Abstract
B7-H3 is a member of the B7 superfamily and a putative inhibitory immune checkpoint molecule. Several early-phase clinical trials have reported promising anti-tumor activity and safety of anti-cancer drugs targeting B7-H3, suggesting that it may be a promising target for a potential next-generation immune checkpoint inhibitor. Despite ongoing clinical studies, most B7-H3-targeted drugs being currently investigated rely on direct cytotoxicity as their mechanisms of action rather than modulating its function as an immune checkpoint, at least in part due to its incompletely understood immune regulatory function. Recent studies have begun to elucidate the role of B7-H3 in regulating the tumor microenvironment (TME). Emerging evidence suggests that B7-H3 may regulate the interferon-STAT1 axis in the TME and promote immune suppression. Similarly, increasing evidence shows B7-H3 may be implicated in promoting M1 to M2 polarization of tumor-associated macrophages (TAMs). There is also accumulating evidence suggesting that B7-H3 may play a role in the heterotypic fusion of cancer stem cells and macrophages, thereby promoting tumor invasion and metastasis. Here, we review the recent advances in the understanding of B7-H3 cancer immunobiology with a focus on highlighting its potential role in the interferon priming of TAMs and the heterotypic fusion of TAMs with cancer stem cells and suggest future direction in elucidating its immune checkpoint function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immune checkpoint molecules are cell surface transmembrane proteins that provide modulatory signals to immune cells such as T cells to inhibit or stimulate immune responses. In the context of cancer, inhibitory immune checkpoints are hijacked by cancer cells to promote immune evasion, which is one of the hallmarks of cancer, permitting uncontrolled growth and proliferation [1, 2]. Over the past decade, immune checkpoint inhibitors (ICIs), which are monoclonal antibodies (mAb) targeting inhibitory immune checkpoints such as programmed death-1 or ligand-1 (PD-(L)1), cytotoxic lymphocyte antigen-4 (CTLA)-4, and lymphocyte activation antigen 3 (LAG)-3, have demonstrated clinical efficacy across an ever-expanding number of different human cancers and revolutionized the paradigm of anti-cancer therapies [3, 4]. Consequently, these successes have spurred further investigation into uncovering novel inhibitory immune checkpoints that represent therapeutic vulnerabilities and in developing additional therapeutic agents aimed at such molecular targets.
B7 superfamily transmembrane proteins comprise a group of stimulatory and inhibitory immune checkpoints. So far, ten members of B7 family proteins have been identified including B7-H1 (PD-L1), B7-1 (CD80), B7-2 (CD86), B7-DC (PD-L2), B7-H2 (CD275), B7-H3 (CD276), B7-H4, B7-H5, B7-H6 (NCR3LG1), and B7-H7 (HHLA2) [5,6,7]. Since its discovery in 2001, B7-H3 has garnered increasing attention in the past few years as a potential therapeutic target in anti-cancer therapy [7]. Although initial studies ascribed a stimulatory function to B7-H3, subsequent research and accumulating evidence have suggested that in the context of cancer, B7-H3 behaves predominantly as an inhibitory immune checkpoint and promotes immune evasion [8,9,10]. In addition to its role as an immune checkpoint promoting immune evasion, B7-H3 has also been implicated in promoting various pro-tumorigenic processes including cell proliferation, invasion, metastasis, dysregulated metabolism, angiogenesis, as well as chemo-resistance [8, 11]. B7-H3 is expressed in two isoforms (2IgB7-H3, 4IgB7-H3) in humans, whereas in mice, it is expressed only as a single isoform (2IgB7-H3) which consists of a single extracellular variable- and constant-like immunoglobulin, transmembrane, and cytoplasmic tail domains [8, 11, 12]. The major isoform expressed in humans is 4IgB7-H3 which has two identical pairs of IgC-like and V-like domains. The binding partner of B7-H3 has remained elusive and controversial, although several putative receptors have been reported including triggering receptor expressed on myeloid cells (TREM)-like transcript 2 (TLT-2, TREML2), interleukin-20 receptor subunit alpha (IL20RA), and phospholipase A2 receptor 1 (PLA2R1) [8, 10, 11, 13].
Drugs targeting B7-H3 such as bispecific antibodies, antibody drug conjugates, chimeric antigen receptor T cells, radio-immunoligands, and Fc-enhanced monoclonal antibodies have recently made their way into early-phase trials and have shown promising anti-tumor activity in various cancers including prostate cancer, head and neck squamous cell carcinoma, and small cell lung cancer [14,15,16] [8, 11, 17] (Table 1). In contrast to the increasing number of studies of B7-H3-targeted agents that use direct cytotoxicity as the major mechanism of action, there is a paucity of drugs being developed aimed at modulating B7-H3 function in part due to the elusive nature of its binding partner but also its incompletely elucidated role in tumor-promoting immunological processes. Here, we aim to review the relevant contemporary research that has studied the function of B7-H3 in immune modulation in the tumor microenvironment (TME) with a focus on its role in regulating the interferon (IFN)-STAT-1 axis and differentiation and function of tumor-associated macrophages (TAMs).
Search methodology
A systematic review was conducted according to PRISMA guidelines with the last search update being on December 29, 2023. The search was conducted in PubMed as well as Embase using the following search query: (B7-H3 OR CD276) AND (IFN OR interferon OR STAT). Studies were included if it investigated the mechanistic association between B7-H3 and interferon signaling or macrophage function and/or regulation. Studies were excluded if it reported overlapping data. In the latter case, the study with the most recent and/or most comprehensive data was included. The initial search identified a total of 440 studies. After review by title and abstract, 6 studies remained. Additionally, 0 studies were added via reference review. Following full-text review, 6 studies were included in the final review.
B7-H3 expression and regulation in healthy and malignant tissues
B7-H3 expression in healthy and malignant tissues has been extensively studied and reviewed since its discovery. While it is absent or expressed at very low levels in healthy tissues and body fluids, it is aberrantly expressed at high levels in cancer tissues in various malignancies [8, 9, 11, 18]. Among healthy tissues, its mRNA levels are highest in the placenta, adipose tissue, cervix, endometrium, and adrenal glands, and protein levels are highest in the prostate, adipose tissue, adrenal gland, appendix, and breast [9, 11]. The differences between its mRNA and protein expression levels underscore the importance of post-transcriptional regulation in B7-H3 expression and function. In comparison to the scant expression in normal tissues, high expression is observed across many cancer types. Literature review suggests that an estimated 60% of all tumors demonstrate some level of positivity of B7-H3 protein [9]. Furthermore, B7-H3 protein expression has been noted on both tumor cells and various cells including immune cells and non-immune stromal cells such as endothelial cells and cancer-associated fibroblasts (CAFs) in the TME [19,20,21]. Importantly, B7-H3 expression levels in tumor tissues correlate with adverse clinical outcomes and pathological features in various hematogenous and solid tumor malignancies including acute myeloid leukemia, prostate cancer, sarcomas, and breast cancer [22,23,24,25,26,27].
Regulation of expression of B7-H3 has been reported at the transcriptional, post-transcriptional, and post-translational as well as epigenetic levels have been described [8, 9, 11]. In human ovarian cancer cells, p38 MAPK-eIF4E signaling was identified as a key initiating step in the transcription of B7-H3 [28]. Patients with ankylosing spondylitis, who have high B7-H3 gene expression, have been shown to have a hypomethylated promoter of the B7-H3 gene [29]. In triple-negative breast cancer models, the fucosyltransferase FUT8 has been shown to drive aberrant glycosylation of B7-H3 and maintain high B7-H3 expression and mediate B7-H3 immune suppressive function [30]. Of the modes of gene expression regulation, post-transcriptional regulation of B7-H3 has been studied the most extensively in part due to the discrepancy between the B7-H3 mRNA and protein levels in healthy tissues. Among mechanisms of post-transcriptional regulation of B7-H3, the best studied has been microRNA (miRNA)-mediated regulation, which has shown clinical significance via associations with prognostic outcomes in cancer. For example, miR-124, -29, 199a, -1253, and -187 have been associated with aberrant B7-H3 protein expression in osteosarcoma, neuroblastoma, cervical cancer, medulloblastoma, and colorectal cancer (CRC), respectively [8, 31,32,33,34]. Higher levels of miR-187, -1301-3p, and -335-5p have been associated with lower aggressiveness, less advanced stage, and decreased metastasis [8, 31, 32]. Additionally, epigenetic regulation of B7-H3 expression has been described. For instance, hypomethylation of the B7-H3 promoter in chronic inflammation has been associated with increased B7-H3 expression levels [29]. Post-transcriptional regulation of B7-H3 represents an attractive mechanism for therapeutic development that depends on the function of B7-H3 rather than direct cytotoxicity.
Immunologic and non-immunologic effects of B7-H3 in malignancy
B7-H3 promotes several pro-tumorigenic cellular processes that are implicated in the hallmarks of cancer that are unrelated to immune regulation such as cell proliferation and growth, invasion, deranged metabolism, and angiogenesis as well as chemo-resistance, which have been reviewed extensively [8, 9, 11]. In vitro models of CRC have shown that B7-H3 downregulation leads to reduction of tumor cell proliferation as well as the downregulation of cyclin D1 and cyclin-dependent kinase (CDK)4 [35]. B7-H3 has been linked to cell proliferation and invasion in lung adenocarcinoma cell lines and in breast cancer cell lines specifically via modulation of major vault protein (MVP)-MEK signaling [36]. Furthermore, B7-H3 has been shown to promote epithelial-mesenchymal transition (EMT) in lung cancer as well as hepatocellular carcinoma cell lines and has been implicated in the pro-tumorigenic derangement of glucose metabolism in cancer cells [37]. For instance, B7-H3 has been shown to increase glucose uptake and lactate production by cancer cells, suggesting that it is involved in promoting the Warburg effect and B7-H3 expression levels are correlated with glycolytic capacity in in vitro models of breast cancer [38, 39]. The role of B7-H3 in regulating glucose metabolism in cancer cells may include its effects on the downstream transcriptional target, GLUT1 [40]. The role of B7-H3 in promoting angiogenesis has been described in various pre-clinical in vivo and in vitro studies and is a potential mechanism of regulating the vascular endothelial growth factor (VEGF)-hypoxia-inducible factor (HIF)-1α axis [41, 42]. Notably, B7-H3 has been shown to promote VEGF expression by activating the NF-kβ pathway in in vitro and in vivo studies of CRC [43].
Accumulating evidence suggests that B7-H3 has a multi-faceted role in modulating the immune contexture of the TME. Upregulation of B7-H3 has been noted in tumor cells, immune cells, and other stromal cells including CAFs and endothelial cells as noted previously. Expression of B7-H3 on CAFs has been associated with an immune suppressive phenotype in experimental studies of human breast cancer [20]. B7-H3 expression has been shown to accompany more frequently an immune suppressive TME by promoting the production of immune suppressive cytokines such as IL-10 and TGF-B1, in turn leading to inhibition of immune effector cell function [44,45,46,47]. Notably, B7-H3 has been shown to regulate the differentiation and polarization of TAMs from an M1 to M2 phenotype and has been associated with the frequency of FOXP3 + regulatory T cells [48, 49][50]. While the general consensus is that B7-H3 has an immune suppressive effect in the TME, some studies have shown increased numbers of immune effector cells such as CD4 + , CD8 + , and NK cells as well as increased pro-inflammatory cytokines in patients with high tumor B7-H3 expression [51, 52]. Nonetheless, a potential explanation for such findings is that the upregulation of B7-H3 reflects the presence of increased inflammatory cytokines such as IFNs in the tumor milieu which result in non-specific upregulation of immune checkpoints.
Evidence supporting the regulation of IFN-STAT1 axis by B7-H3
IFNs have been shown to have a dual role in promoting versus inhibiting tumorigenesis in a context-dependent manner. Of note, the IFN-STAT1 axis has both tumor promoting and inhibitory effects that depend at least in part on the strength and duration of the IFN signaling [53,54,55,56]. In other words, acute, short-lived exposure of tumor cells to IFN will result in tumor-inhibitory cytotoxicity, whereas chronic, long-lived exposure will result in tumor-promoting effects including increased tumor growth and proliferation, invasiveness, and immune evasion and immune suppression. For example, cancer cells that are DNA-damage-resistant produce IFNs in sufficient quantity to induce higher levels of STAT1, but not enough to induce cytotoxic genes, compared to DNA-damage-sensitive cell lines. Low levels of IFNs upregulate only the unphosphorylated interferon-stimulated gene factor (U-ISGF)3 dependent subset of ISGs in DNA-damage-resistant cancer cells, without the sustained increase in expression of ISGs that mediate the acute apoptotic, anti-proliferative, and inflammatory responses to IFN [57]. Thus, tumor cells have evolved to hijack the IFN-STAT1 axis to sustain chronic, long-lived signaling to enhance tumorigenesis.
There is a paucity of studies evaluating the role of B7 family molecules in regulating the IFN-STAT1 axis (Table 2). Among the B7 family members, some evidence exists for PD-L1 and its role in modulating STAT1. For example, Cheon et al. demonstrated that IFN-β-induced STAT1 phosphorylation is markedly higher in PD-L1 knockdown cells [58]. Similarly, Gato-Cañas et al. showed that PD-L1 decreases IFN-β cytotoxicity by inhibiting IFN-β-induced STAT3 signaling, although levels of STAT3 and phosphorylated STAT3 were not changed in PD-L1 knockdown cells [59]. Recent studies in the past few years have begun to implicate B7-H3 in regulating the IFN-STAT1 axis. For example, Liu et al. demonstrated that in tumor cells from B7-H3 knockdown or control Tsc2-deficient tumors in mice, IFN-γ response signature was the second-most upregulated pathway. Increased phosphorylated STAT-1 and STAT1 levels were also found in B7-H3 knockdown tumor cells compared to control. Furthermore, a difference in phosphorylated STAT1 and STAT1 was not observed in B7-H3 proficient and deficient cultured cells in the presence of activated T cells, suggesting that the TME is required for the upregulation of IFNγ in response to B7-H3 deficiency [60]. It remains unclear which cells in the TME are responsible for this difference; however, one hypothesis is that TAMs are implicated.
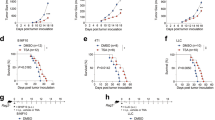
Huang et al. showed that tumor cells from B7-H3 knockout ID8 ovarian tumor-bearing mice, compared to B7-H3 wild type, showed markedly increased expression of IFN-β and its downstream genes which included STAT1 and increased the phosphorylation of STAT1 [61]. Additionally, Purvis et al. showed that transfection of D283 cells in vitro with miR-29, which targets and downregulates B7-H3 expression, led to marked upregulation of STAT1 expression and transcriptional activity [62]. These results are consistent with the results of Oh et al. who showed that the IFN-STAT1 pathway genes were among the top upregulated genes in B7-H3 knockdown mononuclear cells [63]. Consistent with these findings, the cancer genome atlas (TCGA) analysis of cutaneous melanoma patients shows that B7-H3 mRNA expression has an inverse correlation with STAT1 and IFNγ (Fig. 1).
Potential mechanisms of IFN-STAT1 regulation by B7-H3 in the tumor microenvironment
Despite emerging evidence supporting the role of B7-H3 in regulating the IFN-STAT1 axis, the potential mechanism through which this occurs and the overall effect on the TME remains unclear. The role of B7-H3 in activating downstream STAT3 signaling has been well established. Furthermore, STAT1 and STAT3 signaling activation is reciprocally regulated such that STAT1 activation leads to STAT3 downregulation and vice versa [64,65,66,67,68,69]. Thus, the indirect inhibition of STAT1 signaling via B7-H3-mediated STAT3 signaling activation is one theoretical mechanism in which B7-H3 may regulate the IFN-STAT1 axis (Fig. 2). Prior studies that have investigated that B7-H3 shows inconsistent results with respect to its effects on tumorigenesis. In other words, certain studies have demonstrated anti-tumor effects of B7-H3, although most studies show that it has pro-tumorigenic effects [70]. Since STAT1 has also been shown to have both tumor-suppressive and tumor-promoting effects, the opposing roles of B7-H3 on tumorigenesis may be explained at least in part by its effects on regulating the IFN-STAT1 axis [53, 56].
Schematic diagram of the theoretical function of B7-H3 in regulating interferon (IFN) priming of tumor-associated macrophages (TAMs) via reciprocal STAT3 and STAT1 activation. B7-H3-intact tumor cells will exhibit sustained STAT3 activation and reciprocal STAT1 inhibition, which reduce sensitivity to IFN, thereby hampering IFN-mediated tumoricidal effects and enhancing tumor cell growth and proliferation (A). B7-H3-deficient tumor cells will show decreased STAT3-dependent STAT1 inhibition leading to increased sensitivity and induction of IFN-stimulated genes. Consequently, B7-H3-deficient tumor cells will produce more IFN, which will stimulate M1 polarization of TAMs (B). Response to IFN sensitivity will depend on the relative strength of B7-H3-STAT3 signaling intrinsic to tumor cells if B7-H3 deficiency affects the cells in the tumor microenvironment such as TAMs rather than in tumor cells. In other words, enhanced B7-H3-STAT3 signaling in tumor cells will increase resistance to IFN and vice versa (C). The balance of reciprocal STAT3 and STAT1 activation will modulate B7-H3-mediated IFN effects
Forward versus backward signaling of B7-H3
B7-H3 engages unknown binding partners on CD4 + and CD8 + T cells and NK cells in exerting its effects on immune cells via forward signaling [8, 11]. On the other hand, in studies investigating its effects on cancer cells, B7-H3 is studied largely as a receptor rather than a ligand (backward signaling) [8, 10]. However, contemporary studies investigating B7-H3 often overlook this distinction between forward versus backward signaling. Thus, closer attention to this distinction is warranted when investigating B7-H3 function in future studies. While B7-H3-STAT3 and B7-H3-mTOR axes have been described in the context of B7-H3 modulating tumor cell function, the specific motifs responsible for mediating downstream signaling still need to be better understood and studied. Likewise, there is a paucity of studies that have evaluated the specific motifs that mediate downstream signaling in PD-L1, although Gato-Cañas et al. have described a phylogenetically conserved motif in the cytoplasmic domain that may have a role in inhibiting IFN-β-induced STAT3 activation [59]. Thus, identification of specific motifs that mediate downstream signaling in B7-H3 is an area of research that warrant further investigation.
Cellular localization and intracellular compartmentalization of B7-H3
Prior functional studies of B7-H3 show a discrepancy in their experimental results depending on the experimental model and methodology as well as whether the study subject is the TME or the tumor itself. For instance, recent studies showed that B7-H3 expression levels on host cells do not affect tumor growth, indicating the importance of tumor-intrinsic B7-H3; furthermore, others have shown the importance of B7-H3 expression on antigen-presenting cells (APCs) [61, 71, 72]. Notably, in vitro studies showed that suppressing B7-H3 expression in cancer cell lines do not affect cancer cell proliferation and invasion [61, 71, 72]. On the other hand, in vivo studies show that B7-H3 suppression markedly inhibits tumor growth [61, 71, 72]. Thus, the anti-tumor effects of B7-H3 suppression are only manifested when the inhibition occurs in the context of an intact TME.
B7-H3 demonstrates cytoplasmic and nuclear localization in addition to cell surface expression [11]. Immunohistochemical staining show that B7-H3 expression is seen on the cell surface as well as in the cytoplasm and nucleus of cancer tissues [73,74,75]. Furthermore, higher cytoplasmic B7-H3 expression compared to membranous expression has been noted in clear cell renal cell carcinoma (RCC) [76]. Additionally, nuclear B7-H3 expression is seen in an estimated 30% of CRC where nuclear expression is correlated with worse survival [77]. Thus, specific cellular compartmentalization of B7-H3 warrants closer attention in future studies evaluating its associations with clinical outcomes.
Relationship between B7-H3 and other B7 family members including PD-L1 and potential for synergism between PD-L1 and B7-H3 blockade
B7-H3 expression has been correlated with PD-L1 expression in preclinical and clinical studies of various cancers (Table 2). TCGA analysis shows that B7-H3 mRNA expression negatively correlates with PD-L1 mRNA levels in cutaneous melanoma patients (Fig. 1). Furthermore, PD-L1 protein expression has been shown to be upregulated in tumor cells after B7-H3 knockdown in mice [60]. However, other studies have shown inconsistent results. For example, B7-H3 mRNA expression was found to be directly correlated with PD-L1 mRNA levels in a pan-cancer analysis in patient tumor samples [18]. Given that B7-H3 is upregulated by inflammatory cytokines such as IFN and pathological molecular patterns such as lipopolysaccharide, B7-H3 may have been upregulated non-specifically in tissues where inflammatory cells are abundant. PD-L1 is similarly upregulated by IFN signaling non-specifically in such situations due to inflammation [53]. Additionally, Yonesaka et al. have also shown inconsistent results which indicated that anti-B7-H3 treatment had no effect on PD-L1 expression [78]. However, this study observed the results at only one early time point and consequently cannot rule out the possibility that the expression levels of both B7-H3 and PD-L1 recovered at the time point of observation. Furthermore, given the majority of studies that have demonstrated similar results suggesting that PD-L1 expression is upregulated by B7-H3 blockade, it is more likely that B7-H3 and PD-L1 have an inverse correlation that may have a mechanistic underpinning [61, 72, 78].
Inhibition of a single tumor-promoting immune checkpoint has shown to be ineffective if other distinct tumor-promoting immune checkpoints are co-expressed and exerting their effects [68]. For example, anti-PD-1-resistant human ovarian tumors exhibit high B7-H3 expression, and B7-H3 inhibition leads to decreased tumor growth [71]. However, B7-H3 knockdown tumors show upregulation of PD-L1 and IDO transcripts, indicating that inhibition of a single tumor-promoting immune checkpoint leads to compensatory expression of additional distinct tumor-promoting inhibitory immune checkpoints and molecules [60]. Furthermore, Yonesaka et al. showed that anti-B7-H3 monotherapy led to marked tumor growth inhibition until day 31 in NSCLC tumors which was followed by rapid tumor growth due to reduced treatment efficacy [78]. Concomitant targeting of additional inhibitory immune checkpoints with B7-H3 inhibition has shown increased anti-tumor efficacy in animal studies with varying degrees of efficacy depending on the combination regimen. For example, Shi et al. showed that in prostate cancer models, concomitant pharmacological anti-B7-H3 and anti-CTLA4 inhibition led to a greatest tumor shrinkage compared to anti-B7-H3 combined with either anti-PD-1 or anti-PD-L1 inhibition [79]. Thus, successful ICI treatment will necessitate improving the understanding of the temporal evolution of tumor-promoting immune checkpoints by tumors in the face of therapeutic pressure and development of rationally sequenced and combined ICI regimens.
Regulation of tumor-associated macrophage polarization and potential involvement in heterotopic fusion of cancer stem cells and macrophages by B7-H3
APCs expressing B7-H3 have been shown to promote tumor progression via suppression of effector CD8 + and NK cell function [8, 11]. As aforementioned, accumulating evidence has shown that B7-H3 enhances M2 macrophage polarization (Table 2). For example, Miyamoto et al. showed that in B7-H3 knockout tumors, F4/80 + CD206 + M2 macrophages were decreased, whereas M1 macrophages were unchanged. In this study, M2 markers such as Arg1 and IL-10 were decreased in macrophages of B7-H3 knockout tumors and increased IFNγCD8 + T cells were observed [48]. In line with this data, Cai et al. showed that B7-H3 was highly expressed on tumor-infiltrating APCs in human ovarian tumors including CD14 + HLA-Dhi macrophages. Additionally, compared to B7-H3 wild-type tumors, CD8 + T cells isolated from B7-H3 knockout ID8 tumor-bearing mice showed decreased PD-1 expression and increased cytotoxicity as exemplified by IFNγ, TNFα, and granzyme B production upon in vitro stimulation. Furthermore, increased tumor-infiltrating CD4 + T cells and TNFα and IFNγ-expressing NK cells were also observed [71]. Moreover, Kang et al. showed that the number of TAMs in human hepatocellular carcinoma tissue samples was positively correlated with B7-H3 expression. Additionally, B7-H3 promoted PMA-induced THP-1 cells to differentiate into M2-like macrophages, as exemplified by Arg1, VEGF, and CCL22 expression. This polarization was reversed by B7-H3 knock down or via STAT3 inhibition [80]. Other studies have shown that B7-H3 expression on TAMs directly inhibits anti-tumor T cell responses [49].
Cancer stem cells (CSCs) represent long-lived, therapy-resistant, immune-evasive cancer cells that harbor the cellular properties of quiescence, pluripotency, and self-renewal [81]. They have been described in both hematogenous and various solid tumor malignancies and are thought to drive tumor growth, proliferation, and metastasis [82, 83]. Recent studies have described the phenomenon of heterotypic fusion between putative CSCs and macrophages through which CSCs attain migratory and invasive properties as well as immune cell trafficking and homing abilities, thereby driving tumor heterogeneity, metastatic organotropism, and survival in circulation [84,85,86,87]. Ahmadzadeh et al. showed that B7-H3 is upregulated by Langerhans giant cells (LGCs) during multi-nucleation. In this study, increased B7-H3 transcripts and cell surface protein expression were observed in both LGCs and osteoclasts [88]. Similarly, Oh et al. showed that B7-H3 is also upregulated during osteoclast precursor cells during differentiation into osteoclasts [63]. Furthermore, the rate of cell fusion has been shown to be increased during polarization of macrophages to an M2-like phenotype, and during cell fusion, hybrid cells show heightened metabolic activity and demonstrate increased glycolysis to support proliferation [89,90,91]. Thus, multi-nucleation and polarization of macrophages to an M2-like state appear to accompany the heterotypic fusion of CSC and macrophages. Given that IFNγ-STAT1 axis has been shown to increase the metastatic potential of CSCs, IFN-STAT axis is a potential mechanism of regulating this process by B7-H3 that warrants further evaluation [92].
The receptor activator of nuclear factor kappa beta ligand (RANKL) has been implicated in promoting cancer cell migration and bone metastasis [93]. Li et al. showed that tumor cell-macrophage fusion has an important role in the recruitment of stromal and immune cells in the TME and promoting angiogenesis. In this study, bone marrow macrophage growth and cell–cell fusion were stimulated using M-CSF and RANKL media [94]. Thus, like its effects in promoting multi-nucleation in osteoclastogenesis, RANKL may have an important role in the fusogenic mechanism of tumor-macrophage hybrid cells. Furthermore, Gast et al. showed in an in vitro study of macrophage-cancer cell fusion hybrids that a subclone of melanoma cells (B16F10) showed high levels of RANK mRNA expression [86]. Additionally, RANK and RANKL expression has been demonstrated in primary human oral cavity squamous cell carcinoma and showed that RANKL increased tumor cell proliferation [95].
Taken together, B7-H3 stimulates several cellular processes that are also hallmark features of heterotypic fusion of CSCs and TAMs such as M2 polarization of macrophages, which enhanced aerobic glycolysis [38, 40, 48, 80, 96,97,98]. Furthermore, B7-H3 inhibition leads to inhibition of RANKL-induced multi-nucleated osteoclast formation [63]. Additionally, B7-H3 is expressed on CSCs in human head and neck cancer cells, in which it is associated with the promotion of EMT and lymph node metastasis [99]. Thus, B7-H3 appears to be involved in several cellular processes that are key features of heterotypic fusion of CSCs and macrophages, suggesting that B7-H3 may be implicated in this process. Indeed, the role of B7-H3 in the heterotypic fusion of cancer cells and macrophages warrants further investigation.
Conclusion
B7-H3 is a putative inhibitory immune checkpoint that is emerging as a clinically relevant immune checkpoint target in anti-cancer therapy development. In parallel to its known non-immunologic tumor-promoting effects and accumulating clinical evidence supporting the potential safety and efficacy of targeting B7-H3, studies elucidating its immune regulatory function in the TME are emerging. Notably among these studies, the role of B7-H3 in regulating the IFN-STAT1 axis and promoting macrophage polarization to an M2-like phenotype has received increasing attention. The contrasting functions of B7-H3 such as its immune stimulatory and inhibitory functions and its tumor promoting and suppressive effects that have been demonstrated in different contexts such as in autoimmune disorders or in cancer and in different types of cancer models may be explained in part by its fine tuning of the IFN-STAT axis. Moreover, there is supporting evidence to suggest that B7-H3 may be involved in the heterotypic fusion of CSCs and TAMs. Based on the currently available data, the role of B7-H3 in regulating IFN-induced priming of TAMs as well as in regulating the heterotypic fusion of CSCs and TAMs warrants further investigation.
Change history
22 February 2024
A Correction to this paper has been published: https://doi.org/10.1007/s12026-024-09467-8
References
Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer. 2019;19(3):133–50.
Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12(1):31–46.
Kraehenbuehl L, Weng CH, Eghbali S, Wolchok JD, Merghoub T. Enhancing immunotherapy in cancer by targeting emerging immunomodulatory pathways. Nat Rev Clin Oncol. 2022;19(1):37–50.
Korman AJ, Garrett-Thomson SC, Lonberg N. The foundations of immune checkpoint blockade and the ipilimumab approval decennial. Nat Rev Drug Discovery. 2022;21(7):509–28.
Wang Y, Zhang H, Liu C, Wang Z, Wu W, Zhang N, et al. Immune checkpoint modulators in cancer immunotherapy: recent advances and emerging concepts. J Hematol Oncol. 2022;15(1):111.
Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8(6):467–77.
Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, et al. B7–H3: a costimulatory molecule for T cell activation and IFN-γ production. Nat Immunol. 2001;2(3):269–74.
Zhao B, Li H, Xia Y, Wang Y, Wang Y, Shi Y, et al. Immune checkpoint of B7–H3 in cancer: from immunology to clinical immunotherapy. J Hematol Oncol. 2022;15(1):153.
Kontos F, Michelakos T, Kurokawa T, Sadagopan A, Schwab JH, Ferrone CR, et al. B7–H3: an attractive target for antibody-based immunotherapy. Clin Cancer Res. 2021;27(5):1227–35.
Kanchan RK, Doss D, Khan P, Nasser MW, Mahapatra S. To kill a cancer: targeting the immune inhibitory checkpoint molecule, B7–H3. Biochim Biophys Acta Rev Cancer. 2022;1877(5):188783.
Getu AA, Tigabu A, Zhou M, Lu J, Fodstad O, Tan M. New frontiers in immune checkpoint B7–H3 (CD276) research and drug development. Mol Cancer. 2023;22(1):43.
Vigdorovich V, Ramagopal UA, Lazar-Molnar E, Sylvestre E, Lee JS, Hofmeyer KA, et al. Structure and T cell inhibition properties of B7 family member, B7–H3. Structure. 2013;21(5):707–17.
Zhou WT, Jin WL. B7–H3/CD276: an emerging cancer immunotherapy. Front Immunol. 2021;12:701006.
Shenderov E, De Marzo AM, Lotan TL, Wang H, Chan S, Lim SJ, et al. Neoadjuvant enoblituzumab in localized prostate cancer: a single-arm, phase 2 trial. Nat Med. 2023;29(4):888–97.
Aggarwal C, Prawira A, Antonia S, Rahma O, Tolcher A, Cohen RB, et al. Dual checkpoint targeting of B7–H3 and PD-1 with enoblituzumab and pembrolizumab in advanced solid tumors: interim results from a multicenter phase I/II trial. J Immunother Cancer. 2022;10:4.
Patel MR, Doi T, Koyama T, Falchook GS, Friedman CF, Piha-Paul SA, et al. (2023) 690P Ifinatamab deruxtecan (I-DXd; DS-7300) in patients with advanced solid tumors: updated clinical and biomarker results from a phase I/II study. Annals of Oncology. 34:S481-S2
Pulanco MC, Madsen AT, Tanwar A, Corrigan DT, Zang X. Recent advancements in the B7/CD28 immune checkpoint families: new biology and clinical therapeutic strategies. Cell Mol Immunol. 2023;20(7):694–713.
Hwang J, Zorko N, Elliott A, Lozada JA, Radovich M, Heath EI, et al. Pan-cancer associations of B7–H3 (CD276) transcriptional expression across human malignancies. J Clin Oncol. 2023;41(16):2624.
Heather LM, Azin S, Andrew E, Ben XW, Sarah Rachel K, Patricia AS, et al. High expression of B7–H3 on stromal cells defines tumor and stromal compartments in epithelial ovarian cancer and is associated with limited immune activation. J Immunother Cancer. 2019;7(1):357.
Costa A, Kieffer Y, Scholer-Dahirel A, Pelon F, Bourachot B, Cardon M, et al. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell. 2018;33(3):463–7910.
Aung PP, Parra ER, Barua S, Sui D, Ning J, Mino B, et al. B7–H3 Expression in merkel cell carcinoma-associated endothelial cells correlates with locally aggressive primary tumor features and increased vascular density. Clin Cancer Res. 2019;25(11):3455–67.
Zhang X, Fang C, Zhang G, Jiang F, Wang L, Hou J. Prognostic value of B7–H3 expression in patients with solid tumors: a meta-analysis. Oncotarget. 2017;8(54):93156–67.
Zang X, Thompson RH, Al-Ahmadie HA, Serio AM, Reuter VE, Eastham JA, et al. B7–H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc Natl Acad Sci. 2007;104(49):19458–63.
Tyagi A, Ly S, El-Dana F, Yuan B, Jaggupilli A, Grimm S, et al. Evidence supporting a role for the immune checkpoint protein B7–H3 in NK cell-mediated cytotoxicity against AML. Blood. 2022;139(18):2782–96.
Wang L, Zhang Q, Chen W, Shan B, Ding Y, Zhang G, et al. B7–H3 is overexpressed in patients suffering osteosarcoma and associated with tumor aggressiveness and metastasis. PLoS ONE. 2013;8(8):e70689.
Kim Nah I, Park Min H, Kweon S-S, Lee Ji S. B7–H3 and B7–H4 expression in breast cancer and their association with clinicopathological variables and T cell infiltration. Pathobiology. 2020;87(3):179–92.
Lin W, Xu Y, Gao J, Zhang H, Sun Y, Qiu X, et al. Multi-omics data analyses identify B7–H3 as a novel prognostic biomarker and predict response to immune checkpoint blockade in head and neck squamous cell carcinoma. Front Immunol. 2021;12:757047.
Zhao S, Wang Y, Yang N, Mu M, Wu Z, Li H, et al. Genome-scale CRISPR-Cas9 screen reveals novel regulators of B7–H3 in tumor cells. J Immunother Cancer. 2022;10:6.
Chen Y, Wu Y, Yang H, Wang J, Kong J, Yu L, et al. DNA methylation and mRNA expression of B7–H3 gene in ankylosing spondylitis: a case-control study. Immunol Invest. 2022;51(7):2025–34.
Huang Y, Zhang H-L, Li Z-L, Du T, Chen Y-H, Wang Y, et al. FUT8-mediated aberrant N-glycosylation of B7H3 suppresses the immune response in triple-negative breast cancer. Nat Commun. 2021;12(1):2672.
Ahangar NK, Hemmat N, Khalaj-Kondori M, Shadbad MA, Sabaie H, Mokhtarzadeh A, et al. The regulatory cross-talk between microRNAs and novel members of the B7 family in human diseases: a scoping review. Int J Mol Sci. 2021;22:5.
Zhao J, Lei T, Xu C, Li H, Ma W, Yang Y, et al. MicroRNA-187, down-regulated in clear cell renal cell carcinoma and associated with lower survival, inhibits cell growth and migration though targeting B7–H3. Biochem Biophys Res Commun. 2013;438(2):439–44.
Hu X, Xu M, Hu Y, Li N, Zhou L. B7–H3, negatively regulated by miR-128, promotes colorectal cancer cell proliferation and migration. Cell Biochem Biophys. 2021;79(2):397–405.
Yang X, Feng KX, Li H, Wang L, Xia H. MicroRNA-199a inhibits cell proliferation, migration, and invasion and activates AKT/mTOR signaling pathway by targeting B7–H3 in cervical cancer. Technol Cancer Res Treat. 2020;19:1533033820942245.
Wang S, Mou J, Cui L, Wang X, Zhang Z. Astragaloside IV inhibits cell proliferation of colorectal cancer cell lines through down-regulation of B7–H3. Biomed Pharmacother. 2018;102:1037–44.
Liu Z, Zhang W, Phillips JB, Arora R, McClellan S, Li J, et al. Immunoregulatory protein B7–H3 regulates cancer stem cell enrichment and drug resistance through MVP-mediated MEK activation. Oncogene. 2019;38(1):88–102.
Liao H, Ding M, Zhou N, Yang Y, Chen L. B7H3 promotes the epithelialmesenchymal transition of NSCLC by targeting SIRT1 through the PI3K/AKT pathway. Mol Med Rep. 2022;25:3.
Lim S, Liu H, Madeira da Silva L, Arora R, Liu Z, Phillips JB, et al. Immunoregulatory protein B7–H3 reprograms glucose metabolism in cancer cells by ROS-mediated stabilization of HIF1alpha. Cancer Res. 2016;76(8):2231–42.
Nunes-Xavier CE, Karlsen KF, Tekle C, Pedersen C, Oyjord T, Hongisto V, et al. Decreased expression of B7–H3 reduces the glycolytic capacity and sensitizes breast cancer cells to AKT/mTOR inhibitors. Oncotarget. 2016;7(6):6891–901.
Li Z, Liu J, Que L, Tang X. The immunoregulatory protein B7–H3 promotes aerobic glycolysis in oral squamous carcinoma via PI3K/Akt/mTOR pathway. J Cancer. 2019;10(23):5770–84.
Cheng N, Bei Y, Song Y, Zhang W, Xu L, Zhang W, et al. B7–H3 augments the pro-angiogenic function of tumor-associated macrophages and acts as a novel adjuvant target for triple-negative breast cancer therapy. Biochem Pharmacol. 2021;183:114298.
Cheng R, Chen Y, Zhou H, Wang B, Du Q, Chen Y. B7–H3 expression and its correlation with clinicopathologic features, angiogenesis, and prognosis in intrahepatic cholangiocarcinoma. APMIS. 2018;126(5):396–402.
Wang R, Ma Y, Zhan S, Zhang G, Cao L, Zhang X, et al. B7–H3 promotes colorectal cancer angiogenesis through activating the NF- kappaB pathway to induce VEGFA expression. Cell Death Dis. 2020;11(1):55.
Han S, Wang Y, Shi X, Zong L, Liu L, Zhang J, et al. Negative roles of B7–H3 and B7–H4 in the microenvironment of cervical cancer. Exp Cell Res. 2018;371(1):222–30.
Lu H, Shi T, Wang M, Li X, Gu Y, Zhang X, et al. B7–H3 inhibits the IFN-gamma-dependent cytotoxicity of Vgamma9Vdelta2 T cells against colon cancer cells. Oncoimmunology. 2020;9(1):1748991.
Si S, Wang L, Cao H, Xu Y, Zhan Q. Co-deficiency of B7–H3 and B7–H4 identifies high CD8 + T cell infiltration and better prognosis in pancreatic cancer. BMC Cancer. 2022;22(1):211.
Lee CC, Ho KH, Huang TW, Shih CM, Hsu SY, Liu AJ, et al. A regulatory loop among CD276, miR-29c-3p, and Myc exists in cancer cells against natural killer cell cytotoxicity. Life Sci. 2021;277:119438.
Miyamoto T, Murakami R, Hamanishi J, Tanigaki K, Hosoe Y, Mise N, et al. B7–H3 suppresses antitumor immunity via the CCL2-CCR2-M2 macrophage axis and contributes to ovarian cancer progression. Cancer Immunol Res. 2022;10(1):56–69.
Jian An H, Cheng C, Yibei Z, Xueguang Z. Induced expression of B7–H3 on the lung cancer cells and macrophages suppresses tumor-specific T cell immunity. Eur Respir J. 2012;40(Suppl 56):P1221.
Inamura K, Amori G, Yuasa T, Yamamoto S, Yonese J, Ishikawa Y. Relationship of B7–H3 expression in tumor cells and tumor vasculature with FOXP3+ regulatory T cells in renal cell carcinoma. Cancer Manag Res. 2019;11:7021–30.
Yim J, Koh J, Kim S, Song SG, Ahn HK, Kim YA, et al. Effects of B7–H3 expression on tumour-infiltrating immune cells and clinicopathological characteristics in non-small-cell lung cancer. Eur J Cancer. 2020;133:74–85.
Luo L, Chapoval AI, Flies DB, Zhu G, Hirano F, Wang S, et al. B7–H3 enhances tumor immunity in vivo by costimulating rapid clonal expansion of antigen-specific CD8+ cytolytic T cells. J Immunol. 2004;173(9):5445–50.
Cheon H, Wang Y, Wightman SM, Jackson MW, Stark GR. How cancer cells make and respond to interferon-I. Trends Cancer. 2023;9(1):83–92.
Boukhaled GM, Harding S, Brooks DG. Opposing roles of type i interferons in cancer immunity. Annu Rev Pathol. 2021;16:167–98.
Jorgovanovic D, Song M, Wang L, Zhang Y. Roles of IFN-gamma in tumor progression and regression: a review. Biomark Res. 2020;8:49.
Meissl K, Macho-Maschler S, Muller M, Strobl B. The good and the bad faces of STAT1 in solid tumours. Cytokine. 2017;89:12–20.
Cheon H, Holvey-Bates EG, Schoggins JW, Forster S, Hertzog P, Imanaka N, et al. IFNbeta-dependent increases in STAT1, STAT2, and IRF9 mediate resistance to viruses and DNA damage. Embo j. 2013;32(20):2751–63.
Cheon H, Holvey-Bates EG, McGrail DJ, Stark GR. PD-L1 sustains chronic, cancer cell-intrinsic responses to type I interferon, enhancing resistance to DNA damage. Proc Natl Acad Sci U S A. 2021;118:47.
Gato-Canas M, Zuazo M, Arasanz H, Ibanez-Vea M, Lorenzo L, Fernandez-Hinojal G, et al. PDL1 signals through conserved sequence motifs to overcome interferon- mediated cytotoxicity. Cell Rep. 2017;20(8):1818–29.
Liu HJ, Du H, Khabibullin D, Zarei M, Wei K, Freeman GJ, et al. mTORC1 upregulates B7–H3/CD276 to inhibit antitumor T cells and drive tumor immune evasion. Nat Commun. 2023;14(1):1214.
Huang M, Luo J, Ji X, Hu M, Xue Y, Liu Q. Deficiency of tumor-expressed B7–H3 augments anti-tumor efficacy of anti- PD-L1 monotherapy rather than the combined chemoimmunotherapy in ovarian cancer. Pharmacol Res. 2022;186:106512.
Purvis IJ, Avilala J, Guda MR, Venkataraman S, Vibhakar R, Tsung AJ, et al. Role of MYC-miR-29-B7-H3 in medulloblastoma growth and angiogenesis. J Clin Med. 2019;8:8.
Oh Y, Park R, Kim SY, Park SH, Jo S, Kim TH, et al. B7–H3 regulates osteoclast differentiation via type I interferon- dependent IDO induction. Cell Death Dis. 2021;12(11):971.
Costa-Pereira AP, Tininini S, Strobl B, Alonzi T, Schlaak JF, Is’harc H, et al. Mutational switch of an IL-6 response to an interferon-gamma-like response. Proc Natl Acad Sci U S A. 2002;99(12):8043–7.
Herrero C, Hu X, Li WP, Samuels S, Sharif MN, Kotenko S, et al. Reprogramming of IL-10 activity and signaling by IFN-gamma. J Immunol. 2003;171(10):5034–41.
Sharif MN, Tassiulas I, Hu Y, Mecklenbrauker I, Tarakhovsky A, Ivashkiv LB. IFN-alpha priming results in a gain of proinflammatory function by IL-10: implications for systemic lupus erythematosus pathogenesis. J Immunol. 2004;172(10):6476–81.
Ho HH, Ivashkiv LB. Role of STAT3 in type I interferon responses .Negative regulation of STAT1-dependent inflammatory gene activation. J Biol Chem. 2006;281(20):14111–8.
Yan J, Wang ZY, Yang HZ, Liu HZ, Mi S, Lv XX, et al. Timing is critical for an effective anti-metastatic immunotherapy: the decisive role of IFNgamma/STAT1-mediated activation of autophagy. PLoS ONE. 2011;6(9):e24705.
Regis G, Pensa S, Boselli D, Novelli F, Poli V. Ups and downs: the STAT1:STAT3 seesaw of Interferon and gp130 receptor signalling. Semin Cell Dev Biol. 2008;19(4):351–9.
Li G, Quan Y, Che F, Wang L. B7–H3 in tumors: friend or foe for tumor immunity? Cancer Chemother Pharmacol. 2018;81(2):245–53.
Cai D, Li J, Liu D, Hong S, Qiao Q, Sun Q, et al. Tumor-expressed B7–H3 mediates the inhibition of antitumor T-cell functions in ovarian cancer insensitive to PD-1 blockade therapy. Cell Mol Immunol. 2020;17(3):227–36.
Lee YH, Martin-Orozco N, Zheng P, Li J, Zhang P, Tan H, et al. Inhibition of the B7–H3 immune checkpoint limits tumor growth by enhancing cytotoxic lymphocyte function. Cell Res. 2017;27(8):1034–45.
Sun Y, Wang Y, Zhao J, Gu M, Giscombe R, Lefvert AK, et al. B7–H3 and B7–H4 expression in non-small-cell lung cancer. Lung Cancer. 2006;53(2):143–51.
Deng J, Ma M, Wang D, Zhu H, Hua L, Sun S, et al. Expression and clinical significance of immune checkpoint regulator B7–H3 (CD276) in human meningioma. World Neurosurg. 2020;135:e12–8.
Qin X, Sun W, Wang C, Li M, Zhao X, Li C, et al. Mifepristone inhibited the expression of B7–H2, B7–H3, B7–H4 and PD-L2 in adenomyosis. Reprod Biol Endocrinol. 2021;19(1):114.
Saeednejad Zanjani L, Madjd Z, Axcrona U, Abolhasani M, Rasti A, Asgari M, et al. Cytoplasmic expression of B7–H3 and membranous EpCAM expression are associated with higher grade and survival outcomes in patients with clear cell renal cell carcinoma. Ann Diagn Pathol. 2020;46:151483.
Ingebrigtsen VA, Boye K, Tekle C, Nesland JM, Flatmark K, Fodstad O. B7–H3 expression in colorectal cancer: nuclear localization strongly predicts poor outcome in colon cancer. Int J Cancer. 2012;131(11):2528–36.
Yonesaka K, Haratani K, Takamura S, Sakai H, Kato R, Takegawa N, et al. B7–H3 negatively modulates CTL-mediated cancer immunity. Clin Cancer Res. 2018;24(11):2653–64.
Shi W, Wang Y, Zhao Y, Kim JJ, Li H, Meng C, et al. Immune checkpoint B7–H3 is a therapeutic vulnerability in prostate cancer harboring PTEN and TP53 deficiencies. Sci Transl Med. 2023;15(695):eadf6724.
Kang FB, Wang L, Li D, Zhang YG, Sun DX. Hepatocellular carcinomas promote tumor-associated macrophage M2-polarization via increased B7–H3 expression. Oncol Rep. 2015;33(1):274–82.
Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–11.
Ailles LE, Weissman IL. Cancer stem cells in solid tumors. Curr Opin Biotechnol. 2007;18(5):460–6.
Vetrie D, Helgason GV, Copland M. The leukaemia stem cell: similarities, differences and clinical prospects in CML and AML. Nat Rev Cancer. 2020;20(3):158–73.
Zhang L-N, Zhang D-D, Yang L, Gu Y-X, Zuo Q-P, Wang H-Y, et al. Roles of cell fusion between mesenchymal stromal/stem cells and malignant cells in tumor growth and metastasis. FEBS J. 2021;288(5):1447–56.
Dittmar T, Hass R. Intrinsic signalling factors associated with cancer cell-cell fusion. Cell Commun Signal. 2023;21(1):68.
Gast CE, Silk AD, Zarour L, Riegler L, Burkhart JG, Gustafson KT, et al. Cell fusion potentiates tumor heterogeneity and reveals circulating hybrid cells that correlate with stage and survival. Sci Adv. 2018;4(9):eaat7828.
Cozzo AJ, Coleman MF, Hursting SD. You complete me: tumor cell-myeloid cell nuclear fusion as a facilitator of organ-specific metastasis. Front Oncol. 2023;13:1191332.
Ahmadzadeh K, Pereira M, Vanoppen M, Bernaerts E, Ko JH, Mitera T, et al. Multinucleation resets human macrophages for specialized functions at the expense of their identity. EMBO Rep. 2023;24(3):e56310.
Clawson GA, Matters GL, Xin P, Imamura-Kawasawa Y, Du Z, Thiboutot DM, et al. Macrophage-tumor cell fusions from peripheral blood of melanoma patients. PLoS ONE. 2015;10(8):e0134320.
Aguirre LA, Montalban-Hernandez K, Avendano-Ortiz J, Marin E, Lozano R, Toledano V, et al. Tumor stem cells fuse with monocytes to form highly invasive tumor-hybrid cells. Oncoimmunology. 2020;9(1):1773204.
Brito A, Merle C, Lagarde P, Faustin B, Devin A, Lartigue L, et al. Cell fusion enhances energy metabolism of mesenchymal tumor hybrid cells to sustain their proliferation and invasion. BMC Cancer. 2021;21(1):863.
Beziaud L, Young CM, Alonso AM, Norkin M, Minafra AR, Huelsken J. IFNγ-induced stem-like state of cancer cells as a driver of metastatic progression following immunotherapy. Cell Stem Cell. 2023;30(6):818-31.e6.
Jones DH, Nakashima T, Sanchez OH, Kozieradzki I, Komarova SV, Sarosi I, et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature. 2006;440(7084):692–6.
Li M, Basile JR, Mallya S, Lin YL. The impact and outcomes of cancer-macrophage fusion. BMC Cancer. 2023;23(1):497.
Sambandam Y, Ethiraj P, Hathaway-Schrader JD, Novince CM, Panneerselvam E, Sundaram K, et al. Autoregulation of RANK ligand in oral squamous cell carcinoma tumor cells. J Cell Physiol. 2018;233(8):6125–34.
Gao Y, Fang P, Li WJ, Zhang J, Wang GP, Jiang DF, et al. LncRNA NEAT1 sponges miR-214 to regulate M2 macrophage polarization by regulation of B7–H3 in multiple myeloma. Mol Immunol. 2020;117:20–8.
Zuo J, Wang B, Long M, Gao Z, Zhang Z, Wang H, et al. The type 1 transmembrane glycoprotein B7–H3 interacts with the glycolytic enzyme ENO1 to promote malignancy and glycolysis in HeLa cells. FEBS Lett. 2018;592(14):2476–88.
Shi T, Ma Y, Cao L, Zhan S, Xu Y, Fu F, et al. B7–H3 promotes aerobic glycolysis and chemoresistance in colorectal cancer cells by regulating HK2. Cell Death Dis. 2019;10(4):308.
Wang C, Li Y, Jia L, Kim JK, Li J, Deng P, et al. CD276 expression enables squamous cell carcinoma stem cells to evade immune surveillance. Cell Stem Cell. 2021;28(9):1597-613.e7.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and Future (2022R1A2C1003860 to Jong Dae Ji).
Author information
Authors and Affiliations
Contributions
Conceptualization: R.P. and J.J.; validation: R.P., J.Y., M.S., S.L., and J.J.; writing—original draft preparation: R.P. and J.J.; writing—review and editing: R.P., J.Y., M.S., S.L., and J.J.; visualization: R.P.; supervision: J.J.; project administration: R.P. and J.J. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: A spelling error was identified in an author’s name, which has been corrected. Namely, Dr. Moazzam Shazad’s name has been corrected to “Shahzad”. The affiliation 1 were corrected from “Department of Hematology/Oncology, Moffitt Cancer Center, Tampa, FL, USA” to “Department of Hematology/Oncology, Moffitt Cancer Center/University of South Florida, Tampa, FL, USA.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Park, R., Yu, J., Shahzad, M. et al. The immune regulatory function of B7-H3 in malignancy: spotlight on the IFN-STAT1 axis and regulation of tumor-associated macrophages. Immunol Res 72, 526–537 (2024). https://doi.org/10.1007/s12026-024-09458-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-024-09458-9