Abstract
Background
The VASOGRADE is a simple aneurysmal subarachnoid hemorrhage (aSAH) grading scale that combines the modified Fisher scale (mFisher) and the World Federation of Neurological Societies (WFNS) grading system, allowing the stratification of delayed cerebral ischemia (DCI) risk. However, the VASOGRADE accuracy in predicting functional outcomes is still to be determined.
Methods
We retrospectively evaluated a multiethnic cohort of consecutive patients with aSAH admitted to a high-volume center in Brazil from January 2016 to January 2019. Patients were classified according to the severity of the clinical presentation (WFNS), the amount of blood in the initial head computerized tomography (mFisher) scan, and the VASOGRADE (green, yellow, red). The primary outcome was to detect DCI-related cerebral infarction, and the secondary outcome was the functional outcome at hospital discharge according to the modified Rankin scale (mRs). Univariate and multivariate logistic regression models were employed.
Results
A total of 212 patients (71.7% female, mean age 52.7 ± 12.8) were included. Sixty-nine patients were classified as VASOGRADE-Green (32.5%), 98 patients as VASOGRADE-Yellow (46.9%), and 45 patients as VASOGRADE-Red (20.6%). DCI-related infarction was present in 39 patients (18.9%). The proportions of patients in the VASOGRADE-Green, VASOGRADE-Yellow, and VASOGRADE-Red categories with DCI-related infarction were 7.7, 61.5, and 30.8%, respectively. After a multivariable analysis including age, sex, aneurysm location, and the VASOGRADE classification as variables, both VASOGRADE-Yellow and VASOGRADE-Red were independently associated with DCI-related infarction (odds ratio [OR] 7.69, 95% confidence interval [CI] 2.13–27.8, and OR 8.07, 95% CI 2.03–32.11, respectively) and unfavorable outcome (OR 4.16, 95% CI 1.33–13.03, and OR 25.57, 95% CI 4.45–147.1, respectively). The VASOGRADE discrimination performance for DCI-related infarction (area under the receiver operating characteristic curve) was 0.67 ± 0.04 (95% CI 0.58–0.75; p = 0.001). VASOGRADE-Red had 97.5% specificity for predicting an unfavorable mRs score at discharge (95% CI 92.8–99.5%). Conversely, VASOGRADE-Green had an excellent specificity for predicting favorable outcome at discharge (mRs score 0–2, 95% CI 82.6–95.5%).
Conclusions
In conclusion, in a multiethnic cohort of patients with aSAH, VASOGRADE-Green predicted the absence of DCI and good clinical outcome at discharge with very high specificity, and patients in this category might be selected for early intensive care unit (ICU) discharge, minimizing costs and medical complications associated with prolonged hospital stay. On the other hand, patients categorized as VASOGRADE-Yellow and VASOGRADE-Red were at the highest risk for DCI. They should, therefore, be selected as a priority for care in high-volume aSAH centers, being aggressively monitored for DCI at the ICU. Such stratification methods are crucial, especially in countries with low financial resources and high health care services demand.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aneurysmal subarachnoid hemorrhage (aSAH) is the cause of 3% of all stroke cases in the United States, and it has a major societal impact because it reaches economically active people, with peak of incidence at the fifth decade of life [1]. Mortality ranges from 8 to 67%, and approximately 50% of patients will have permanent neurological deficits [2].
After rebleeding, delayed cerebral ischemia (DCI) is a major cause of unfavorable outcomes, mainly in the first 2 weeks after the initial bleeding. It occurs in up to 30% of patients, and half of it evolves with an unfavorable outcome, death, or dependence on activities of daily living [3, 4]. Although DCI has been traditionally associated with angiographic vasospasm, its pathophysiology seems multifactorial, including large vessel spasms, microthrombosis, microcirculation dysfunction, inflammation, and cortical spreading depression [4,5,6,7].
DCI is defined as a new neurological focal sign or a worsening of the level of consciousness, presumably related to ischemia, that lasts for at least 1 h and that cannot be explained by other causes [8, 9]. Concern for this major complication is one of the main reasons why patients are kept in the intensive care unit (ICU) for several days for close monitoring, even when the aneurysm is secured [3, 10]. Monitoring of the occurrence of vasospasm includes using high-cost tools such as transcranial Doppler (TCD), cerebral angiography, and computed tomography (CT), CT angiography, and CT perfusion of the head [8].
Thus, an accurate method to predict DCI risk and clinical outcomes at discharge is useful not only for standardizing treatment protocols, but also to safely discharge patients with a low risk of DCI earlier. Such a method could potentially decrease costs associated with DCI monitoring and complications associated with prolonged hospital length of stay [3, 10, 11].
Poor clinical status on admission, a large amount of blood on the initial head CT scan, and younger age are the major risk factors for DCI-related cerebral infarction [3, 10]. De Oliveira et al. [11] evaluated 746 patients and derived a classification that combines the modified Fisher scale (mFisher) and the World Federation of Neurological Societies (WFNS) grading system, the VASOGRADE (Fig. 1). The VASOGRADE accurately predicted DCI with good discrimination and good calibration, with an area under the receiver operating characteristic curve (AUC) of 0.63 (95% confidence interval [CI] 0.58–0.68). Compared with the green VASOGRADE, the yellow grade tended to increase the risk of DCI (odds ratio [OR] 1.31, 95% CI 0.77–2.23) and the red one was associated with three times the risk (OR 3.19, 95% CI 2.07–4.50) [11].
De Oliveira et al. [11] mentioned that besides DCI prediction, VASOGRADE could also be validated for different purposes, such as prognostication. However, since the original publication, this scale has never been validated in another cohort of patients and has never been correlated with a functional outcome. Therefore, we evaluated the VASOGRADE prognostic performance for DCI and functional outcome at discharge in a multiethnic cohort of consecutive patients with aSAH admitted to a high-volume tertiary center in Brazil.
Methods
Study Population
We retrospectively evaluated study participants from a consecutive multiethnic cohort of patients with subarachnoid hemorrhage (SAH) admitted to our hospital, a high-volume academic tertiary center in Brazil with an average of 70 patients with SAH per year, from January 2016 to January 2019. Because the design requirements for studies that attempt to evaluate the performance of prognostic models in new data have not been widely explored, we used a sample size close to that of the two studies from which the VASOGRADE was previously derived [3, 10, 12].
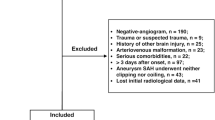
We included adult patients (≥ 18 years old) with aSAH confirmed by head CT scan or lumbar puncture and aneurysm proved through CT angiography, magnectic resonance angiography, or catheter angiography. Exclusion criteria were traumatic SAH and pregnancy. Patients were classified according to the severity of the clinical presentation and the amount of blood seen at the initial CT scan using the WFNS scores, Hunt–Hess grade, the mFisher score, and the VASOGRADE by a single investigator not involved in the patient’s clinical care using data from electronic medical records (EMRs). We evaluated risk factors for aSAH (age, sex, smoking status, history of hypertension, alcohol intake, and use of illicit drugs), the site of the aneurysm in accordance with the PHASES (Population, Hypertension, Age, Earlier aSAH from another aneurysm, Site of aneurysm) study [13], the time from ictus to admission and to aneurysm treatment, the treatment modality (clinical, surgical, or endovascular treatment), and the ICU and hospital length of stay in days. The clinical treatment cohort included patients who did not undergo surgical or endovascular treatment mainly because of rebleeding and death.
The following neurological and clinical complications were evaluated: DCI-related infarction, hydrocephalus, need for an external ventricular drain and ventricular peritoneal shunt, meningitis or ventriculitis, seizures, intracranial hypertension, decompressive craniectomy, infections (pneumonia, bloodstream infection, urinary tract infection, and infection of undetermined focus), sodium disturbances (hyponatremia and hypernatremia), acute kidney injury (AKI), cardiac complications (arrhythmias and acute myocardial infarction), and pulmonary complications (pulmonary thromboembolism, acute respiratory distress syndrome, and acute pulmonary edema). Risk factors, aneurysm location, and complications were extracted from EMRs and neuroimaging and neuroradiology reports.
At our hospital, patients are closely monitored for vasospasm and DCI with daily TCD and serial neurological examinations. When patients are comatose or under sedation, besides TCD and the neurological examination, perfusion CT is used. Once a diagnosis of DCI is established, hypertension is induced with norepinephrine. In case of failure or contraindication, patients are managed with intravenous milrinone [5, 14]. If DCI is still present after the milrinone infusion, treatment with intra-arterial milrinone is considered [5, 14].
We chose DCI-related infarcts (defined as new spontaneous ischemic lesions on at least one follow-up scan) as a primary outcome measure, as was previously suggested by Vergouwen et al. [9], because of the retrospective nature of our study. Unlike infarcts on CT scans, clinical deterioration caused by DCI is notorious for low interobserver agreement and, therefore, was not used as a primary or secondary outcome [9]. Only spontaneous infarcts not related to clipping or coiling of the ruptured aneurysm within 28 days after SAH were included; lesions caused by ventriculostomies, preexisting infarcts, and hypodensities around a hematoma or in the vicinity of the operation were not considered as new infarcts.
The diagnosis of cerebral ischemia was based on neuroimaging reports by certified neuroradiologists who were unaware of the clinical status of each patient. EMRs were reviewed, and the neuroimaging report that prompted the DCI-related infarction diagnosis was the one ordered because clinical deterioration correlated with the diagnosis of DCI.
Functional outcomes were evaluated using the modified Rankin scale (mRs) on discharge based on the review of EMRs by a certified investigator. A favorable outcome was defined as mRs score 0–2, and an unfavorable outcome was defined as mRs score 3–6.
Statistical Analysis
For descriptive purposes, categorical variables are presented through relative and absolute frequencies and compared using the χ2 or Fisher exact test, as appropriate. Continuous variable distributions were assessed for normality by skewness and kurtosis and by graphical methods for the decision of the use of parametric or nonparametric tests. Those with normal distribution are presented as means and standard deviations and compared using the independent samples Student’s t-test. Otherwise, they are presented as medians and interquartile ranges and compared using the Mann–Whitney nonparametric test.
A multivariable logistic regression analysis was employed to verify the independence of the association between the VASOGRADE and the dichotomous outcome DCI-related infarction and the mRs score at discharge. The significant variables at the 0.10 level on the univariate analysis were considered for inclusion in the multivariable models. Age, sex, aneurysm location, and VASOGRADE were defined a priori for inclusion on the multivariable models because of biological plausibility. The AUC assessed discrimination performance with a corresponding 95% CI.
All tests were two-sided, and final p values less than 0.05 were considered statistically significant. All analyses were conducted with the software SPSS (SPSS Statistics para Windows, version 24.0; IBM Corp., Armonk, NY).
Ethical approval/informed consent
The current study has been approved by the appropriate institutional and national research ethics committee and has been performed in accordance with the ethical standards as laid out in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent has been provided.
Results
A total of 212 consecutive patients with acute SAH were included in the final analysis; however, six patients had missing data regarding DCI-related infarction.
The clinical and epidemiological characteristics of the patients are depicted in Table 1. Among 212 patients (71.7% female, mean age 52.7 ± 12.8), most patients were classified as VASOGRADE-Yellow (46.9%), followed by VASOGRADE-Green (32.5%) and VASOGRADE-Red (20.6%). The location of the aneurysms were as follows: anterior communicating artery (52 patients, 24.5%), posterior communicating artery (47 patients, 22.2%), middle cerebral artery (40 patients, 19.4%), internal carotid artery (37 patients, 18%), and posterior circulation arteries (23 patients, 10.8%). In most patients, surgical clipping was the modality of aneurysm repair (67%).
DCI-related infarction was present in 39 patients (incidence 18.9%, 95% CI 14.3–25.1%), and the epidemiological characteristics and risk factors were similar between those with or without DCI-related infarction. There was no difference in DCI incidence in patients treated with clipping or coiling (p = 0.652). The proportions of patients in the VASOGRADE-Green, VASOGRADE-Yellow, and VASOGRADE-Red groups who developed DCI-related infarction were 7.7%, 61.5%, and 30.8%, respectively.
On the univariate analysis, the mRs score, Glasgow Coma Scale (GCS), Hunt–Hess grade, WFNS score, and VASOGRADE were all associated with DCI-related infarction. Because of a high correlation between the VASOGRADE and the GCS (ρ = 0.654), Hunt–Hess grade (ρ = 0.604), WFNS score (ρ = 0.718), and mFisher score (ρ = 0.741), only the VASOGRADE was kept in the multivariable model, which already included information regarding the level of consciousness, motor status, and the amount of blood on the head CT scan (see Table 1).
On the multivariable analysis, adjusted for age, sex, and aneurysm location, compared with VASOGRADE-Green, VASOGRADE-Yellow and VASOGRADE-Red were significantly associated with DCI-related infarction (OR 7.69, 95% CI 2.13–27.8, and OR 8.07, 95% CI 2.03–32.11, respectively; Hosmer and Lemeshow test χ2 = 5.57, degrees of freedom (df) = 8, p = 0.695) (Table 2).
The VASOGRADE AUC for predicting DCI-related infarction was 0.67 ± 0.04 (95% CI 0.58–0.75; p = 0.001). The predicted probability of DCI is presented in Fig. 2. Table 3 presents the VASOGRADE accuracy (sensitivity, specificity, and likelihood ratios). VASOGRADE-Red had a sensitivity of 30.8% and a specificity of 82% for DCI-related infarction. VASOGRADE-Green had a specificity of 92.3% for identifying patients without DCI-related infarction. Overall, VASOGRADE-Yellow and VASOGRADE-Red had a combined sensitivity of 92.31% for predicting DCI (Table 3).
Favorable outcome (mRs score 0–2) occurred in 118 patients (51.9%), whereas 112 patients (48.1%) had an unfavorable outcome. In a multivariate analysis including DCI-related infarction and other neurological and systemic complications (Fig. 3), compared with VASOGRADE-Green, VASOGRADE-Yellow and VASOGRADE-Red presented a higher chance of an unfavorable mRS score (OR 4.16, 95% CI 1.33–13.03, and OR 25.57, 95% CI 4.45–147.1, respectively; Hosmer Lemeshow χ2 = 13,770, df 8, p = 0.088). VASOGRADE-Red had a specificity of 97.5% for predicting an unfavorable mRs score at discharge (95% CI 92.8–99.5%). Combined, VASOGRADE-Yellow and VASOGRADE-Red predicted unfavorable outcomes at discharge with 90.43% sensitivity (Table 4). Conversely, VASOGRADE-Green had an excellent specificity for predicting favorable outcome at discharge (mRs score 0–2, 95% CI 82.6–95.5%).
Forest plot of the multivariate analysis of the VASOGRADE as a predictor of unfavorable outcome (mRs score 3–6) at discharge, adjusted for age, sex, DCI-related infarction, aneurysm treatment modality, hydrocephalus, presence of epileptic seizures, ICH, hypernatremia, AKI, infections, and cardiac complications. AKI acute kidney injury, CI confidence interval, DCI delayed cerebral ischemia, ICH intracranial hypertension, mRs modified Rankin score. Conservative treatment/clinical treatment: clinical treatment was considered when patients did not receive any modality of surgical or endovascular treatment, mainly because of rebleeding and death
Discussion
In our study evaluating a multiethnic population with SAH, VASOGRADE-Green had more than 90% specificity of predicting no DCI and favorable outcome (mRs score 0–2) at discharge, whereas VASOGRADE-Yellow and VASOGRADE-Red categories together predicted DCI with high sensitivity.
Our study’s AUC was similar to the AUC in VASOGRADE’s original publication (AUC = 0.63 in the original cohort and AUC = 0.67 in our cohort). However, VASOGRADE-Yellow was no different from VASOGRADE-Red, which might be due to an insufficient sample size and a small number of patients in the red category. Although an AUC of 0.68 might be considered only moderate, other methods that tried to predict DCI used technical demanding tools (i.e., Lindegaard ratio using cerebral blood flow evaluation with Xenon clearance technique) or data not readily available at hospital admission [3, 15,16,17]. Therefore, although not a perfect tool, the VASOGRADE is a simple, easy to apply score built with data immediately available at hospital admission. The original VASOGRADE results validated previously published risk charts in a large and diverse sample of patients with SAH, and now our data presented similar results in a real-world scenario of SAH management in a middle-income country, suggesting that it can help to select patients who are at high risk for the development of DCI as well as standardize treatment protocols and research studies.
In our cohort, patients with VASOGRADE-Yellow and VASOGRADE-Red had the highest medical and neurological complications rates, with a higher chance of unfavorable outcome at discharge (mRs score 3–6). A previous study described that each day of hospital stay increases the cost by $3,228/day (± $19; p < 0.001), and those patients who developed DCI had longer hospital and ICU lengths of stay (p = 0.034 and p < 0.001, respectively) [18]. De Rooij et al. [3] and Crobeddu et al. [10] identified that the strongest predictors of DCI in a multivariate model were a clinical condition on admission (WFNS score), the amount of cisternal and intraventricular blood on the CT scan, and age (older age was associated with lower incidence of DCI). The authors indicated the importance of a reliable model to predict DCI because it might allow early ICU discharge and cost savings [3, 10]. Advanced age, although it has been consistently associated with lower incidence of angiographic vasospasm and DCI, was not included in the predicting model of VASOGRADE mainly because elderly patients (> 60 years) also have significantly increased risk of poor outcome due to higher rates of medical complications [3, 10, 19, 20].
The VASOGRADE is not a perfect tool; however, it has the benefit of using simple data readily available at hospital admission, a significant advantage compared to other prediction tools. In our series, three patients classified as VASOGRADE-Green developed DCI-related infarction. Although not flawless, the VASOGRADE had a specificity of 92.3% to predict the absence of DCI, with an AUC similar to that noted in the original VASOGRADE article and good calibration. In scenarios of low resources, such as in most hospitals of the world, keeping a patient with VASOGRADE-Green in a step-down unit or even in a ward with close monitoring 7 days after the initial bleeding seems reasonable. Besides, the VASOGRADE evaluates the probability of DCI with a higher AUC than the mFisher (AUC = 0.608) [21], maintaining its good usability and good correlation with outcome at discharge. Thus, as suggested in the original cohort, the VASOGRADE might be used to stratify the risk of DCI and the chance of a favorable outcome at hospital discharge.
The VASOGRADE, as a DCI risk stratification and functional outcome scale, may allow a better allocation of resources, including patient disposition (e.g., transitional care unit vs. ICU admission), and the prioritization of referrals to a high-volume center (VASOGRADE-Yellow and VASOGRADE-Red). Additionally, it may also help in the decision of early ICU discharge of those patients with low risk of DCI and unfavorable outcomes (i.e., VASOGRADE-Green).
Our study has some limitations. First, it is a single-center retrospective study; therefore, some baseline data, such as family history of SAH, were not available, and outcomes were not evaluated blindly. Although using DCI-related infarction as the primary outcome allows consistency with previous and future studies [5], it underdiagnoses DCI (around 20% incidence in our sample and 30% in previous literature) [4, 22]. Additionally, in our cohort, unlike in previous reports of lower incidence of DCI after endovascular treatment [23], the treatment method had no impact on the presence of DCI in our cohort. This may be due to our lower rate of endovascular treatment (only 18% of patients were treated with coiling), whereas, for instance, 65% of patients in the United Kingdom were treated with the endovascular method [24], which reflects costs and availability of methods in our service. Inclusion of patients who did not undergo any aneurysm treatment, mainly because of rebleeding and death [25], may have also played a role. Furthermore, the WFNS score is a qualitative categorical classification criterion collected by medical records analysis, carrying a considerable risk of interobserver variability [17]. Finally, the sample size, although sufficient to achieve the same AUC as that in the original VASOGRADE publication [9], proved insufficient to discriminate differences between VASOGRADE-Yellow and VASOGRADE-Red. However, when considered together, VASOGRADE-Yellow and VASOGRADE-Red had a high sensitivity for the presence of DCI (Table 4, row 2, column 1).
In conclusion, in a multiethnic cohort of patients with aSAH, VASOGRADE-Green predicted absence of DCI and good clinical outcome at discharge with very high specificity, and patients in this category might be selected for early ICU discharge, minimizing costs and medical complications associated with long hospital length of stay. On the other hand, patients categorized as VASOGRADE-Yellow and VASOGRADE-Red are at the highest risk for DCI and should, therefore, be selected as a priority for care in high-volume aSAH centers, being aggressively monitored with noninvasive and invasive methods for DCI in the ICU. Therefore, not only is the VASOGRADE an excellent clinical tool for predicting DCI but it might also allow better allocation of resources, which is even more critical in low-income countries [4].
References
Shea AM, Reed SD, Curtis LH, Alexander MJ, Villani JJ, Schulman KA. Characteristics Of Nontraumatic Subarachnoid Hemorrhage In The United States In 2003. Neurosurgery. 2007;61(6):1131–8.
Perry JJ, Stiell IG, Sivilotti MLA, Bullard MJ, Hohl CM, Sutherland J, et al. Clinical decision rules to rule out subarachnoid hemorrhage for acute headache. JAMA - J Am Med Assoc. 2013;310(12):1248–55.
de Rooij NK, Greving JP, Rinkel GJE, Frijns CJM. Early prediction of delayed cerebral ischemia after subarachnoid hemorrhage: development and validation of a practical risk chart. Stroke. 2013;44(5):1288–94.
de Oliveira Manoel AL, Goffi A, Marotta TR, Schweizer TA, Abrahamson S, Macdonald RL. The critical care management of poor-grade subarachnoid haemorrhage. Crit Care BioMed Central Ltd. 2016. https://doi.org/10.1186/s13054-016-1193-9.
Rouanet C, Chaddad F, Freitas F, Miranda M, Vasconcellos N, Valiente R, et al. Kinetics of cerebral blood flow velocities during treatment for delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2022;36(1):226–39.
Francoeur CL, Mayer SA. Management of delayed cerebral ischemia after subarachnoid hemorrhage. Crit Care BioMed Central Ltd. 2016. https://doi.org/10.1186/s13054-016-1447-6.
Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol. 2014;10:44–58.
Diringer MN, Bleck TP, Hemphill JC, Menon D, Shutter L, Vespa P, et al. Critical care management of patients following aneurysmal subarachnoid hemorrhage: Recommendations from the neurocritical care society’s multidisciplinary consensus conference. Neurocrit Care. 2011;15:211–40.
Vergouwen MDI, Vermeulen M, van Gijn J, Rinkel GJE, Wijdicks EF, Muizelaar JP, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke; J Cereb Circ. 2010;41(10):2391–5.
Crobeddu E, Mittal MK, Dupont S, Wijdicks FM, Lanzino G, et al. Predicting the lack of development of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Stroke. 2011. https://doi.org/10.1161/STROKEAHA.111.
de Oliveira Manoel AL, Jaja BN, Germans MR, Yan H, Qian W, Kouzmina E, et al. The vasograde. Stroke. 2015;46(7):1826–31.
Collins GS, Ogundimu EO, Altman DG. Sample size considerations for the external validation of a multivariable prognostic model: a resampling study. Stat Med. 2016;35(2):214–26.
Greving JP, Wermer MJH, Brown RD, Morita A, Juvela S, Yonekura M, et al. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol. 2014;13(1):59–66.
Lannes M, Teitelbaum J, del Pilar CM, Cardoso M, Angle M. Milrinone and homeostasis to treat cerebral vasospasm associated with subarachnoid hemorrhage: the montreal neurological hospital protocol. Neurocrit Care. 2012;16(3):354–62.
Dumont TM, Rughani AI, Tranmer BI. Prediction of symptomatic cerebral vasospasm after aneurysmal subarachnoid hemorrhage with an artificial neural network: feasibility and comparison with logistic regression models. World Neurosurg. 2011;75(1):57–63.
Gonzalez NR, Boscardin WJ, Glenn T, Vinuela F, Martin NA. Vasospasm probability index: a combination of transcranial Doppler velocities, cerebral blood flow, and clinical risk factors to predict cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2007;107(6):1101–12.
Rosen DS, Loch Macdonald R. Subarachnoid hemorrhage grading scales: a systematic review. Neurocrit Care. 2005;2(2):110–8.
Modi S, Shah K, Schultz L, Tahir R, Affan M, Varelas P. Cost of hospitalization for aneurysmal subarachnoid hemorrhage in the United States. Clin Neurol Neurosurg. 2019;182:167–70.
Torbey MT, Hauser TK, Bhardwaj A, Williams MA, Ulatowski JA, Mirski MA, et al. Effect of age on cerebral blood flow velocity and incidence of vasospasm after aneurysmal subarachnoid hemorrhage. Stroke. 2001;32(9):2005–11.
Magge SN, Chen HI, Ramakrishna R, Cen L, Chen Z, Elliott JP, et al. Association of a younger age with an increased risk of angiographic and symptomatic vasospasms following subarachnoid hemorrhage. J Neurosurg. 2010;112(6):1208–15.
Eagles ME, Jaja BNR, Macdonald RL. Incorporating a modified graeb score to the modified fisher scale for improved risk prediction of delayed cerebral ischemia following aneurysmal subarachnoid hemorrhage. Neurosurgery. 2018;82(3):299–305.
Kassell NF, Torner JC, Haley EC, Jane JA, Adams HP, Kongable GL, et al. The international cooperative studyon the timing of aneurysm surgery. J Neurosurg. 1990;73(1):18–36.
Mees SMD, Kerr RS, Rinkel GJE, Algra A, Molyneux AJ. Occurrence and impact of delayed cerebral ischemia after coiling and after clipping in the International Subarachnoid Aneurysm Trial (ISAT). J Neurol. 2012;259(4):679–83.
Molyneux AJ, Birks J, Clarke A, Sneade M, Kerr RSC. The durability of endovascular coiling versus neurosurgical clipping of ruptured cerebral aneurysms: 18 year follow-up of the UK cohort of the International Subarachnoid Aneurysm Trial (ISAT). Lancet. 2015;385(9969):691–7.
Buscot MJ, Chandra RV, Maingard J, Nichols L, Blizzard L, Stirling C, et al. Association of onset-to-treatment time with discharge destination, mortality, and complications among patients with aneurysmal subarachnoid hemorrhage. JAMA Netw Open. 2022;5(1):e2144039. https://doi.org/10.1001/jamanetworkopen.2021.44039.
Acknowledgements
The present work was conducted with the support of the National Council for Scientific and Technological Development–Brazil.
Funding
No funding was required.
Author information
Authors and Affiliations
Contributions
NVOS: was responsible for conception, organization, and execution of the case report project and writing of the first draft of the manuscript, and review and critique of the manuscript. CR: was responsible for organization and execution of the case report project and writing of the first draft of the manuscript. DJFS: was responsible for organization and execution of the case report project and review and critique of the manuscript. CVBL: was responsible for organization and execution of the case report project and review and critique of the manuscript. CAS: was responsible for organization and execution of the case report project and review and critique of the manuscript. FR: was responsible for conception of the case report project and review and critique of the manuscript. MMA: was responsible for conception of the case report project and review and critique of the manuscript. ALOM: was responsible for organization and execution of the case report project and review and critique of the manuscript. FCN: was responsible for conception of the case report project and review and critique of the manuscript. MF: was responsible for conception of the case report project and review and critique of the manuscript. GSS: was responsible for conception, organization, and execution of the case report project, writing of the first draft of the manuscript, and review and critique of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
All authors declare no conflicts of interest.
Ethical approval/informed consent
Full consent was obtained from the patients and/or family members, and this study was approved by the Ethical Committee of São Paulo Hospital, in accordance with the ethical standards as laid out in the 1964 Declaration of Helsinki and its later amendments.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oliveira Souza, N.V., Rouanet, C., Solla, D.J.F. et al. The Role of VASOGRADE as a Simple Grading Scale to Predict Delayed Cerebral Ischemia and Functional Outcome After Aneurysmal Subarachnoid Hemorrhage. Neurocrit Care 38, 96–104 (2023). https://doi.org/10.1007/s12028-022-01577-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-022-01577-1