures compared with the ≤ 1000 mg/day levetiracetam group, respectively, but these differences were not statistically significant.
Overlap weights (OW)-weighted cumulative incidences for posttraumatic seizures and death by daily levetiracetam maintenance dose. a OW-weighted cumulative incidence of early posttraumatic seizures based on total daily maintenance levetiracetam dose (≤ 1000 mg/day, 1500 mg/day, and ≥ 2000 mg/day). b OW-weighted cumulative incidence of death based on total daily maintenance levetiracetam dose (≤ 1000 mg/day, 1500 mg/day, and ≥ 2000 mg/day)
Secondary Outcomes
Secondary outcomes are reported in Table 3. In the original cohort, seizures were validated with EEG in 33 of the 51 patients who experienced early posttraumatic seizures (64.7%). The majority of EEG-validated seizures were prolonged/continuous EEG (97.1%); only one seizure was validated with routine EEG (2.94%). The remaining seizures were validated with a clinical diagnosis (35.3%). The mean time to early posttraumatic seizure was 45.9 h (± 29.4 h) in the ≤ 1000 mg/day group, 72.6 h (± 44.8 h) in the 1500 mg/day group, and 57.3 h (± 44.9 h) in the ≥ 2000 mg/day group. Death without seizure occurred in 12 patients (4.2%) in the ≤ 1000 mg/day group, 7 patients (5.1%) in the 1500 mg/day group, and 35 patients (8%) in the ≥ 2000 mg/day group. In-hospital mortality occurred in 19 patients (6.6%) in the ≤ 1000 mg/day group, 13 patients (9.5%) in the 1500 mg/day group, and 74 patients (16.8%) in the ≥ 2000 mg/day group. Hospital LOS was similar among the groups but longest in the ≥ 2000 mg/day group (9.5 days [interquartile range 4–23 days]). Of the patients who experienced early posttraumatic seizures, 31 of 52 needed operative intervention (59.6%). However, the majority of posttraumatic seizures occurred prior to operative intervention (77.4%). Seven early posttraumatic seizures occurred after operative intervention (22.6%). Overall, after weighting, the ≤ 1000 mg/day group had the lowest rates of early posttraumatic seizures, death without seizure, and in-hospital mortality.
In the unweighted Fine–Gray multivariable regression model, there were no statistically significant differences in the cumulative incidences of early posttraumatic seizures between the three levetiracetam dosing groups (Table 4). Older age, higher head AIS score, prehospital GCS score ≤ 10, and need for operative intervention were associated with a significant increase in the subdistribution hazard rate of early posttraumatic seizures. Additional factors, such as head CT scan findings and admission CrCl, were not associated with the incidence of early posttraumatic seizures. Higher head AIS score, prehospital GCS score ≤ 10, and CT scan finding of penetrating head injury were associated with a significant increase in the subdistribution hazard rate of death.
Discussion
In this retrospective single-center cohort study, there were no significant differences in the cumulative incidences of early posttraumatic seizures or death within 7 days of TBI between three different levetiracetam dosing strategies. These results remained consistent in both the original and weighted cohorts. Additionally, rates of early posttraumatic seizures, death without seizure, in-hospital mortality, and hospital LOS remained lowest in the ≤ 1000 mg/day levetiracetam group. Although the implications of patient-specific factors on levetiracetam dosing strategy are still exploratory, the results of this study demonstrate that higher total daily maintenance doses of levetiracetam were not associated with a reduced incidence of early posttraumatic seizures as hypothesized. Although causality cannot be established, the results of this study are hypothesis generating in that lower total daily maintenance doses of levetiracetam (≤ 1000 mg/day) could be effective in preventing early posttraumatic seizures following TBI.
Posttraumatic seizures are estimated to occur in approximately 1.5–3.6% of patients who receive antiseizure drugs for prophylaxis following severe TBI [3, 5, 9, 10]. The prospective observational multicenter study conducted by Inaba and colleagues [9] (n = 813) compared levetiracetam with phenytoin for the prevention of early posttraumatic seizures. This cohort of patients experienced similar TBI severity as our study cohort, in which patients in the levetiracetam group received levetiracetam at 1000 mg every 12 h intravenously or enterally for 7 days. There were no significant differences in seizure rates (1.5% vs. 1.5%, p = 0.997), adverse drug reactions, or mortality. Similarly, the prospective, randomized, single-blinded comparative trial conducted by Szaflarski and colleagues [10] (n = 52) demonstrated no difference in early posttraumatic seizures occurrence in patients receiving phenytoin compared with levetiracetam for prophylaxis following TBI with a starting dose of 1,000 mg every 12 h intravenously (16.7% vs. 14.7%, p > 0.99). In the prospective observational cohort study conducted by Jones and colleagues [5] (n = 27), EEG findings in patients receiving phenytoin were compared with those in patients receiving levetiracetam for seizure prophylaxis following severe TBI. Patients in the levetiracetam group received levetiracetam at 500 mg every 12 h intravenously for 7 days. Overall, patients had equivalent incidence of seizure activity (p = 0.556), but patients receiving levetiracetam had a higher incidence of abnormal EEG findings (p = 0.003) [5]. Although this study is limited by the small sample size, it highlights the need for further investigation into the implications of increased seizure tendency and epileptiform activity observed with levetiracetam when used as prophylaxis following TBI.
The cumulative incidence of early posttraumatic seizures was similar in the present study compared to currently published literature [3, 5, 9, 10]. Patients who experienced early posttraumatic seizures in our cohort had similar baseline injury severity and operative intervention to those included in previously published studies [3, 5, 9, 10]. Although there may have been patient-specific unanticipated confounders that could not be controlled for prospectively in our cohort, the unweighted multivariable regression model used in our study demonstrated that older age, head AIS score > 3, prehospital GCS score ≤ 10, and operative interventions were associated with increased risk of early posttraumatic seizures. These findings are consistent with the currently published literature [3, 5, 6, 9, 10]. Additionally, prolonged EEG monitoring was obtained for the majority of patients who experienced early posttraumatic seizures in our study, which may have contributed to the increased incidence reported, particularly for nonconvulsive seizures.
Limitations of this study include those inherent to retrospective evaluations, including reliance on accurate documentation in the electronic medical record. Levetiracetam dosing was not standardized during the study period, and causality cannot be determined. Administration of a levetiracetam loading dose was not standardized practice at the study institution. Additionally, levetiracetam dose adjustments, including adjustments after the occurrence of early posttraumatic seizures, were not collected retrospectively. Aside from new seizure occurrence, adjustments to levetiracetam dosing for the indication of early posttraumatic seizure prophylaxis were anticipated to be minimal at the study institution. However, levetiracetam dose adjustments that were not accounted for may have impacted study findings. Similarly, concomitant medications that may have provided additional antiseizure benefit, including benzodiazepines, propofol, and gabapentin, were not accounted for retrospectively. Although these medications certainly could have impacted early posttraumatic seizure occurrence, the anticipated impact is expected to be minimal given the small percentage of patients undergoing treatment for alcohol withdrawal as well as mechanical ventilation during the study time frame. Barbiturates were not routinely used at the study institution during the study time frame. Because of the low incidence of observed early posttraumatic seizures in this study cohort, differences have marginal significance owing to the small sample size. An a priori sample size calculation was not performed because all patients who met inclusion criteria were included in the study cohort. Given the retrospective nature of this study, it remains unclear whether clinician selection bias may have influenced the observed higher total daily maintenance doses of levetiracetam in patients who experienced early posttraumatic seizures compared with those who did not. Similarly, without prospective randomization, there may have been unanticipated confounders that were not accounted for. Moreover, patients perceived to be more severely ill and thus more prone to posttraumatic seizures by the clinical team may have received higher doses of levetiracetam but experienced similar rates of posttraumatic seizures owing to the severity of their underlying disease. Lastly, because of retrospective study design, we could not reliably capture adverse drug events associated with levetiracetam. Pertaining to EEG monitoring, if there was a concern for early posttraumatic seizure, routine or prolonged EEG monitoring was obtained per the treating clinician. The majority of early posttraumatic seizures were confirmed by prolonged EEG. However, there was no empiric EEG monitoring conducted at the study institution, so there is a possibility of uncaptured subclinical nonconvulsive seizures.
Strengths of this study include the novel association of various levetiracetam dosing strategies with cumulative incidence of early posttraumatic seizures. Additionally, the use of OW weighting and completion of a subdistributional hazard regression analysis improve the validity of this study’s findings. On the basis of the results of this study, higher total daily maintenance doses of levetiracetam (≥ 1500 mg/day) commonly used in clinical practice may not be more effective in preventing early posttraumatic seizures compared with lower doses (≤ 1000 mg/day). Additional prospective data are needed to further explore these findings and validate these results.
Conclusions
Overall, the results of this study demonstrate no statistically significant difference in the cumulative incidence of early posttraumatic seizures within 7 days of TBI between three different levetiracetam dosing strategies. Older age, head AIS score > 3, prehospital GCS score ≤ 10, and need for operative intervention were associated with an increased risk of early posttraumatic seizures in this cohort. Prospective studies are needed to further validate these findings by including a control group, standardizing levetiracetam dosing, and assessing adverse drug events.
Source of Support
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award UL1TR002553. Additionally, research reported in this publication was supported in part by a Pfizer Foundation grant and the Duke Clinical and Translational Science Institute (CTSI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, Pfizer Foundation, or the Duke CTSI.
References
Carney N, Totten AM, O’Reilly C, et al. Guidelines for the management of severe traumatic brain injury. Neurosurgery. 2017;80(1):6–15. https://doi.org/10.1227/NEU.0000000000001432.
Ferguson PL, Smith GM, Wannamaker BB, Thurman DJ, Pickelsimer EE, Selassie AW. A population-based study of risk of epilepsy after hospitalization for traumatic brain injury. Epilepsia. 2010;51(5):891–8. https://doi.org/10.1111/j.1528-1167.2009.02384.x.
Temkin NR, Dikmen SS, Wilensky AJ, Keihm J, Chabal S, Winn HR. A randomized, double-blind study of phenytoin for the prevention of post-traumatic seizures. N Engl J Med. 1990;323(8):497–502. https://doi.org/10.1056/NEJM199008233230801.
Gabriel WM, Rowe AS. Long-term comparison of GOS-E scores in patients treated with phenytoin or levetiracetam for posttraumatic seizure prophylaxis after traumatic brain injury. Ann Pharmacother. 2014;48(11):1440–4. https://doi.org/10.1177/1060028014549013.
Jones KE, Puccio AM, Harshman KJ, et al. Levetiracetam versus phenytoin for seizure prophylaxis in severe traumatic brain injury. Neurosurg Focus. 2008;25(4):E3. https://doi.org/10.3171/FOC.2008.25.10.E3.
Khan NR, VanLandingham MA, Fierst TM, et al. Should levetiracetam or phenytoin be used for posttraumatic seizure prophylaxis? A Systematic review of the literature and meta-analysis. Neurosurgery. 2016;79(6):775–82. https://doi.org/10.1227/NEU.0000000000001445.
Kruer RM, Harris LH, Goodwin H, et al. Changing trends in the use of seizure prophylaxis after traumatic brain injury: a shift from phenytoin to levetiracetam. J Crit Care. 2013;28(5):883.e9-13. https://doi.org/10.1016/j.jcrc.2012.11.020.
Wilson CD, Burks JD, Rodgers RB, Evans RM, Bakare AA, Safavi-Abbasi S. Early and late posttraumatic epilepsy in the setting of traumatic brain injury: a meta-analysis and review of antiepileptic management. World Neurosurg. 2018;110:e901–6. https://doi.org/10.1016/j.wneu.2017.11.116.
Inaba K, Menaker J, Branco BC, et al. A prospective multicenter comparison of levetiracetam versus phenytoin for early posttraumatic seizure prophylaxis. J Trauma Acute Care Surg. 2013;74(3):766–71. https://doi.org/10.1097/TA.0b013e3182826e84.
Szaflarski JP, Sangha KS, Lindsell CJ, Shutter LA. Prospective, randomized, single-blinded comparative trial of intravenous levetiracetam versus phenytoin for seizure prophylaxis. Neurocrit Care. 2010;12(2):165–72. https://doi.org/10.1007/s12028-009-9304-y.
Wat R, Mammi M, Paredes J, et al. The effectiveness of antiepileptic medications as prophylaxis of early seizure in patients with traumatic brain injury compared with placebo or no treatment: a systematic review and meta-analysis. World Neurosurg. 2019;122:433–40. https://doi.org/10.1016/j.wneu.2018.11.076.
Owen E, Creel-Bulos C, Willman M, Keeperman J. High-versus low-dose levetiracetam seizure prophylaxis for mild to moderate traumatic brain injury. Crit Care Med. 2018;46(1):455. https://doi.org/10.1097/01.ccm.0000528950.75491.1b.
Udy AA, Jarrett P, Lassig-Smith M, et al. Augmented renal clearance in traumatic brain injury: a single-center observational study of atrial natriuretic peptide cardiac output and creatinine clearance. J Neurotrauma. 2017;34(1):137–44. https://doi.org/10.1089/neu.2015.4328.
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. https://doi.org/10.1159/000180580.
Shiue HJ, Taylor M, Sands KA. Comparison of levetiracetam dosing regimens in end-stage renal disease patients undergoing intermittent hemodialysis. Ann Pharmacother. 2017;51(10):862–5. https://doi.org/10.1177/1060028017713294.
Li F, Li F. Propensity score weighting for causal inference with multiple treatments. Annals Appl Stat. 2019;13(4):2389–415.
Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661–79. https://doi.org/10.1002/sim.6607.
Granger E, Watkins T, Sergeant JC, Lunt M. A review of the use of propensity score diagnostics in papers published in high-ranking medical journals. BMC Med Res Methodol. 2020;20(1):132. https://doi.org/10.1186/s12874-020-00994-0.
Chattopadhyay A, Hase CH, Zubizarreta JR. Balancing vs modeling approaches to weighting in practice. Stat Med. 2020;39(24):3227–54.
H W. Data from: ggplot2: Elegant graphics for data analysis. 2016;ISBN 978–3–319–24277–4. Springer-Verlag New York.
Zhou T, Tong G, Li F, Thomas L, Li F. PSweight: Propensity score weighting for causal inference with observational studies and randomized trials. R package version 1.1.5. . 2021.
Author information
Authors and Affiliations
Contributions
Authorship requirements have been met, and the final manuscript was approved by all authors. All authors (KO, BK, J. S, J. S, SK, ZY, HJL, CV, and JK) contributed to study design, analysis of study results, and writing of the manuscript. In addition, KO and BK contributed to data collection, and ZY and HJL contributed to statistical analysis.
Corresponding author
Ethics declarations
Conflict of Interest
None.
Ethical Approval/Informed Consent
The institutional review board approved the study prior to data collection and waived the need for informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ohman, K., Kram, B., Schultheis, J. et al. Evaluation of Levetiracetam Dosing Strategies for Seizure Prophylaxis Following Traumatic Brain Injury. Neurocrit Care 38, 345–355 (2023). https://doi.org/10.1007/s12028-022-01599-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-022-01599-9