Abstract
Background
B7 homolog 3 (B7-H3), a member of the immunoregulatory ligand B7 family, is pivotal in T-cell-mediated immune response. It is widely expressed in diverse human tumors and its high expression indicates the poor prognosis of the patients. Nonetheless, B7-H3’s role in colorectal cancer (CRC) needs to be further explored.
Methods
Western blot and immunohistochemistry were employed for detecting B7-H3 protein expression in CRC tissues and cell lines, respectively. Quantitative real-time polymerase chain reaction (qRT-PCR) was utilized for detecting B7-H3 mRNA and miR-128 expression levels. CRC cell lines SW620 and HT29 were used to construct B7-H3 overexpression or knockdown cell models, respectively. Cell counting kit-8 (CCK-8), 5-bromo-2′-deoxyuridine (BrdU), and scratch wound healing assays were employed for evaluating the effects of B7-H3 on CRC cell multiplication and migration. Besides, the regulatory relationship between miR-128 and B7-H3 was validated through dual-luciferase reporter gene assay, qRT-PCR, and western blotting.
Results
B7-H3 expression level was remarkably elevated in CRC tissues and cell lines, and its high expression level was associated with increased tumor size, positive lymph node metastasis, and increased T stage. In CRC cells, B7-H3 overexpression significantly facilitated the cell multiplication and migration, while B7-H3 knockdown worked oppositely. Moreover, B7-H3 was identified as a target of miR-128, and miR-128 negatively regulated B7-H3 expression in CRC cells.
Conclusion
B7-H3 expression is upregulated in CRC tissues and cell lines, and B7-H3 participates in promoting the proliferation and migration of CRC cells. Besides, B7-H3 expression is negatively regulated by miR-128 in CRC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As a common tumor in the digestive tract, colorectal cancer (CRC) poses a threat to human health [1]. In 2018, the global cancer statistics suggests that the morbidity of CRC ranks third, and its mortality ranks second among all cancers [2]. At present, surgery, radiotherapy, and chemotherapy are common treatment strategies for CRC, but its metastasis, recurrence, and drug resistance will lead to the failure of treatment [3, 4]. It is imperative to find novel therapy targets for CRC.
B7 homolog 3 (B7-H3), also called CD276, is known as a kind of immunoregulatory factor that regulates immune responses [5,6,7]. B7-H3 inhibits CD4+ T cells proliferation [8]. A recent study shows that B7-H3 may be involved in chronic inflammation induced dysplasia, and it promotes tumorigenesis [9]. B7-H3 is confirmed to be highly expressed in various human malignancies, including CRC [10]. In addition, it is reported that B7-H3 upregulates Smad1 expression through activating the PI3K-Akt pathway, so as to facilitate the epithelial–mesenchymal transition (EMT) of CRC cells [11]. Nevertheless, the biological functions of B7-H3 in CRC are not completely understood.
MicroRNAs (miRNAs) recognize and bind to the 3′-untranslated region (3′-UTR) of target mRNA to degrade the mRNA or inhibit its translation [12, 13]. Accumulating studies indicate that miRNAs are widely implicated in cancer biology. For instance, miR-21 modulates the prostaglandin signaling pathway via targeting 15-PGDH and promotes gastric cancer progression [14]. MiR-142-5p suppresses pancreatic cancer cell multiplication and apoptosis via modulating RAP1A expression [15]. In addition, miR-128 expression level is reported to be decreased in CRC tissues, and miR-128 suppresses CRC cell growth and migration via targeting RPN2 through the Akt-p53-cyclin pathway [16]. Nonetheless, the underlying mechanism of miR-128 in CRC progression warrants further investigation.
The present work aimed at exploring the role of B7-H3 in CRC progression and clarifying the interaction between B7-H3 and miR-128.
Materials and Methods
Tissue Samples
This research was approved by the Ethics Committee of the Affiliated Hospital of Xiangnan University, and strictly followed the Declaration of Helsinki. Fifty subjects pathologically diagnosed with CRC were enrolled, and none of the subjects had undergone radiotherapy, chemotherapy, or other neoadjuvant therapies prior to surgery. The cancerous tissues and adjacent tissues were collected during the surgery and then immediately frozen in liquid nitrogen at −196 °C.
Immuniohistochemical Staining
The resected tissue specimens were fixed with formaldehyde and embedded in paraffin. Tissue slices with a thickness of 4 μm were dewaxed with xylene. Subsequently, the sections were hydrated in gradient ethanol and heated in microwave (900 W, 2 s). Following antigen retrieval, the sections were incubated with 0.3% H2O2 for 30 min for the purpose of blocking endogenous peroxidase. After being washed three times with PBS, the slices were incubated with 10% goat serum for blocking the nonspecific antigens. Next, the slices were incubated overnight with the primary antibody (anti-B7-H3, Abcam, ab134161, 1:100) and then with secondary antibodies at room temperature for 30 min. Then, the slices were thoroughly cleaned using PBS solution. At last, DAB solution (Aithen Biotechnology Co., Ltd., Beijing, China) was utilized for color developing, and the slides were washed two to three times with distilled water. Two pathologists scored the staining, respectively, to evaluate the protein expression of B7-H3 in CRC tissues.
Cell Culture
Human CRC cell lines (HT29, SW620, SW480, and LoVo) and normal colonic epithelial cell lines (NCM460) were obtained at the American Type Culture Collection (Manassas, VA, USA). The cells were cultured in RPMI-1640 medium (Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (Gibco, Grand Island, NY, USA) and antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin) in 5% CO2 and 95% humidity at 37 °C.
Cell Transfection
B7-H3 overexpression plasmids, B7-H3 siRNA, and the corresponding negative controls were bought from Genechem Co., Ltd. (Shanghai, China). MiRNA control (miR-NC), miR-128 mimic, miR-128 inhibitor, and inhibitor control (miR-in) were purchased from RiboBio Co., Ltd. (Guangzhou, China). SW620 and HT29 cells were transferred into 60 mm culture plates (1 × 106 mL/well) and cultured at 37 °C in 5% CO2 for 24 h. Then, transfection was performed with LipofectamineTM 2000 (Invitrogen; Thermo Fisher Scientific, Inc., Grand Island, NY, USA) according to the manufacturer’s instruction. After 36 h, western blot was employed for detecting the transfection efficiency.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA extraction from cells and tissues was performed with RNAiso Plus (Takara, Dalian, China). Next, PrimeScript RT Master Mix reverse transcription kit (Takara, Shiga, Japan) was employed for synthesizing cDNA, and then SYBR Premix Ex TaqTM (Takara, Dalian, China) was adopted for conducting real-time PCR. The primer sequences were shown below (‘F’ for ‘forward’ and ‘R’ for ‘reverse’): B7-H3 F, 5′-CAAAGGATGCGATACACAGACCAC-3′ and R, 5′-CAGCAGGCAGGATGACTTAGAGAA-3′; β-actin F, 5′-ACTCGTCATACTCCTGCT-3′ and R, 5′-GAAACTACCTTCAACTCC-3′; miR-128 F, 5′-TTTTCGAGGCGAGAAAATCG-3′ and R, 5′-ATGGAGGCTAGGGCGAAATC-3′; and U6 F, 5′-CTCGCTTCGGCAGCACA-3′ and R, 5′-AACGCTTCACGAATTTGCGT-3′. U6 and β-actin were used as internal reference. The data analysis was conducted by 2−ΔΔCt method, in which ΔΔCT = (CTmiR-128/B7-H3 mRNA − CTU6/β-actin) tumor (or cells) − (CTmiR-128/B7-H3 mRNA − CTU6/β-actin) normal (or cells).
Western Blotting
RIPA lysis buffer (Beyotime Biotechnology, Shanghai, China) containing protease inhibitors and phosphatase inhibitors was employed to lyse cells. In line with the manufacturer’s instructions, the enhanced BCA protein detection kit (Beyotime, Shanghai, China) was utilized for determining the protein concentration. Then, 15 μg of total protein was dissolved with 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred to PVDF membranes (Beyotime, Shanghai, China). After that, 5% BSA (Fcmacs, Nanjing, China) was utilized to block the membranes for 1 h at room temperature, and they were then incubated overnight with primary antibodies at 4 °C, including anti-B7-H3 antibody (Abcam, ab134161, 1:1000) and anti-β-actin antibody (Abcam, ab8227, 1:1000). The next day, the membranes were incubated with the corresponding HRP-conjugated secondary antibodies (Beyotime, Shanghai, China) at room temperature for 1 h. Ultimately, with Chemi DocTM MP imaging system (Bio-Rad, Shanghai, China), ECL reagent (NCM Biotech, Suzhou, China) was utilized for visualizing the protein bands.
Cell Proliferation Assay
Overall, 2 × 103 cells in each group were inoculated into each well of the 96-well plates. After 24 h, each well was added with 10 μL of cell counting kit-8 (CCK-8, Beyotime, Shanghai, Chin) solution, with which the cells were incubated for 4 h at 37 °C, and a microplate reader was employed for measuring the absorbance value at 450 nm wavelength. Similarly, the absorbance of SW620 and HT29 cells was detected at 48, 72, and 96 h after inoculation, respectively. Ultimately, the proliferation curve was plotted, with the absorbance as the vertical axis, and time as the abscissa axis.
5-Bromo-2′-deoxyuridine (BrdU) Assay
HT29 and SW620 cells during logarithmic growth phase were inoculated into 96-well plates (6 × 104 cells/mL), and the cells were cultured overnight in 5% CO2 at 37 °C. After 24 h, each well was added with 20 μL of BrdU solution (Beyotime, Shanghai, China) and incubated for another 12 h. Subsequently, the cells were fixed with methanol. After being washed with PBS, the cells were incubated with prediluted detection antibodies for 1 h at room temperature. Next, the cell nuclei were stained with DAPI staining solution. After stop solution was added, fluorescence microscope was employed for observing the cells and the number of BrdU-positive cells was counted.
Wound Healing Assay
CRC cells were inoculated in six-well plates, and each well was added with 2 mL of complete medium. When the cells reached 80–90% confluence, a pipette tip was employed for scratching across the cells, with the long axis of the tip always perpendicular to culture plate. After cells were rinsed with PBS twice, the wells were added with serum-free medium. Then, an inverted microscope was utilized to take photos, and this time point was defined as 0 h. Next, cell culture was continued at 37 °C in 5% CO2. Twenty four hours later, the wound healing was observed at the same observation point. Wound healing rate (%) = (scratch wound width at 0 h − scratch wound width at 24 h)/scratch wound width at 0 h × 100%.
Luciferase Reporter Assay
Shanghai Genechem Co., Ltd. (Shanghai, China) designed and synthesized the luciferase reporter vectors carrying mutant type (MUT) B7-H3 sequence or wild-type B7-H3 sequence. SW620 and HT29 cells were transferred into 24-well plates (5000 cells/well). Twenty four hours later, miR-128 mimics or NC was transfected into the cells, respectively, together with the reporter vectors, and the cell culture was continued for 48 h. Subsequently, based on the manufacturer’s instruction, the luciferase activity of the cells in each group was examined by the dual-luciferase reporter gene detection kit (Promega, Madison, WI, USA).
Statistical Analysis
GraphPad Prism (Version 8.0; La Jolla, CA, USA) was used to draw the graphs. The statistical analysis tool was SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). Mean ± standard deviation (x ± s) was the expression form of measurement data. The comparison between the two groups was performed with t-test, while the comparison among multiple groups was performed with one-way analysis of variance. Furthermore, enumeration data were expressed in fourfold table, and the differences between the two groups were analyzed through χ2 test. P < 0.05 meant that the difference was of statistical significance.
Results
B7-H3 Expression Characteristics and the Clinical Significance in CRC
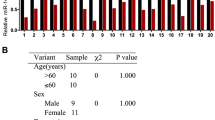
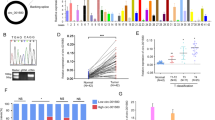
To study B7-H3 expression in CRC tissues, firstly, we used immunohistochemistry for detecting B7-H3 expression in cancerous and paracancerous tissues of 50 CRC patients, and the statistical results indicated that the percentage of B7-H3 positive samples in CRC tissues was significantly higher than that in adjacent tissues (Fig. 1A, B). Subsequently, B7-H3 mRNA expression in CRC tissues and cells was detected through qRT-PCR, and the results revealed that in CRC tissues and cells, B7-H3 mRNA was significantly highly expressed, compared with that in paracancerous tissues or NCM460 cell line (Fig. 1C, D). Next, western blot was employed for detecting B7-H3 protein expression in CRC cells, and it was revealed that B7-H3 protein expression in CRC cells was markedly enhanced in comparison to NCM460 cells (Fig. 1E). In addition, the GEPIA database (http://gepia.cancer-pku.cn/) was searched for analyzing the association between the CRC patient’s prognosis and B7-H3 expression level, and it was suggested that the overall survival time of CRC patients with highly expressed B7-H3 was markedly shorter as against those with low B7-H3 expression (Fig. 1F). In addition, to pinpoint the clinical significance of highly expressed B7-H3 in CRC progression, the aforementioned 50 CRC tissue samples were used for analyzing the correlation of B7-H3 expression with pathological indicators (Table 1). χ2 test indicated that highly expressed B7-H3 in cancer tissues was closely related to relatively larger tumor size (P = 0.0470), increased T stage (P = 0.0367), and positive lymph node metastasis (P = 0.0099) of CRC patients. The above data indicated that B7-H3 could probably promote CRC progression and may be a biomarker for CRC.
B7-H3 was highly expressed in CRC tissues and cells and was associated with poor prognosis. A, B Immunohistochemistry was utilized for detecting B7-H3 protein expression in cancerous tissue samples and corresponding adjacent tissues of 50 CRC patients, the results of which showed that the expression of B7-H3 protein was significantly upregulated in CRC samples. C qRT-PCR was utilized for detecting B7-H3 mRNA expression in CRC tissues and adjacent tissues, and it showed that B7-H3 expression was upregulated in CRC tissues. D qRT-PCR was utilized for detecting B7-H3 mRNA expression in normal colonic epithelial cell line NCM460 and four kinds of CRC cell lines, and it showed that B7-H3 expression was upregulated in all of the CRC cell lines. E Western blotting was employed for detecting B7-H3 protein expression in four kinds of CRC cells and normal colonic epithelial cell line NCM460, and it showed that B7-H3 expression was upregulated in all of the CRC cell lines. F GEPIA database was employed for analyzing the correlation between B7-H3 mRNA expression level and the CRC patient’s prognosis, and it indicated that the patients with higher B7-H3 expression had a shorter overall survival time. All experiments were performed in triplicate. **P < 0.01 and ***P < 0.001
B7-H3 Facilitated CRC Cell Proliferation and Migration
To study B7-H3’s functions in regulating the malignant phenotypes of CRC cells, SW620 cells with the lower B7-H3 expression in CRC cells were selected to construct an overexpression model, and HT29 cells with the higher B7-H3 expression were used to construct a knockdown model, and cell transfection effect was detected by western blot (Fig. 2A). Subsequently, CRC cell growth was examined by CCK-8 and BrdU assays, and the results displayed that B7-H3 overexpression observably facilitated SW620 cell proliferation in comparison to the control group, and B7-H3 knockdown inhibited the growth of HT29 cells (Fig. 2B, C). Furthermore, wound healing assay suggested that B7-H3 overexpression significantly promoted SW620 cell migration ability and B7-H3 knockdown exerted the opposite effect on HT29 cells (Fig. 2D). These data indicated that B7-H3 facilitated CRC cell multiplication and migration.
B7-H3 promoted CRC cell proliferation and migration. A Western blot was utilized for detecting B7-H3 protein expression in CRC cells after overexpression or knockdown of B7-H3. B, C CCK-8 and BrdU assays were employed for detecting the cell proliferation after overexpression or knockdown of B7-H3, and the results implied that B7-H3 overexpression promoted the proliferation of CRC cells, but the transfection of siRNAs targeting B7-H3 markedly inhibited the proliferation of CRC cells. D Wound healing assay was utilized for detecting the cell migration capability after overexpression or knockdown of B7-H3. The results showed that B7-H3 overexpression promoted the migration of CRC cells, while B7-H3 depletion suppressed the migration of CRC cells. All experiments were performed in triplicate. *P < 0.05, **P < 0.01, and ***P < 0.001
B7-H3 was a Downstream Target of MiR-128
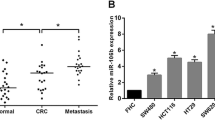
To probe into the mechanism of B7-H3 dysregulation in CRC progression, potential miRNAs complementary to B7-H3 3′-UTR were predicted through StarBase (http://starbase.sysu.edu.cn), and the results showed that there were multiple complementary base binding sites between miR-128 and B7-H3 3′-UTR (Fig. 3A). Subsequently, the correlation of B7-H3 mRNA expression with miR-128 expression in CRC tissues was analyzed, the findings of which indicated that their expression levels were inversely correlated (Fig. 3B). Next, dual-luciferase reporter assay was utilized for verifying the targeted relationship between B7-H3 and miR-128 (Fig. 3C). Furthermore, qRT-PCR illustrated that miR-128 expression in CRC tissues and cell lines was remarkably upregulated (Fig. 3D, E). We also discovered that the transfection of miR-128 mimics decreased B7-H3 mRNA and protein expression, while the inhibition of miR-128 expression exerted adverse effects in CRC cells (Fig. 3F, G). All these findings implied that B7-H3 was the target of miR-128, and in CRC, miR-128 negatively regulated B7-H3 expression.
B7-H3 was a target of miR-128. A Bioinformatics analysis was employed for predicting the binding sequence between miR-128 and B7-H3 3′-UTR. There are three potential binding sites on B7-H3 3′-UTR for miR-128. B qRT-PCR data were utilized for examining the correlation between miR-128 expression with B7-H3 mRNA expression in CRC tissues, and it showed that miR-128 expression was negatively correlated with B7-H3 expression. C Luciferase reporter gene assay was utilized for validating the binding sites between miR-128 and B7-H3 3′-UTR. The luciferase activity of wild-type reporter was suppressed by miR-128, but miR-128 could not repress the luciferase activity of the reporter after all of the three binding sites were mutated. D, E qRT-PCR was used for detecting miR-128 expression in CRC tissues and cell lines. The results showed that miR-128 expression was decreased in both CRC tissue samples and cell lines. F qRT-PCR was utilized for detecting the impacts of the transfection of miR-128 inhibitors or mimics on B7-H3 mRNA expression. It showed that miR-128 could negatively regulate B7-H3 expression. G Western blot was utilized for detecting the impacts of the transfection of miR-128 inhibitors or mimics on B7-H3 protein expression. It showed that miR-128 could negatively regulate B7-H3 expression. All experiments were performed in triplicate. **P < 0.01, ***P < 0.001, and ns: P > 0.05
MiR-128 Contributed to Inhibiting CRC Cell Proliferation and Migration via Targeting B7-H3
Next, SW620 cells were cotransfected with miR-128 mimics and B7-H3 overexpression plasmids, and miR-128 inhibitors and B7-H3 siRNA were cotransfected into HT29 cells (Fig. 4A). Subsequently, CRC cell multiplication and migration abilities were detected through CCK-8, BrdU, and wound healing assays, the results of which indicated that compared with the control group, miR-128 mimics markedly repressed SW620 cell multiplication and migration abilities, and the inhibition of miR-128 expression facilitated HT29 cell multiplication and migration (Fig. 4B–D). Meanwhile, B7-H3 overexpression weakened the inhibiting impacts of the transfection of miR-128 mimics on SW620 cell multiplication and migration, while the promoting effects of miR-128 inhibitors on HT29 cell proliferation and migration were partially counteracted by the knockdown of B7-H3 (Fig. 4B–D). The above evidence displayed that miR-128 repressed CRC cell multiplication and migration via regulating B7-H3.
MiR-128 suppressed the malignant biological behaviors of CRC cells via targeting B7-H3. A HT29 cells were transfected with miR-128 inhibitors and B7-H3 siRNA, and miR-128 mimics and B7-H3 overexpression plasmid were cotransfected into SW620 cells, and the transfection efficiency was detected by qRT-PCR. B, C CCK-8 and BrdU assays were employed to detect SW620 and HT29 cell proliferation. The results showed that B7-H3 counteracted the effects of miR-128 on the proliferation of CRC cells. D Wound healing assay was utilized for detecting the migration capacity of CRC cells. The results showed that B7-H3 counteracted the effects of miR-128 on the migration of CRC cells. All experiments were performed in triplicate. *P < 0.05, **P < 0.01, ***P < 0.001, and ns: P > 0.05
Discussion
Belonging to the costimulatory B7 superfamily, B7-H3 is composed of an extracellular immunoglobulin-like region IgV-IgC, an N-terminal signal peptide, a cytoplasmic tail containing 45 amino acid residues, and a transmembrane region, and it regulates immune responses [5, 6, 17]. B7-H3 has both negative and positive regulatory effects in T-cell-mediated immune responses [18, 19]. Previous studies authenticates that as a coregulatory molecule of immune cells, B7-H3 is significantly highly expressed in various human tumors, and contributes to promoting tumor cell proliferation, metastasis, antiapoptosis, chemotherapy resistance, etc. For example, B7-H3 modulates drug resistance of breast cancer (BC) stem cells through regulating MVP-mediated MEK activation [20]. In pancreatic cancer, the expression of B7-H3 is regulated by BRD4, and B7-H3 increases TLR4 expression and facilitates cancer cell proliferation and metastasis [21]. In addition, it is confirmed that B7-H3 is highly expressed in CRC, and may be a diagnostic marker and therapeutic target for CRC [10]. Other studies also prove that B7-H3 promotes CRC cell EMT and upregulates thymidine expression via the PI3K/AKT pathway [11, 22]. In addition, B7-H3 overexpression increases the antiapoptosis effect of CRC cells via enhancing the JAK2-STAT3 signaling pathway [23]. Consistently, this study confirmed that in CRC tissues and cells, B7-H3 was significantly highly expressed. Functional experiments confirmed that B7-H3 overexpression markedly facilitated CRC cell multiplication and migration in vitro while its knockdown worked oppositely. Moreover, the high expression of B7-H3 was strongly related to the CRC patient’s unfavorable pathological characteristics and poor prognosis. The above results fully confirmed the cancer-promoting role of B7-H3 in CRC.
As a category of small endogenous noncoding RNAs, miRNAs modulate the expression of various genes and can exert effects as tumor suppressors or oncomiRs [14, 15]. Multiple studies show that miR-128 is dysregulated in several human tumors and exerts an inhibitory effect on tumor progression. For instance, miR-128 expression is decreased in pancreatic cancer and miR-128 induces pancreatic cancer cell apoptosis via targeting MDM4 [24]. MiR-128-3p inhibits BC progression by modulating the LIMK1/CFL1 signaling pathway [25]. Furthermore, miR-128 expression is decreased in CRC, which is strongly associated with the proliferation, metastasis, apoptosis, and chemoresistance of CRC cells. For example, the SIRT1/ROS/DR5 pathway is targeted by miR-128, and miR-128 promotes TRAIL-induced CRC cell apoptosis [26]. By targeting IRS1, miR-128 represses CRC cell growth and metastasis [27]. Besides, miR-128-3p transferred by exosome can increase the chemosensitivity of L-OHP-resistant CRC cells [28]. The present study also displayed that miR-128 expression was markedly decreased in CRC tissues and cells. In addition, the transfection of miR-128 mimics observably repressed CRC cell multiplication and migration while downregulating miR-128 expression significantly exerted the opposite effects. The above findings indicated that miR-128 suppressed CRC progression, which are consistent with the previous reports.
Previous studies unmask that B7-H3 expression is negatively regulated by multiple miRNAs in various human tumors. For instance, miR-124 represses osteosarcoma cell proliferation and invasion via targeting B7-H3 [29]. MiR-506 suppresses mantle cell lymphoma cell proliferation and metastasis through inhibiting B7-H3 expression [30]. It is noteworthy that in this study, bioinformatics analysis revealed the presence of three binding sites between B7-H3 3′-UTR and miR-128. It was hypothesized that miR-128 might function as an upstream molecule of B7-H3 in CRC. The dual-luciferase reporter gene assay validated that miR-128 could bind directly to B7-H3 3′-UTR, and their expression levels were negatively correlated in CRC tissues. Furthermore, it was revealed that B7-H3 overexpression counteracted the inhibiting effects of the transfection of miR-128 mimics on CRC cell multiplication and migration, while B7-H3 knockdown counteracted the promoting impacts of the inhibition of miR-128 on CRC cell multiplication and migration. These findings manifested that miR-128 modulated CRC cell proliferation and migration via targeting B7-H3.
To sum up, B7-H3 promotes CRC progression, and its expression is negatively modulated by miR-128. This work reveals a new mechanism in the progression of CRC and lays a new theoretical foundation for clinical CRC diagnosis and treatment.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
DeSantis, C. E., Lin, C. C., Mariotto, A. B., Siegel, R. L., Stein, K. D., & Kramer, J. L., et al. (2014). Cancer treatment and survivorship statistics, 2014. CA: A Cancer Journal for Clinicians, 64(4 Jul–Aug), 252–271.
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., & Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 68(6 Nov), 394–424.
Kuipers, E. J., Grady, W. M., Lieberman, D., Seufferlein, T., Sung, J. J., & Boelens, P. G., et al. (2015). Colorectal cancer. Nature Review Disease Primers, 1(Nov), 15065.
Martini, G., Troiani, T., Cardone, C., Vitiello, P., Sforza, V., & Ciardiello, D., et al. (2017). Present and future of metastatic colorectal cancer treatment: a review of new candidate targets. World Journal of Gastroenterology, 23(26 Jul), 4675–4688.
Collins, M., Ling, V., & Carreno, B. M. (2005). The B7 family of immune-regulatory ligands. Genome Biology, 6(6), 223.
Hansen, J. D., Du Pasquier, L., Lefranc, M. P., Lopez, V., Benmansour, A., & Boudinot, P. (2009). The B7 family of immunoregulatory receptors: a comparative and evolutionary perspective. Molecular Immunology, 46(3 Jan), 457–472.
Sun, X., Vale, M., Leung, E., Kanwar, J. R., Gupta, R., & Krissansen, G. W. (2003). Mouse B7-H3 induces antitumor immunity. Gene Therapy, 10(20 Sep), 1728–1734.
Ling, V., Wu, P. W., Spaulding, V., Kieleczawa, J., Luxenberg, D., & Carreno, B. M., et al. (2003). Duplication of primate and rodent B7-H3 immunoglobulin V- and C-like domains: divergent history of functional redundancy and exon loss. Genomics, 82(3 Sep), 365–377.
Hasselbalch, H. C. (2012). Perspectives on chronic inflammation in essential thrombocythemia, polycythemia vera, and myelofibrosis: is chronic inflammation a trigger and driver of clonal evolution and development of accelerated atherosclerosis and second cancer? Blood, 119(14 Apr), 3219–3225.
Ingebrigtsen, V. A., & Boye, K. (2014). B7-H3 expression in colorectal cancer: associations with clinicopathological parameters and patient outcome. BMC Cancer, 14(Aug), 602.
Jiang, B., Zhang, T., Liu, F., Sun, Z., Shi, H., & Hua, D., et al. (2016). The co-stimulatory molecule B7-H3 promotes the epithelial-mesenchymal transition in colorectal cancer. Oncotarget, 7(22 May), 31755–31771.
Xue, J., Niu, J., Wu, J., & Wu, Z. H. (2014). MicroRNAs in cancer therapeutic response: friend and foe. World Journal of Clinical Oncology, 5(4 Oct), 730–743.
Friedman, R. C., Farh, K. K., Burge, C. B., & Bartel, D. P. (2009). Most mammalian mRNAs are conserved targets of microRNAs. Genome Research, 19(1 Jan), 92–105.
Li, L., Wang, X., Li, W., Yang, L., Liu, R. & Zeng, R. et al.(2018). miR-21 modulates prostaglandin signaling and promotes gastric tumorigenesis by targeting 15-PGDH. Biochemical and Biophysical Research Communications, 495(1 Jan), 928–934.
Yao, R., Xu, L., Wei, B., Qian, Z., Wang, J., & Hui, H., et al. (2019). miR-142-5p regulates pancreatic cancer cell proliferation and apoptosis by regulation of RAP1A. Pathology, Research and Practice, 215(6 Jun), 152416.
Zhou, T., Wu, L., Wang, Q., Jiang, Z., Li, Y., & Ma, N., et al. (2018). MicroRNA-128 targeting RPN2 inhibits cell proliferation and migration through the Akt-p53-cyclin pathway in colorectal cancer cells. Oncology Letters, 16(6 Dec), 6940–6949.
Zhou, Y. H., Chen, Y. J., Ma, Z. Y., Xu, L., Wang, Q., & Zhang, G. B., et al. (2007). 4IgB7-H3 is the major isoform expressed on immunocytes as well as malignant cells. Tissue Antigens, 70(2 Aug), 96–104.
Prasad, D. V., Nguyen, T., Li, Z., Yang, Y., Duong, J., & Wang, Y., et al. (2004). Murine B7-H3 is a negative regulator of T cells. The Journal of Immunology, 173(4 Aug), 2500–2506.
Suh, W. K., Gajewska, B. U., Okada, H., Gronski, M. A., Bertram, E. M., & Dawicki, W., et al. (2003). The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nature Immunology, 4(9 Sep), 899–906.
Liu, Z., Zhang, W., Phillips, J. B., Arora, R., McClellan, S., & Li, J., et al. (2019). Immunoregulatory protein B7-H3 regulates cancer stem cell enrichment and drug resistance through MVP-mediated MEK activation. Oncogene, 38(1 Jan), 88–102.
Zhao, J., Meng, Z., Xie, C., Yang, C., Liu, Z., & Wu, S., et al. (2019). B7-H3 is regulated by BRD4 and promotes TLR4 expression in pancreatic ductal adenocarcinoma. The International Journal of Biochemistry & Cell Biology, 108(Mar), 84–91.
Jiang, B., Liu, F., Liu, Z., Zhang, T., & Hua, D. (2016). B7-H3 increases thymidylate synthase expression via the PI3k-Akt pathway. Tumour Biology, 37(7 Jul), 9465–9472.
Zhang, T., Jiang, B., Zou, S. T., Liu, F., & Hua, D. (2015). Overexpression of B7-H3 augments anti-apoptosis of colorectal cancer cells by Jak2-STAT3. World Journal of Gastroenterology, 21(6 Feb), 1804–1813.
Han, H., Wang, L., Xu, J., & Wang, A. (2018). miR-128 induces pancreas cancer cell apoptosis by targeting MDM4. Experimental and Therapeutic Medicine, 15(6 Jun), 5017–5022.
Zhao, J., Li, D., & Fang, L. (2019). MiR-128-3p suppresses breast cancer cellular progression via targeting LIMK1. Biomedicine & Pharmacotherapy, 115(Jul), 108947.
Lian, B., Yang, D., Liu, Y., Shi, G., Li, J., & Yan, X., et al. (2018). miR-128 targets the SIRT1/ROS/DR5 pathway to sensitize colorectal cancer to TRAIL-induced apoptosis. Cellular Physiology and Biochemistry, 49(6), 2151–2162.
Wu, L., Shi, B., Huang, K., & Fan, G. (2015). MicroRNA-128 suppresses cell growth and metastasis in colorectal carcinoma by targeting IRS1. Oncology Reports, 34(5 Nov), 2797–2805.
Liu, T., Zhang, X., Du, L., Wang, Y., Liu, X., & Tian, H., et al. (2019). Exosome-transmitted miR-128-3p increase chemosensitivity of oxaliplatin-resistant colorectal cancer. Molecular Cancer, 18(1 Mar), 43.
Wang, L., Kang, F. B., Sun, N., Wang, J., Chen, W., & Li, D., et al. (2016). The tumor suppressor miR-124 inhibits cell proliferation and invasion by targeting B7-H3 in osteosarcoma. Tumour Biology, 37(11 Nov), 14939–14947.
Zhu, X. W., Wang, J., Zhu, M. X., Wang, Y. F., Yang, S. Y., & Ke, X. Y. (2019). MicroRNA-506 inhibits the proliferation and invasion of mantle cell lymphoma cells by targeting B7H3. Biochemical and Biophysical Research Communications, 508(4 Jan), 1067–1073.
Acknowledgements
The authors thank Hubei Yican Health Industry Co., Ltd. for its linguistic assistance during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Our study was approved by the Ethics Review Board of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hu, X., Xu, M., Hu, Y. et al. B7-H3, Negatively Regulated by miR-128, Promotes Colorectal Cancer Cell Proliferation and Migration. Cell Biochem Biophys 79, 397–405 (2021). https://doi.org/10.1007/s12013-021-00975-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-021-00975-0