Abstract
Background
Early hyperoxia may be an independent risk factor for mortality in critically ill traumatic brain injury (TBI) patients, although current data are inconclusive. Accordingly, we conducted a retrospective cohort study to determine the association between systemic oxygenation and in-hospital mortality, in critically ill mechanically ventilated TBI patients.
Methods
Data were extracted from the Australian and New Zealand Intensive Care Society Centre for Outcome and Resource Evaluation Adult Patient Database. All adult TBI patients receiving mechanical ventilation in 129 intensive care units between 2000 and 2016 were included in analysis. The following data were extracted: demographics, illness severity scores, physiological and laboratory measurements, institutional characteristics, and vital status at discharge. In-hospital mortality was used as the primary study outcome. The primary exposure variable was the ‘worst’ partial arterial pressure of oxygen (PaO2) recorded during the first 24 h in ICU; hyperoxia was defined as > 299 mmHg. Adjustment for illness severity utilized multivariable logistic regression, the results of which are reported as the odds ratio (OR) 95% CI.
Results
Data concerning 24,148 ventilated TBI patients were extracted. By category of worst PaO2, crude in-hospital mortality ranged from 27.1% (PaO2 40–49 mmHg) to 13.3% (PaO2 140–159 mmHg). When adjusted for patient and institutional characteristics, the only PaO2 category associated with a significantly greater risk of death was < 40 mmHg [OR 1.52, 1.03–2.25]. A total of 3117 (12.9%) patients were hyperoxic during the first 24 h in ICU, with a crude in-hospital mortality rate of 17.8%. No association was evident in between hyperoxia and mortality in adjusted analysis [OR 0.97 (0.86–1.11)].
Conclusions
In this large multicenter cohort of TBI patients, hyperoxia in the first 24 h after ICU admission was not independently associated with greater in-hospital mortality. Hypoxia remains associated with greater in-hospital mortality risk and should be avoided where possible.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The administration of oxygen is widespread in medical practice [1] and has the potential to be lifesaving in a number of critical conditions. However, resuscitation using oxygen frequently exceeds the patient’s physiological needs. Albeit this is usually accepted to avoid hypoxia, the concept of oxygen toxicity is well described, whereby harmful effects occur due to formation of reactive oxygen species, cell damage by inflammation, and cell death by apoptosis. For each organ system, the threshold where harm surpasses benefits is unknown [2], although any injurious effects are assumed to be dose and time dependent [3].
In the lungs, hyperoxia impairs mucociliary clearance and alters the metabolism of surfactant. This may lead to edema initially and later pulmonary fibrosis. In the cardiovascular system, hyperoxia increases systemic vascular resistance, which can decrease perfusion to vital organs, particularly when there are concomitant vascular abnormalities. In the AVOID (Air Versus Oxygen in ST-Segment Elevation Myocardial Infarction) trial, the administration of supplemental oxygen (in comparison with room air) to patients with ST-elevation acute myocardial infarction resulted in inferior clinical outcomes, as demonstrated by greater infarct size and likelihood of arrhythmia [4]. In pediatric medicine, the use of 100% oxygen for resuscitation of neonates has been abandoned due to greater morbidity and mortality [5].
In the central nervous system, hyperoxia has been associated with delayed cerebral ischemia after stroke [6], although the impact of hyperoxia in other forms of brain injury is uncertain. In traumatic brain injury (TBI), a number of studies have already been published, with mixed results [7,8,9,10,11,12], such that there is no consensus on the ideal oxygen target in TBI. Moreover, the most recent version of the Brain Trauma Foundation Guidelines makes no recommendation on this subject [13].
Accordingly, our aim was to explore the relationship between early hyperoxia (as measured by the partial arterial pressure of oxygen [PaO2]) and in-hospital mortality, in mechanically ventilated TBI patients admitted to an intensive care unit (ICU) in Australia and New Zealand. We hypothesized that ‘excessive’ systemic oxygenation may be a modifiable risk factor, whereby PaO2 values above a specific threshold are associated with greater mortality.
Methods
Study Design
This was a multicenter retrospective cohort study, designed to evaluate the association between early hyperoxia and in-hospital mortality in mechanically ventilated TBI patients admitted to ICU in Australia and New Zealand. Ethics approval was obtained from the Alfred Hospital Human Research Ethics Committee (reference number 162/17), with a waiver of individual patient informed consent.
Database and Case Identification
The Australian and New Zealand Intensive Care Society (ANZICS) Centre for Outcome and Resource Evaluation Adult Patient Database (APD) is a binational voluntary database containing data on more than 1.5 million ICU admissions [14]. De-identified data are imputed on a quarterly basis and principally used for quality-assurance and benchmarking activities. Over 80% of public and private institutions throughout Australia and New Zealand contribute to the ANZICS-APD, including all major tertiary trauma centers.
Ventilated adult patients (> 17 years of age) who were admitted to the ICU with a TBI at one of 129 participating centers between January 1, 2000 and December 31, 2016 were included. In order to identify suitable patients, we used the primary acute physiology and chronic health evaluation (APACHE) III-J codes for head injury ± multitrauma. We excluded patients with readmission, patients whose records did not contain arterial blood gas (ABG) values, those who were transferred to another ICU, and those where mortality outcomes were unknown. We also excluded patients who were admitted to ICU solely for the purposes of palliative care or for consideration of organ donation. Access to the data was granted by the ANZICS Centre for Outcome and Resource Evaluation Management Committee.
Oxygen Values
Consistent with the APACHE III scoring system, the most abnormal set of ABG measurements from the first 24 h in ICU are entered into the ANZICS-APD. These are calculated by simultaneously recording the patients fraction inspired oxygen (FiO2) and PaO2. If the FiO2 is ≥ 0.5, the PaO2 associated with the highest A-a gradient is selected. If the FiO2 is < 0.5, the lowest PaO2 is used. We defined these values as the ‘worst’ PaO2. Of note, if multiple ABGs are obtained during the first 24 h, and the patient is receiving a variable FiO2 (e.g., both ≥ 0.5 and < 0.5), the PaO2 derived from measurements taken on ≥ 0.5 is used. Of note, Bellomo et al. have previously demonstrated that PaO2 values determined in this fashion more closely represent overall mean oxygenation status in ICU patients during the first 24–48 h after admission [15].
Data Extraction
Both hospital and patient level data were extracted from the ANZICS-APD. The following patient characteristics were recorded: year of admission, physiological and ABG measurements during the first 24 h, Glasgow Coma Scale (GCS), age, APACHE II and III scores with component sub-scores, APACHE III risk of death, Australian and New Zealand Risk of Death (ANZROD), hospital and ICU admission source, hospital and ICU length of stay, and vital status on ICU and hospital discharge. Hospital data included location, size, and type of institution. In-hospital mortality was used as the primary study outcome. GCS in the ANZICS-APD either represents the lowest score recorded in the first 24 h in ICU (where the patient is free from sedation and/or paralysis) or is the value recorded at the time of, or just prior to, the institution of sedation and/or neuromuscular blockade.
Statistical Analysis
All analyses were performed using Stata® version 14 (StataCorp LLC, College Station, TX USA). Continuous data are presented as mean (SD) or median (interquartile range) depending upon the distribution. All categorical data are reported counts (%). T test, Wilcoxon, and Chi-square test (depending on distribution and type of data) were used to compare characteristics of survivors to non-survivors. To explore the relationship between oxygenation and mortality, logistic regression models were used with the worst PaO2 values divided into 17 categories (< 40 to > 500 mmHg) and referenced against a value of 100–109 mmHg. In subsequent sensitivity analyses, PaO2 values were collapsed into three groups: Hypoxia was classified as a worst PaO2 < 60 mmHg, normoxia as 60–299 mmHg, and hyperoxia as greater than or equal to 300 mmHg. Specific subgroups explored included those patients coded as having an isolated head injury, non-operative versus postoperative admissions, and on the basis of TBI severity (GCS < 9, 9–12, and > 12).
Multivariable models were constructed adjusting for severity of illness using ANZROD methodology [16] and included acute and chronic illness severity, operative status, treatment limitations at ICU admission, hospital type, age, gender, and year of admission, with results reported as odds ratios (95% CI). To avoid confounding through the inclusion of oxygenation and/or neurological status in overall severity of illness scores, sub-scores were utilized in model development, with these covariates entered independently for each patient. No imputation was made for missing data.
Assuming a baseline mortality of 20%, 20,000 patients were required to detect an absolute mortality difference of 1.5% or greater, with alpha of 0.05 and power of 0.8. A two-sided P value of 0.05 was considered statistically significant. Data are reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines [17].
Results
Data Selection and Baseline Characteristics
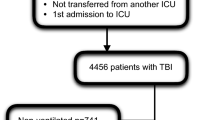
Data pertaining to 1,756,035 patients admitted to ICU between 2001 and 2016 were available for analysis. A total of 37,532 were coded as operative or non-operative head injury ± multitrauma using the ANZICS version of the APACHE III-J scoring system. A number of patients were then excluded (see Fig. 1). This left a cohort of 24,148 patients for analysis. Baseline characteristics are provided in Table 1. Young men were overrepresented, with the vast majority being admitted to ICU either from the emergency department or operating theater. Almost 90% were cared for at a tertiary institution. The median ‘worst’ PO2 was 130 (90–216) mmHg. Observed in-hospital mortality was 17.2% (n = 4150). Survivors tended to be younger, with higher coma and lower illness severity scores, less comorbidity, and less frequently were admitted postoperatively (P < 0.01).
Primary Analysis
The crude relationship between in-hospital mortality and PaO2 category (using the worst value recorded in the first 24 h in ICU) is presented in Fig. 2a. Mortality ranged from 27.1% (PaO2 40–49 mmHg) to 13.3% (PaO2 140–159 mmHg). Figure 2b displays the adjusted ORs (95% CI) for in-hospital mortality by PaO2 category in multivariable analysis. As illustrated, the only PaO2 category associated with a significantly greater adjusted risk of death was < 40 mmHg (OR 1.52 [1.03–2.25]). Additional details concerning the overall performance of the logistic regression model are provided in Supplementary Appendix.
Sensitivity Analysis
In the overall cohort, 1172 (4.9%) patients were hypoxic (PaO2 < 60 mmHg), 19,859 (82.2%) were normoxic (60–299 mmHg), and 3117 (12.9%) hyperoxic (PaO2 > 299 mmHg), during the first 24 h in ICU. A total of 4013 patients were identified as having an isolated head injury on the basis of their APACHE III-J sub-code. In 13,443 cases, the sub-code was missing. Figure 3a displays crude in-hospital mortality according to oxygenation status in both the overall cohort and patients coded as suffering an isolated head injury. Crude in-hospital mortality was 17.8% in hyperoxic patients overall, and 24.0% in those with an isolated head injury. Figure 3b displays the adjusted ORs (95% CI) from multivariable analysis. As illustrated, hyperoxia was not identified as an independent risk factor for mortality [OR 0.97 (0.86–1.11)—all patients, OR 1.21 (0.90–1.64)—isolated head injury].
Non-operative admissions accounted for 17,800 cases; the remainder (n = 6348) were postoperative (admitted from the operating theater). Figure 4a displays crude in-hospital mortality according to operative status and oxygenation. Postoperative hyperoxic patients had an in-hospital mortality rate of 21%. This was 16.5% in non-operative patients. Adjusted ORs (95% CI) are provided in Fig. 4b. As illustrated, hyperoxia was not independently associated with greater in-hospital mortality in either operative group [OR 0.96 (0.77–1.19)—postoperative, OR 1.00 (0.85–1.18)—non-operative].
The GCS was < 9 in 12,974 patients, between 9 and 12 in 4380, and > 12 in 6794. Figure 5a displays crude in-hospital mortality according to GCS category and oxygenation status. In-hospital mortality in hyperoxic patients with a GCS < 9, between 9 and 12, and > 12 was 28.5, 6.5, and 4.9%, respectively. Adjusted ORs (95% CI) are provided in Fig. 5b. Hyperoxia was not independently associated with greater in-hospital mortality in any group [OR 0.99 (0.84–1.13)—GCS < 9, OR 1.01 (0.67–1.51)—GCS 9–12, OR 1.1 (0.75–1.63)—GCS > 12]. Further details concerning the regression models employed are provided in Supplementary Appendix.
Discussion
Key Findings
In a large retrospective multicenter cohort of traumatic brain injury patients admitted to ICUs in Australia and New Zealand, we were unable to identify any association between early (during the first 24 h of ICU admission) hyperoxia and in-hospital mortality. Importantly, this result was consistent across multiple subgroups, including isolated head injury, postoperative and non-operative patients, and GCS category. Indeed, only markedly low PaO2 values (worst PaO2 < 40 mmHg) were associated with a significantly higher adjusted mortality risk overall, whereas in non-operative cases and those with a GCS > 12, this was observed when defining hypoxia as < 60 mmHg.
Relationship with Previous Studies
Our results are consistent with many other studies [18, 19], suggesting an association between hypoxia and increased mortality in TBI patients. However, in contrast to previous work, we did not find any association between hyperoxia and mortality. Davis et al. [8] in 2009 concluded that both hypoxemia (OR 0.54; 95% CI 0.42–0.69; P < 0.001) and extreme hyperoxemia (OR 0.50; 95% CI 0.36–0.71; P < 0.001) were associated with an increased mortality; however, hypoxemia in this case was defined as PaO2 < 110 mmHg and extreme hyperoxemia defined as PaO2 ≥ 487 mmHg. They used only the first PaO2 measurement obtained and deduced that a PaO2 between 110 and 487 mmHg was optimal.
Similar results were reported by Brenner et al. [10], where both low and high PaO2 values were associated with increased mortality (P < 0.05), although hypoxia was defined as a PaO2 < 100 mmHg and hyperoxia as PaO2 ≥ 200 mmHg. This study used the mean PaO2 compared to our ‘worst’ value. Their study was not restricted to patients receiving mechanical ventilation however; therefore, it is likely that they enrolled patients with less severe TBI and who were less likely to be hyperoxemic. In contrast, Raj et al. [11] used the ‘worst’ PaO2 reading, with hyperoxia defined as a PaO2 > 100 mmHg and hypoxia defined as < 75 mmHg, and found no independent relationship between hyperoxia and mortality after adjusting for markers of illness severity. We defined hypoxia as a ‘worst’ PaO2 value < 60 mmHg and hyperoxia as ≥ 300 mmHg, as previously described by Rincon et al. [12]. This was the most recent study examining the relationship between hyperoxia and mortality in TBI patients. These values for hypoxia and hyperoxia have also been used in three previous studies examining the relationship between hyperoxia and mortality after cardiac arrest or myocardial infarction [15, 20, 21] where hyperoxia was associated with increased hospital mortality.
Study Implications
Our findings imply that hypoxia in patients with TBI has potentially significant prognostic implications and should be robustly avoided where possible. However, avoiding hyperoxia is more contentious, as we were unable to establish any association between high PaO2 values and mortality.
Strengths and Limitations
Our study has several strengths. It used a large, independent, binational database, which included over 1.7 million critically ill patients. The final cohort of 24,148 TBI patients is the largest to date exploring this topic and is likely to be reflective of all ICUs in Australia and New Zealand. By only including patients receiving mechanical ventilation, we have selected a cohort with significant injury, and where oxygen exposure has been accurately recorded. The data were collected by trained collectors for the purposes of audit and for this reason, are unlikely to be subject to bias. We used validated markers for severity of illness, which allowed us to calculate adjusted mortality risk with varying PaO2 categories. We also performed sensitivity analysis in multiple subgroups to further substantiate our findings.
There are a number of limitations. The study was retrospective, so we were unable to assess long-term neurological outcome, instead using in-hospital mortality as the primary endpoint. This was dictated by the nature of the dataset, as functional status and/or quality of life is not routinely recorded in the ANZICS-APD. This is important, as arguably hyperoxia may have a greater impact on functional outcomes. The study was also observational, which limits any robust assessment of causation.
There were also patients whose outcomes were missing (n = 267), and therefore excluded. These data are likely to be missing at random and therefore should not have generated any selection bias. We used PaO2 values to describe hypoxia, normoxia, and hyperoxia as per recent literature. While these are relatively coarse and may therefore have obscured a relationship between a high PaO2 and mortality, our primary analysis (using 17 categories of PaO2) did not suggest any such association. In addition, by using the worst recorded PaO2 value over the first 24 h in ICU, high values (> 299 mmHg) are likely to be reflective of a substantial period of extreme hyperoxia.
We used APACHE III-J codes to select patients; however, some codes may be entered in error or not sub-coded appropriately. In this respect, sub-codes were missing in a substantial number of cases. As such, the primary analysis (n = 24,148) includes patients with varying degrees of multisystem trauma where the effects of hyperoxia are unknown. Although this may have weakened any signal overall, in those cases recorded as having isolated head injury, no significant association was noted between hyperoxia and in-hospital mortality. Indeed, this subgroup analysis has utilized the largest cohort (n = 4013) of isolated TBI patients reported to date and is also consistent with our findings in those with severe TBI (GCS < 9). We also acknowledge that TBI encompasses a number of unique pathologies (e.g., subdural hematoma versus diffuse axonal injury) and that although no differences were evident between non-operative and postoperative patients (in terms of the association between hyperoxia and in-hospital mortality), it is highly conceivable that hyperoxia may impact each injury sub-type differently.
The ANZICS-APD collects data for the first 24 h in ICU only, and it is unknown what FiO2 patients were exposed to later in their ICU stay, in the pre-hospital setting, or in the emergency department. As such, our data are limited in determining what duration of hyperoxia may be associated with adverse outcomes. Finally, we made no assessment of the partial arterial pressure of carbon dioxide, which is known to influence cerebral blood flow.
Future Research
The results from our study add substantially to the current literature examining this topic. The lack of consistent results suggests a prospective cohort study exploring the association between oxygen exposure and clinical outcomes is now urgently needed. Any subsequent work might also explore brain tissue oxygenation tensions (PbtO2), particularly as PbtO2-targeted therapy has been shown to improve outcome in select studies [22,23,24]. Assessments of long-term neurological outcome as a primary endpoint rather than in-hospital mortality would also be beneficial.
Conclusion
In a large multicenter cohort of TBI patients, hyperoxia in the first 24 h after ICU admission was not independently associated with greater in-hospital mortality. Conversely, hypoxia should be robustly avoided where possible.
References
Bitterman H. Bench-to-bedside review: oxygen as a drug. Crit Care. 2009;13(1):205.
Habre W, Petak F. Perioperative use of oxygen: variabilities across age. Br J Anaesth. 2014;113:26–36.
Asfar P, Singer M, Radermacher P. Understanding the benefits and harms of oxygen therapy. Intensive Care Med. 2015;41(6):1118–21.
Stub D, Smith K, Bernard S, et al. Air versus oxygen in ST-segment elevation myocardial infarction. Circulation. 2015;131(24):2143–50.
Tan A, Schulze A, O’Donnell CP, Davis PG. Air versus oxygen for resuscitation of infants at birth. Cochrane Database Syst Rev. 2004;3:CD002273.
Rincon F, Kang J, Maltenfort M, et al. Association between hyperoxia and mortality after stroke: a multicenter cohort study. Crit Care Med. 2014;42(2):387–96.
Quintard H, Patet C, Suys T, Marques-Vidal P, Oddo M. Normobaric hyperoxia is associated with increased cerebral excitotoxicity after severe traumatic brain injury. Neurocrit Care. 2015;22(2):243–50.
Davis DP, Meade W, Sise MJ, et al. Both hypoxemia and extreme hyperoxemia may be detrimental in patients with severe traumatic brain injury. J Neurotrauma. 2009;26(12):2217–23.
Asher SR, Curry P, Sharma D, et al. Survival advantage and PaO2 threshold in severe traumatic brain injury. J Neurosurg Anesthesiol. 2013;25(2):168–73.
Brenner M, Stein D, Hu P, Kufera J, Wooford M, Scalea T. Association between early hyperoxia and worse outcomes after traumatic brain injury. Arch Surg. 2012;147(11):1042–6.
Raj R, Bendel S, Reinikainen M, et al. Hyperoxemia and long-term outcome after traumatic brain injury. Crit Care. 2013;17(4):R177.
Rincon F, Kang J, Vibbert M, Urtecho J, Athar MK, Jallo J. Significance of arterial hyperoxia and relationship with case fatality in traumatic brain injury: a multicentre cohort study. J Neurol Neurosurg Psychiatry. 2014;85(7):799–805.
https://braintrauma.org/uploads/03/12/Guidelines_for_Management_of_Severe_TBI_4th_Edition.pdf. Accessed 17 Apr 2018.
Stow PJ, Hart GK, Higlett T, et al. Development and implementation of a high-quality clinical database: the Australian and New Zealand intensive care society adult patient database. J Crit Care. 2006;21(2):133–41.
Bellomo R, Bailey M, Eastwood GM, et al. Arterial hyperoxia and in-hospital mortality after resuscitation from cardiac arrest. Crit Care. 2011;15(2):R90.
Paul E, Bailey M, Pilcher D. Risk prediction of hospital mortality for adult patients admitted to Australian and New Zealand intensive care units: development and validation of the Australian and New Zealand risk of death model. J Crit Care. 2013;28(6):935–41.
von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–7.
Davis DP, Dunford JV, Poste JC, et al. The impact of hypoxia and hyperventilation on outcome after paramedic rapid sequence intubation of severely head-injured patients. J Trauma. 2004;57(1):1–10.
Chi JH, Knudson MM, Vassar MJ, et al. Prehospital hypoxia affects outcome in patients with traumatic brain injury: a prospective multicenter study. J Trauma. 2006;61:1134–41.
Kilgannon JH, Jones AE, Shapiro NI, et al. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA. 2010;303(21):2165–71.
Elmer J, Scutella M, Pullalarevu R, et al. The association between hyperoxia and patient outcomes after cardiac arrest: analysis of a high-resolution database. Intensive Care Med. 2015;41(1):49–57.
Spiotta AM, Stiefel MF, Gracias VH, et al. Brain tissue oxygen-directed management and outcome in patients with severe traumatic brain injury. J Neurosurg. 2010;113(3):571–80.
Stiefel MF, Spiotta A, Gracias VH, et al. Reduced mortality rate in patients with severe traumatic brain injury treated with brain tissue oxygen monitoring. J Neurosurg. 2005;103(5):805–11.
Bohman LE, Heuer GG, Macyszyn L, et al. Medical management of compromised brain oxygen in patients with severe traumatic brain injury. Neurocrit Care. 2011;14(3):361–9.
Acknowledgements
The authors wish to acknowledge the participation of the contributing hospitals: Albury Base Hospital ICU, Alfred Hospital ICU, Alice Springs Hospital ICU, Allamanda Private Hospital ICU, Armadale Health Service ICU, Armidale Rural Referral Hospital ICU, Auckland City Hospital DCCM, Austin Hospital ICU, Ballarat Health Services ICU, Bankstown-Lidcombe Hospital ICU, Bathurst Base Hospital ICU, Bendigo Health Care Group ICU, Box Hill Hospital ICU, Bunbury Regional Hospital ICU, Bundaberg Base Hospital ICU, Caboolture Hospital HDU, Cairns Hospital ICU, Calvary Hospital (Canberra) ICU, Calvary Hospital (Lenah Valley) ICU, Calvary Mater Newcastle ICU, Campbelltown Hospital ICU, Canberra Hospital ICU, Central Gippsland Health Service ICU, Christchurch Hospital ICU, Coffs Harbour Health Campus ICU, Concord Hospital (Sydney) ICU, Dandenong Hospital ICU, Dubbo Base Hospital ICU, Dunedin Hospital ICU, Epworth Freemasons Hospital ICU, Fiona Stanley Hospital ICU, Flinders Medical Centre ICU, Footscray Hospital ICU, Frankston Hospital ICU, Fremantle Hospital ICU, Gold Coast University Hospital ICU, Gosford Hospital ICU, Goulburn Valley Health ICU, Grafton Base Hospital ICU, Greenslopes Private Hospital ICU, Griffith Base Hospital ICU, Hawkes Bay Hospital ICU, Hervey Bay Hospital ICU, Holy Spirit Northside Hospital ICU, Hornsby Ku-ring-gai Hospital ICU, Ipswich Hospital ICU, John Flynn Private Hospital ICU, John Hunter Hospital ICU, Joondalup Health Campus ICU, Knox Private Hospital ICU, Latrobe Regional Hospital ICU, Launceston General Hospital ICU, Lismore Base Hospital ICU, Liverpool Hospital ICU, Logan Hospital ICU, Lyell McEwin Hospital ICU, Mackay Base Hospital ICU, Macquarie University Private Hospital ICU, Manly Hospital & Community Health ICU, Manning Rural Referral Hospital ICU, Maroondah Hospital ICU, Mater Adults Hospital (Brisbane) ICU, Mater Private Hospital (Brisbane) ICU, Mater Private Hospital (Sydney) ICU, Mersey Community Hospital ICU, Middlemore Hospital ICU, Mildura Base Hospital ICU, Monash Medical Centre-Clayton Campus ICU, Mount Isa Hospital ICU, Nambour General Hospital ICU, Nelson Hospital ICU, Nepean Hospital ICU, North Shore Hospital ICU, North West Regional Hospital (Burnie) ICU, Northeast Health Wangaratta ICU, Orange Base Hospital ICU, Port Macquarie Base Hospital ICU, Prince of Wales Hospital (Sydney) ICU, Prince of Wales Private Hospital (Sydney) ICU, Princess Alexandra Hospital ICU, Queen Elizabeth II Jubilee Hospital ICU, Redcliffe Hospital ICU, Robina Hospital ICU, Rockhampton Hospital ICU, Rockingham General Hospital ICU, Rotorua Hospital ICU, Royal Adelaide Hospital ICU, Royal Brisbane and Women’s Hospital ICU, Royal Darwin Hospital ICU, Royal Hobart Hospital ICU, Royal Melbourne Hospital ICU, Royal North Shore Hospital ICU, Royal Perth Hospital ICU, Royal Prince Alfred Hospital ICU, Shoalhaven Hospital ICU, Sir Charles Gairdner Hospital ICU, South West Healthcare (Warrnambool) ICU, St Andrew’s War Memorial Hospital ICU, St George Hospital (Sydney) ICU, St George Hospital (Sydney) ICU2, St George Private Hospital (Sydney) ICU, St Vincent’s Hospital (Melbourne) ICU, St Vincent’s Hospital (Sydney) ICU, St Vincent’s Hospital (Toowoomba) ICU, St Vincent’s Private Hospital (Sydney) ICU, Sunshine Hospital ICU, Sutherland Hospital & Community Health Services ICU, Sydney Adventist Hospital ICU, Tamworth Base Hospital ICU, Taranaki Health ICU, Tauranga Hospital ICU, The Northern Hospital ICU, The Prince Charles Hospital ICU, The Queen Elizabeth (Adelaide) ICU, The Townsville Hospital ICU, The Wesley Hospital ICU, Timaru Hospital ICU, Toowoomba Hospital ICU, Tweed Heads District Hospital ICU, University Hospital Geelong ICU, Wagga Wagga Base Hospital & District Health ICU, Waikato Hospital ICU, Wellington Hospital ICU, Western District Health Service (Hamilton) ICU, Westmead Hospital ICU, Westmead Private Hospital ICU, Whangarei Area Hospital, Northland Health Ltd ICU, Wimmera Health Care Group (Horsham) ICU, Wollongong Hospital ICU, Wyong Hospital ICU.
Funding
Professor Andrew Udy gratefully acknowledges salary support from the National Health and Medical Research Council of Australia (Early Career Fellowship; GNT1124532). ANZICS CORE is funded by the State and Territory Health Departments of Australia and the New Zealand Ministry of Health to monitor performance and provide benchmarking services to ICUs and Health Departments throughout both countries.
Author information
Authors and Affiliations
Contributions
DO, DP, AU, and CN designed the study. DP/AU had full access to the raw data and take responsibility for the integrity of the data and the accuracy of the data analysis. Statistical analysis was performed by DP. DO/AU drafted the initial manuscript. All authors critically revised the manuscript for important intellectual content and read and approved the final version.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
Ethics approval was obtained from the Alfred Hospital Human Research Ethics Committee (HREC reference number 162/17), with a waiver of individual patient informed consent.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ó Briain, D., Nickson, C., Pilcher, D.V. et al. Early Hyperoxia in Patients with Traumatic Brain Injury Admitted to Intensive Care in Australia and New Zealand: A Retrospective Multicenter Cohort Study. Neurocrit Care 29, 443–451 (2018). https://doi.org/10.1007/s12028-018-0553-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-018-0553-5