Abstract
Background
The aim of this study was to describe the utilization patterns of brain tissue oxygen (PbtO2) monitoring following severe traumatic brain injury (TBI) and determine associations with mortality, health care use, and pulmonary toxicity.
Methods
We conducted a retrospective cohort study of patients from United States trauma centers participating in the American College of Surgeons National Trauma Databank between 2008 and 2016. We examined patients with severe TBI (defined by admission Glasgow Coma Scale score ≤ 8) over the age of 18 years who survived more than 24 h from admission and required intracranial pressure (ICP) monitoring. The primary exposure was PbtO2 monitor placement. The primary outcome was hospital mortality, defined as death during the hospitalization or discharge to hospice. Secondary outcomes were examined to determine the association of PbtO2 monitoring with health care use and pulmonary toxicity and included the following: (1) intensive care unit length of stay, (2) hospital length of stay, and (3) development of acute respiratory distress syndrome (ARDS). Regression analysis was used to assess differences in outcomes between patients exposed to PbtO2 monitor placement and those without exposure by using propensity weighting to address selection bias due to the nonrandom allocation of treatment groups and patient dropout.
Results
A total of 35,501 patients underwent placement of an ICP monitor. There were 1,346 (3.8%) patients who also underwent PbtO2 monitor placement, with significant variation regarding calendar year and hospital. Patients who underwent placement of a PbtO2 monitor had a crude in-hospital mortality of 31.1%, compared with 33.5% in patients who only underwent placement of an ICP monitor (adjusted risk ratio 0.84, 95% confidence interval 0.76–0.93). The development of the ARDS was comparable between patients who underwent placement of a PbtO2 monitor and patients who only underwent placement of an ICP monitor (9.2% vs. 9.8%, adjusted risk ratio 0.89, 95% confidence interval 0.73–1.09).
Conclusions
PbtO2 monitor utilization varied widely throughout the study period by calendar year and hospital. PbtO2 monitoring in addition to ICP monitoring, compared with ICP monitoring alone, was associated with a decreased in-hospital mortality, a longer length of stay, and a similar risk of ARDS. These findings provide further guidance for clinicians caring for patients with severe TBI while awaiting completion of further randomized controlled trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traumatic brain injury (TBI) is a significant public health challenge. The scope of the problem is only in part captured by the more than 2.5 million emergency department visits, 252,000 hospitalizations, and 56,000 deaths attributable to TBI in a single year in the United States [1]. These numbers do not encompass the long lasting societal and economic strain resulting from TBI. Additionally, TBI-related deaths have increased in recent years, emphasizing the need for further therapies and advances in management strategies [2]. Critical care of severe TBI focuses on limiting the damage of the primary injury to the brain and on preventing secondary brain injuries [3], and the monitoring of intracranial pressure (ICP) has become a standard means of reducing secondary injury. Despite a relative paucity of prospective clinical trials demonstrating efficacy, ICP measurements are widely used and are included as part of the Brain Trauma Foundation [4], Seattle International Severe Traumatic Brain Injury Consensus Conference [5], and Emergency Neurological Life Support [6] guidelines for the management of severe TBI.
More directly relevant physiological markers of secondary brain injury exist to complement ICP measurements. Brain tissue oxygenation (PbtO2) has been proposed and studied as such a marker to detect cerebral hypoxia that may precede, or occur independently to, elevations in ICP levels. A decrease in PbtO2 is associated with cerebral ischemia and cell death [7]. Over the last two decades, observational studies have shown that low PbtO2 is associated with poor outcomes [8,9,10,11], and a recent randomized controlled trial has demonstrated the safety and feasibility of a monitoring strategy aimed at correcting PbtO2 [12]. Brain tissue hypoxia has been shown to occur independently of changes in ICP or cerebral perfusion pressure, raising the question of whether current intracranial monitoring methods are adequate to prevent adverse events [13]. Despite this, equipoise remains, as optimal thresholds for PbtO2 remain controversial, and the interpretation of PbtO2 varies depending on placement location and whether there is focal or diffuse pathology [14,15,16]. Furthermore, there is a paucity of data on the use of PbtO2 across United States hospitals and its impact on clinical outcomes in severe TBI. To address these gaps, we examined PbtO2 monitoring in severe TBI by using the National Trauma Data Bank. The primary outcome we examined was mortality. We additionally examined health care use and pulmonary toxicity as secondary outcomes.
Methods
Study Design and Database
We conducted a retrospective cohort study using the National Trauma Data Bank (NTDB) from 2008 to 2016. The NTDB research data sets include information on more than 7.5 million patients with trauma from 595 trauma facilities and houses the largest collection of hospital trauma data in the United States. The data are divided into distinct files, comprising emergency department data, demographic and clinical data, injury mechanism and severity, comorbidities, hospital complications, procedures performed, and hospital disposition. The NTDB is fully deidentified and, therefore, exempt from institutional review board review by the Duke University Health System Institutional Review Board.
Population
The NTDB data set files were used to identify adult patients who suffered from severe TBI and required ICP monitoring. We used International Classification of Disease 9th Revision (ICD-9) and International Classification of Disease 10th Revision (ICD-10) diagnosis codes to identify patients with a diagnosis of TBI (Supplemental Table 1). Severity of TBI was assessed by using the emergency department Glasgow Coma Scale (GCS) score. We included patients with a GCS ≤ 8 who were at least 18 years old. We excluded patients who died in the emergency department or within 24 h of hospital admission (as this likely indicates death from head injury alone, without time for a PbtO2 monitor to be placed or contribute meaningfully to clinical outcome), and patients who did not require ICP monitoring (identified by ICD-9 and ICD-10 procedure codes, listed in Supplementary Data).
Exposures, Outcomes, and Covariates
To describe the use patterns of PbtO2 monitoring among patients with severe TBI, our first research question examined the use of PbtO2 monitoring (compared with ICP monitoring alone) as the outcome, ascertained by examination of ICD-9 and ICD-10 procedure codes (listed in the Supplementary Data). For our second research question, the exposure was the use of PbtO2 monitoring (compared with ICP monitoring alone) and the primary outcome was hospital mortality, defined as death during the hospitalization or discharge to hospice. Secondary outcomes were examined to determine the association of PbtO2 monitoring with health care use and pulmonary toxicity and included the following: (1) intensive care unit length of stay, (2) hospital length of stay, and (3) development of acute respiratory distress syndrome (ARDS), ascertained from the hospital complications file of the NTDB database. The decision to analyze development of ARDS was based on the incidence of ARDS following severe isolated TBI and the concern that PbtO2 monitoring might lead to increased oxygen use and subsequent worsening ventilator-induced lung injury [17].
Covariates included demographic characteristics (sex, age, race, insurance status), time (calendar year), medical comorbidities (prior cerebrovascular insult, respiratory disease, hypertension, diabetes, Do Not Resuscitate status, bleeding disorder, and disseminated cancer or active chemotherapy), clinical characteristics (Injury Severity Score, TBI characteristics [blunt vs. penetrating], and injury mechanism), facility characteristics (bed size, trauma designation, teaching status), and geographic characteristics (census region).
Statistical Analysis
We used descriptive statistics to examine the demographic and clinical characteristics of our cohort of patients with severe TBI with ICP monitors, stratified by using PbtO2 monitoring. Continuous data were summarized by using means and standard deviations, and categorical data were summarized as counts and percentages. For examination of factors associated with PbtO2 use, we fit a mixed-effects logistic regression model, adjusted for the above covariates, with the individual hospital modeled as a random effect to account for clustering. The model’s intraclass correlation coefficient was used to examine the percentage of variation in PbtO2 use at the level of the individual hospital. We further examined BtO2 use over time (calendar year), as well as identified changes in use with a multivariable joinpoint regression.
We examined the association of PbtO2 use with clinical outcomes using a propensity-weighted analysis for primary and secondary outcomes. First, we built propensity scores for treatment (receipt of a PbtO2 monitor) using a logistic regression model adjusted for the following variables, selected a priori based on prior literature, directed acyclic graphs, and study team subject matter expertise: age, sex, race, admission GCS score, injury severity score, admission hypotension, admission heart rate, need for mechanical ventilation, comorbidities (respiratory disease, diabetes, bleeding disorder, and “Do not resuscitate” status), and facility variables (region, hospital size, hospital teaching status, and hospital level I trauma designation). Next, we assessed for adequate covariate balance following propensity weighting by examining changes in standardized mean differences between covariates prepropensity and postpropensity score weighting; standardized mean difference values of < 0.1 suggested adequate covariate balance. Last, we calculated the average treatment effect risk estimates for the primary and secondary outcome using inverse probability of treatment weighting [18] and reported this as a risk ratio (for binary outcome) and mean difference (for continuous outcomes), with 95% confidence intervals. Given missingness of less than 5% in all variables used for analysis, a complete case analysis was performed. All analyses were conducted by using STATA 15.0 (College Station, TX), using the Treatment-Effects package for propensity-weighted analyses.
Results
Population
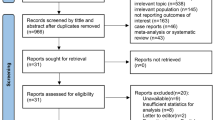
The selection of study participants is depicted in Fig. 1. The study population included 35,501 patients who presented with severe TBI and underwent placement of an ICP monitor. Through the entire period of study (2008–2016), 1,346 (3.8%) patients had a PbtO2 monitor placed. Details on the demographic, clinical, and facility characteristics of the study population are found in Supplemental Table 2. The mean (standard deviation) presenting GCS was 3.9 (1.6). The mean age of a patient in the study population was 40.3 years, with 77% being men and 65% identifying their race as White.
Use of PbtO2 Monitoring
Use of PbtO2 monitoring ranged from a low of 2.68% in 2008 to a high of 5.47% in 2011. Further details regarding variation in the use of PbtO2 monitoring by year is shown in Fig. 2. Multivariable joinpoint regression showed that the break point in utilization change occurred in 2011 (p < 0.0001). Risk factors for the use of PbtO2 monitoring are shown in Table 1 and Supplementary Table 3. We observed that variation in use of PbtO2 monitoring was explained more by the treating facility than by patient-level characteristics. The mixed-effects logistic regression model demonstrated an intraclass correlation coefficient of 0.71 (0.63–0.77), indicating that 71% of the variation in PbtO2 monitor placement occurred at the individual hospital level and was not explained by patient-level characteristics alone.
Association of PbtO2 Monitoring with Clinical Outcomes
Clinical outcomes are shown in Table 2. Patients who underwent placement of PbtO2 monitor in addition to an ICP monitor had a crude in-hospital mortality of 31.1%, compared with 33.5% in patients who underwent placement of an ICP monitor alone. In propensity-weighted analysis, exposure to PbtO2 in addition to ICP monitoring, compared with ICP monitoring alone, was associated with a decreased risk of hospital mortality (adjusted risk ratio 0.84, 95% CI 0.76–0.93). PbtO2 monitoring was not associated with the development of the ARDS (9.2% vs. 9.8%, respectively: adjusted risk ratio 0.89, 95% CI 0.73–1.09). Hospital length of stay was longer for patients who underwent PbtO2 monitoring (24.2 days vs. 22.6 days; p < 0.001), and intensive care unit length of stay was longer for patients who underwent PbtO2 monitoring, compared with patients exposed to ICP monitoring alone (16.5 days vs 14.5. days; p < 0.001).
Discussion
Severe TBI is a significant public health problem associated with a high mortality. Investigations into interventions to prevent secondary brain injury have consistently not shown a benefit (progesterone [19, 20], steroids [21, 22], and cooling [23]). Recent efforts have focused on the direct measurement and then intervention on cerebral ischemia. In this study, we found (1) significant variation in the use of PbtO2 monitoring, the majority of which is not explained by patient-level characteristics, and (2) the use of PbtO2 monitoring in addition to ICP monitoring, compared with ICP monitoring alone, is associated with decreased hospital mortality, increased health care use, and similar risk for development of ARDS.
Observational studies have demonstrated an association between reduced PbtO2 and poor outcomes following TBI [9,10,11]. A management strategy including the use of PbtO2 measurements has been proposed as safe and possibly efficacious in reducing mortality [12]. These studies, and other work, led to the Brain Oxygen Optimization in Severe TBI Phase 2 (BOOST-2) trial, which demonstrated reduced brain hypoxia in patients managed with a combined ICP and PbtO2 directed strategy [12]. This finding, coupled with a trend toward reduced mortality associated with the use of a PbtO2 monitoring strategy, prompted the larger Brain Oxygen Optimization in Severe TBI Phase 3 (BOOST-3) study, which is currently enrolling nationally to assess functional outcomes when a PbtO2 monitoring strategy is employed. In addition to BOOST-3, the Impact of Early Optimization of Brain Oxygenation on Neurological Outcome After Severe Traumatic Brain Injury (OXY-TC) study is currently underway to test the impact of PbtO2 management on outcomes after severe TBI [24].
We observed temporal variation in the use of PbtO2 monitors, which peaked in 2011 to 2012. It is not clear what drove these year-by-year changes. BOOST-2, the most recent randomized trial involving PbtO2 monitors, was published in 2017, making it difficult to link the peak in observed use to the study. In addition, our analysis indicated that the vast majority (71%) of variation in the decision to place a PbtO2 monitor was center-dependent as opposed to driven by the clinical characteristics of the individual patient. Previous studies have shown significant variation in adherence to guideline-based TBI care including ICP monitor placement [25], consistent with the significant variation observed in our study that was unexplained by patient characteristics. Therefore, a hospital’s culture (or availability of technology) is likely the most important contributors to using PbtO2 as part of the routine care of patients with severe TBI. This observation is broadly consistent with findings regarding variation in the decision to intervene on severe TBI surgically [25].
Our analysis indicates that placement of a PbtO2 monitor was associated with a reduced risk of hospital mortality. The nature of this study, which does not include details regarding the interventions made in response to data provided by the PbtO2 monitors, limits our ability to ascertain the mechanism by which PbtO2 monitoring reduces mortality following severe TBI. The reduction in mortality we observed may reflect that the devices are functioning as theorized by alerting clinicians to brain tissue hypoxemia in an early enough manner to intervene. Alternatively, placement of a PbtO2 monitor may reflect an institution’s broader efforts toward aggressive care for severe TBI, and thus serve as an indirect marker of multimodal severe TBI management.
Placement of PbtO2 monitors was associated with an increased length of hospitalization, potentially due to longer periods of clinical monitoring and additional inventions based on the additional clinical data. A possible contributing factor of increased health care use is related to an institutional culture on prolonged aggressive care of patients with severe TBI. In other words, institutions that routinely place a PbtO2 monitor may be more likely to counsel caregivers toward aggressive care resulting in a prolonged hospitalization. There have been concerns that management guided by PbtO2 may be associated with increased pulmonary complications. The data presented in this study do not support that conclusion, as it showed a similar rate of ARDS between the two groups. In this study, we observed a 9.2–9.8% rate of ARDS. Previous work by our group showed a slightly lower rate of TBI-associated ARDS of 7%, but overall is consistent with what has been reported in the literature [17].
Although this study is the largest that, we are aware of, examines the use of PbtO2 monitoring in patients with TBI, there are several limitations. Data available in the NTDB indicate only whether a device capable of monitoring PbtO2 was placed. No information is available to the length of time it provided functional data or how the information it provided were used in patient care. The NTDB does not provide information on whether protocolized management was carried out in response to the PbtO2 information provided, representing an important consideration in interpreting our results. While BOOST-2 provided a standardized approach to the management of brain tissue hypoxemia, there are no data available on whether the patients captured in NTDB were managed in this fashion. Therefore, our analysis cannot draw conclusions on what aspect of placement or resultant management of a PbtO2 monitor may have resulted in reduced rates of mortality. Additionally, although we included respiratory disease as a covariate in our outcome analysis of ARDS, we did not excluded patients with acute lung pathology (i.e., pulmonary contusion), which can be risk factors for ARDS [26]. The NTDB is limited in the number of covariates available, which may result in residual confounding. A final limitation to the present study is the lack of neurobehavioral outcomes. We are therefore unable to draw conclusions about whether management involving PbtO2 monitoring results in improved quality of life. The ongoing BOOST-3 study should help elucidate this by including Glasgow Outcome Score Extended as part of its outcome measures.
Conclusions
In conclusion, we present data demonstrating significant variation in the use of PbtO2 monitoring as part of the care of patients with severe TBI. The use of PbtO2 was associated with a decreased risk of hospital mortality, increased health care use, and no association with the development of ARDS in patients with severe TBI. Given the variation in use and possible benefit of therapy, our analysis confirms the need for large multicenter trials, which are currently being conducted.
References
Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic brain injury-related emergency department visits, hospitalizations, and deaths - United States, 2007 and 2013. MMWR Surveill Summ. 2017;66:1–16.
Daugherty J, Waltzman D, Sarmiento K, Xu L. Traumatic brain injury-related deaths by race/ethnicity, sex, intent, and mechanism of injury - United States, 2000–2017. MMWR Morb Mortal Wkly Rep. 2019;68:1050–6.
Krishnamoorthy V, Komisarow JM, Laskowitz DT, Vavilala MS. Multi-organ dysfunction following severe traumatic brain injury: epidemiology, mechanisms, and clinical management. Chest 2021.
Carney N, Totten AM, O’Reilly C, et al. Guidelines for the management of severe traumatic brain injury. Fourth Edition Neurosurgery. 2017;80:6–15.
Hawryluk GWJ, Aguilera S, Buki A, et al. A management algorithm for patients with intracranial pressure monitoring: the Seattle international severe traumatic brain injury consensus conference (SIBICC). Intensive Care Med. 2019;45:1783–94.
Garvin R, Mangat HS. Emergency neurological life support: severe traumatic brain injury. Neurocrit Care. 2017;27:159–69.
Hlatky R, Valadka AB, Goodman JC, Contant CF, Robertson CS. Patterns of energy substrates during ischemia measured in the brain by microdialysis. J Neurotrauma. 2004;21:894–906.
Maloney-Wilensky E, Gracias V, Itkin A, et al. Brain tissue oxygen and outcome after severe traumatic brain injury: a systematic review. Crit Care Med. 2009;37:2057–63.
Oddo M, Levine JM, Mackenzie L, et al. Brain hypoxia is associated with short-term outcome after severe traumatic brain injury independently of intracranial hypertension and low cerebral perfusion pressure. Neurosurgery. 2011;69:1037–45.
Bardt TF, Unterberg AW, Hartl R, Kiening KL, Schneider GH, Lanksch WR. Monitoring of brain tissue PO2 in traumatic brain injury: effect of cerebral hypoxia on outcome. Acta Neurochir Suppl. 1998;71:153–6.
Valadka AB, Gopinath SP, Contant CF, Uzura M, Robertson CS. Relationship of brain tissue PO2 to outcome after severe head injury. Crit Care Med. 1998;26:1576–81.
Okonkwo DO, Shutter LA, Moore C, et al. Brain oxygen optimization in severe traumatic brain injury Phase-II: A Phase II randomized trial. Crit Care Med. 2017;45:1907–14.
Chang JJ, Youn TS, Benson D, et al. Physiologic and functional outcome correlates of brain tissue hypoxia in traumatic brain injury. Crit Care Med. 2009;37:283–90.
Leach MR, Shutter LA. How much oxygen for the injured brain - can invasive parenchymal catheters help? Curr Opin Crit Care 2021.
Hirschi R, Hawryluk GWJ, Nielson JL, et al. Analysis of high-frequency PbtO2 measures in traumatic brain injury: insights into the treatment threshold. J Neurosurg 2018:1–11.
Patchana T, Wiginton JT, Brazdzionis J, et al. Increased brain tissue oxygen monitoring threshold to improve hospital course in traumatic brain injury Patients. Cureus. 2020;12:e7115.
Komisarow JM, Chen F, Vavilala MS, Laskowitz D, James ML, Krishnamoorthy V. Epidemiology and outcomes of acute respiratory distress syndrome following isolated severe traumatic brain injury. J Intensive Care Med 2020:885066620972001.
Desai RJ, Franklin JM. Alternative approaches for confounding adjustment in observational studies using weighting based on the propensity score: a primer for practitioners. BMJ. 2019;367:l5657.
Skolnick BE, Maas AI, Narayan RK, et al. A clinical trial of progesterone for severe traumatic brain injury. N Engl J Med. 2014;371:2467–76.
Wright DW, Yeatts SD, Silbergleit R, et al. Very early administration of progesterone for acute traumatic brain injury. N Engl J Med. 2014;371:2457–66.
Roberts I, Yates D, Sandercock P, et al. Effect of intravenous corticosteroids on death within 14 days in 10008 adults with clinically significant head injury (MRC CRASH trial): randomised placebo-controlled trial. Lancet. 2004;364:1321–8.
Edwards P, Arango M, Balica L, et al. Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury-outcomes at 6 months. Lancet. 2005;365:1957–9.
Cooper DJ, Nichol AD, Bailey M, et al. Effect of early sustained prophylactic hypothermia on neurologic outcomes among patients with severe traumatic brain injury: the POLAR randomized Clinical trial. JAMA. 2018;320:2211–20.
Payen JF, Richard M, Francony G, et al. Comparison of strategies for monitoring and treating patients at the early phase of severe traumatic brain injury: the multicentre randomised controlled OXY-TC trial study protocol. BMJ Open. 2020;10:e040550.
Truong EI, Stanley SP, DeMario BS, et al. Variation in Neurosurgical Intervention for Severe TBI: The Challenge of Measuring Quality in Trauma Center Verification. J Trauma Acute Care Surg 2021.
Eworuke E, Major JM, Gilbert McClain LI. National incidence rates for acute respiratory distress syndrome (ARDS) and ARDS cause-specific factors in the United States (2006–2014). J Crit Care. 2018;47:192–7.
Funding
National Institute of Neurological Disorders and Stroke, K23 NS109274, Vijay Krishnamoorthy.
Author information
Authors and Affiliations
Contributions
Study design: JMK, CT, JC, CC, GM, and VK. Data & statistical analysis: JMK, CT, BM, MV, TO, and VK. Article generation & review: JK, CT, BM, MV, DTL, MLJ, JPM, AH, JS, and VK. Final review: all authors. The final manuscript was approved by all authors.
Corresponding author
Ethics declarations
Conflicts of interest
No conflicts of interest exist for any authors.
Ethical approval/informed consent
The present study was approved by the Institutional Review Board at Duke University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12028_2021_1394_MOESM1_ESM.docx
eTable 1 ICD-9 and ICD-10 diagnosis and procedure codes. ICD-9, International Classification of Disease 9th Revision, ICD-10, International Classification of Disease 10th Revision.
Rights and permissions
About this article
Cite this article
Komisarow, J.M., Toro, C., Curley, J. et al. Utilization of Brain Tissue Oxygenation Monitoring and Association with Mortality Following Severe Traumatic Brain Injury. Neurocrit Care 36, 350–356 (2022). https://doi.org/10.1007/s12028-021-01394-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-021-01394-y