Abstract
MicroRNAs (miRNAs) are small endogenous noncoding RNAs that regulate genome expression posttranscriptionally and are involved in autoimmune diseases. Previous studies have indicated that follicular helper T (Tfh) cells play a critical role in the pathogenesis of Graves’ disease (GD). However, the molecular mechanisms that contribute to circulating Tfh memory cell response in GD patients remain incompletely understood. This study aimed to investigate the role of miRNAs on circulating Tfh memory cells in GD patients. Herein, our data showed that the proportion of circulating Tfh memory cells, the transcript levels of IL-21, and the plasma concentrations of IL-21 were increased in the peripheral blood from GD patients. We also found that inducible co-stimulator (ICOS) expression, an important molecule expressed on Tfh cells, were significantly augmented in the peripheral blood mononuclear cells (PBMCs) from GD patients and positively correlated with the percentage of circulating Tfh memory cells and the transcript levels of IL-21 in GD. Intriguingly, miRNA sequencing screened miR-29a-3p expression was downregulated and inversely correlated with ICOS expression and the frequency of circulating Tfh memory cells in patients with GD. Luciferase assay demonstrated that ICOS was the direct target gene of miR-29a-3p, and miR-29a-3p could inhibit ICOS at both transcriptional and translational levels. Overexpression of miR-29a-3p reduced the proportion of circulating Tfh memory cells. Moreover, miR-29a-3p expression negatively correlated with serum concentrations of TSH receptor antibody (TRAb) in GD patients. Collectively, our results demonstrate that miR-29a-3p emerges as a post-transcriptional brake to limit circulating Tfh memory cell response in GD patients and may be involved in the pathogenesis of GD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Graves’ disease (GD) is an autoimmune disorder which affects the thyroid gland [1]. It is now considered the most common cause of hyperthyroidism and thyrotoxicosis, with an annual incidence of 0.2–0.5/1000 subjects [2, 3]. GD is clinically characterized by goiter, hyperthyroidism, palpitation (tachycardia), and ophthalmopathy [4, 5]. The serological signs of GD patients include elevated levels of free triiodothyronine (FT3) and/or free thyroxine (FT4) as well as low concentration of thyroid-stimulating hormone (TSH); additional manifestation includes positive TSH receptor antibody (TRAb) [6, 7]. Some GD cases also have anti-thyroglobulin antibody (TgAb) and anti-thyroperoxidase antibody (TPOAb) [8]. There is to date consensus as to the pathogenesis of GD that results from complex interactions between genetic and environmental factors [3, 9], but insights into the underlying immunopathogenesis of this thyroid-associated autoimmune disease are not broadly known.
Follicular helper T (Tfh) cells, as a specialized subset of CD4+ T cells, were coined in a series of studies on human tonsillar germinal center (GC) [10]. The prominent role of Tfh cells is providing auxiliary to B cells’ survival, proliferation, migration, and antibody production in GC [11]. Tfh cells express elevated levels of C-X-chemokine receptor type 5 (CXCR5), inducible co-stimulator (ICOS), programmed death-1 (PD-1), and transcription factor B cell lymphoma 6 (Bcl-6) [10, 12,13,14,15,16]. Interleukin 21 (IL-21) is the most effective cytokine secreted by Tfh cells, which play a vital role in the production of pathogenic autoantibodies [17]. Human blood CD4+CXCR5+ cells, with “Tfh” characteristics in CD4+ T cells, are commonly termed circulating Tfh memory cells [18]. These cells have come into the spotlight with the publication of a series of studies linking to them and autoimmune diseases in the past few years [19,20,21]. However, our understanding of Tfh memory cells in the GD patients remains elusive. ICOS is a member of CD28 co-stimulatory receptor family that is induced on Tfh cells upon stimulation [12, 19]. ICOSL is the sole ligand of ICOS that is expressed on the surface of B cells and antigen-presenting cells [22]. Tfh cells express ICOS, a molecule combined with ICOSL that is essential for Tfh generation and GC formation [23]. ICOS/ICOSL signal is particularly important for the maintenance of Tfh cells’ survival [24]. Moreover, ICOS-ICOSL signal plays a direct role in the proliferation and differentiation of thyroid follicular cells and participates in autoimmune response in local thyroid tissue [25]. The studies suggest that ICOS plays an important role in the pathogenesis of GD.
MicroRNAs (miRNAs) are small regulatory RNAs that include ~22 nucleotides in length [26]. The importance of microRNAs in modulating gene expression at the post-transcriptional level inhibiting translation and/or causing degradation of target mRNA transcript has gained great interest among researchers [27, 28]. In humans and other mammals, most of the 90 conserved miRNA families are required for viability or proper development [29]. Evidence has demonstrated that miRNAs are involved in the development and progression of GD, or serve as potential biomarkers of GD [30,31,32]. However, a study focused on miRNAs-ICOS in GD has not been reported.
Herein, we identified whether miRNA regulated circulating Tfh memory cells by targeting ICOS in GD. Using this approach, the present study offers new insight into the role of ICOS-specific miRNA in the pathogenesis of GD.
Methods
Subjects and samples
GD specimens were collected in the department of clinical laboratory of the Affiliated People’s Hospital of Jiangsu University. All of them were diagnosed by clinical signs, symptoms, and laboratory examination including the serum concentration of FT3, FT4, TSH, TgAb, TPOAb, and TRAb. The cases who had recent infection or combined tumors and other inflammatory diseases were excluded. The control specimens were collected in the physical examination center of the Affiliated People’s Hospital of Jiangsu University. The criterions of healthy control group were as follows: (1) normal thyroid function and thyroid B-ultrasound; (2) no occurrence of recent infection; (3) no autoimmune diseases, tumors, and infectious diseases; (4) no recently taken immunosuppressive drugs; (5) the numbers of peripheral leukocytes within the normal range (3.50–9.50 × 109/l); (6) the age and sex matched with GD group. Based on the rigorous screening process, 30 GD patients (24 females and 6 males) and 30 age- and sex-matched healthy controls (22 females and 8 males) were enrolled into the study. The main clinical features are revealed in Table 1. Among them, 11 patients were newly diagnosed GD and the rest were a follow-up visit. Patients on methimazole therapy received 20–30 mg/day for the first phase, and the dose was reduced to 5–15 mg when patients achieved a remission. Patients treated with propylthiouracil took 300–500 mg/day for the first phase and 25–100 mg for maintaining remission. EDTA-K2 anticoagulant (Bio-Rad, California, USA) tube was used to collect the subjects’ peripheral blood.
Laboratory measurements
The serum of participants was used to measure indicators of thyroid function and thyroid autoantibodies. The serum levels of FT3, FT4, TSH, TgAb, and TPOAb were measured by an LDX-800 system (Beckman Coulter, CA, USA) according to the manufacturer’s instructions. The serum levels of TRAb were measured by Cobas 6000 (Roche, Basel, Switzerland) according to the manufacturer’s instructions.
Cell isolation and purification
Human peripheral blood mononuclear cells (PBMCs) were separated by density-gradient centrifugation over Ficoll-Hypaque solution (Tianjin Haoyang Biological Technology Co., Tianjin, China) according to the manufacturer’s instructions and stored at −80 °C for nucleic acids research. Human PBMCs were cultured in RPMI-1640 medium (Gibco, California, USA) supplemented with 10% fetal bovine serum (Gibco, California, USA) at 37 °C under 5% CO2. HEK293T cells were cultured with DMEM (Gibco, California, USA) containing 10% fetal bovine serum at 37 °C under 5% CO2 for luciferase report assay.
MiRNA sequencing
Five adult GD patients and five adult healthy subjects were selected at random for miRNA sequencing analysis. MiRNA sequencing was performed by Cloud-Seq Biotech Ltd. Co. (Shanghai, China). These RNA samples were subjected to sequencing on the Illumina HiSeq4000 platform following the standard procedures. High-quality reads were obtained from raw sequencing reads. Trimmed reads were compared to the consolidated pre-miRNA database using NovoAlign software. The statistically different levels of miRNA expression were identified by fold changes and p values between GD group and control group (fold change >1.5 and p < 0.05).
MiRNA transfection
MiR-29a-3p mimics and negative control (miR-NC) were designed and synthetized by Ribobio Co. (Ribobio, Guangzhou, China). The separated PBMCs were transfected with the miR-29a-3p mimics and miR-NC using the Entranster-R (Engreen Biosystem, Co. Ltd, Beijing, China) according to the manufacturer’s protocol and then cultured in the presence of 0.5 μg/ml functional anti-human CD3 Ab plus 2 μg/ml functional anti-human CD28 Ab (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) for 24 h to detect transcript levels and for 48 h to detect cell proportion or protein levels.
RNA extraction and qRT-PCR
We used RNA-Quick Purification Kit (YiShan Biotech, Shanghai, China) to extract the total RNA and then measured the RNA concentrations with Nano-Drop ND-1000 instrument (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. cDNA reverse transcription and qPCR with reverse transcription were performed according to the manufacturer’s instructions. The primer sequences were as follows: ICOS, sense, 5′-CAGGAGAAATCAATGGTTCTGCC-3′, antisense, 5′-CCTTTTGTCTTAGTGAGATCGCA-3′; IL-21, sense, 5′-CAAGGTCAAGATCGCCACA-3′, antisense, 5′-AGGGACCAAGTCATTCACATAA-3′; β-actin, sense, 5′-CACGAAACTACCTTC AACTCC-3′, antisense, 5′-CATACTCCTGCTTGCTGATC-3′. The primer sequences of miR-29a-3p, miR-29b-3p, miR-98-5p, and U6 primers are designed and synthetized by Ribobio Co. (Ribobio). The transcript levels of mRNAs and miRNAs were normalized to β-actin and U6, respectively, which were measured by 2−∆Ct.
Flow cytometric analysis
The PBMCs were stained using anti-human CD3 PE-Cy5 (Biolegend, San Diego, USA), anti-human CD4 FITC (Biolegend), and anti-human CXCR5 PE (Biolegend) antibodies. Transfected PBMCs were immunostained with anti-human ICOS BV-421 (Biolegend) against the cell surface. All the flow cytometry experiments were performed using FACS Canto (Becton Dickinson, Sparks, USA). The results were analyzed using FlowJo (X 10.0.7 R2) (Stanford University, San Francisco, USA).
Enzyme-linked immunosorbent assay
The plasma was separated from EDTA anticoagulant blood, and the cytokine IL-21 was detected by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (IBL international GmbH, Hamburg, Germany).
Luciferase report assay
The wild-type (WT) sequence of ICOS 3′UTR containing binding site for miR-29a-3p was generated from Sangon Biotech Co. (Sangon, Shanghai, China). Mutant sequence unable to bind miR-29a-3p was also obtained from Sangon Biotech Co. The luciferase report assay experiment was performed as previously described [33].
Statistical analysis
Statistical analyses were determined using Prism 8 (GraphPad Software, Inc., San Diego, USA). The comparisons of two groups were calculated using unpaired two-sided Student’s t-test when variables passed the normal distribution test. Non-normally distributed variables were compared using Mann–Whitney U test. One-way ANOVA was used for comparisons of multiple groups. Tukey’s test was applied for pair-to-pair comparisons of multiple groups after ANOVA. Correlations between the two groups were conducted by using Pearson’s correlation coefficient. A p value <0.05 was considered to indicate a statistically significant difference (*p < 0.05, **p < 0.01, ***p < 0.001).
Results
Increased circulating Tfh memory cells in the peripheral blood of the GD patients
To quantify circulating Tfh memory cells in the peripheral blood, we analyzed the percentage of CD3+CD4+CXCR5+ cells in the PBMCs to distinguish the Tfh memory cells (Fig. 1a). The proportion of CD4+CXCR5+ T cells was significantly increased in GD patients compared with the healthy controls (Fig. 1b). Meanwhile, the expression of IL-21 mRNA (Fig. 1c) and the serum concentrations of IL-21 (Fig. 1d) were increased in GD patients. We subsequently examined the relationship between the percentage of CD4+CXCR5+ T cells and the clinical parameters of GD in GD patients. The proportion of CD4+CXCR5+ T cells positively correlated with the serum levels of TRAb (r = 0.3631; p = 0.0486) (Fig. 1h), but not with the serum concentrations of FT3 (r = 0.2608; p = 0.1639) (Fig. 1e), FT4 (r = 0.2358; p = 0.2098) (Fig. 1f), TSH (r = −0.1245; p = 0.5121) (Fig. 1g), TgAb (r = 0.3011; p = 0.1060) (Fig. 1i), and TPOAb (r = 0.0871; p = 0.6473) (Fig. 1j). These data demonstrated that circulating Tfh memory cells are increased in GD patients.
Increased circulating Tfh memory cells in the peripheral blood of GD patients. Peripheral blood was obtained from 30 GD patients and 30 healthy volunteers. a The values on the upper right correspond to the proportion of circulating Tfh memory cells in the rectangular region and the CD3+CD4+CXCR5+ cells were gated as circulating Tfh memory cells. b Flow cytometry analysis showing the percentage of circulating Tfh memory cells between 30 GD patients and 30 controls. c qRT-PCR analysis of IL-21 mRNA expression in the PBMCs from 30 GD patients and 30 controls. d The plasma levels of IL-21 in 30 GD patients and 30 controls were determined by ELISA. Scatter plots show the correlations between the proportion of circulating Tfh memory cells and the serum concentrations of FT3 (e), FT4 (f), TSH (g), TRAb (h), TgAb (i), and TPOAb (j) in 30 GD patients. Each data point represents an individual subject, and the horizontal lines show the mean. *p < 0.05, ***p < 0.001
The prediction of ICOS-associated miRNAs in GD patients
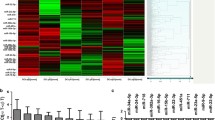
To address the factors contributing to increased circulating Tfh memory cells in GD patients, we focused on ICOS molecule and found that the transcript levels of ICOS were significantly augmented in the PBMCs from GD patients (Fig. 2a). Notably, ICOS expression positively correlated with the percentage of circulating Tfh memory cells (r = 0.3990; p = 0.0290) (Fig. 2b) and the transcript levels of IL-21 (r = 0.7206; p < 0.0001) (Fig. 2c) in GD patients. To further investigate the causes of the elevated ICOS expression, we analyzed miRNAs’ expression profile (GSE183576). Hierarchical clustering analysis (Fig. 2d) and scatter plots (Fig. 2e) results revealed differentially expressed miRNAs between the GD and control samples. A total of 1310 dysregulated miRNAs (490 upregulated and 820 downregulated) were identified between GD and controls. Among them, 31 miRNAs, including 3 upregulated and 28 downregulated, showed fold change ˃1.5, p <0.05 (Fig. 2f). We subsequently predicted miRNAs that targeted ICOS using the prediction programs, and these predicted miRNAs have been reported in other autoimmune diseases. Venn diagram analysis revealed that three shared miRNAs meet the requirement for targeted relationship (miR-29a-3p, miR-29b-3p, and miR-98-5p) (Fig. 2g). Based on the following preliminary experiments, only miR-29a-3p was clearly downregulated in the PBMCs from GD patients compared with miR-29b-3p and miR-98-5p expressions (Fig. S1a–c). Because of the negative regulatory relationship between miRNAs and their target genes, miR-29a-3p was selected for further studies.
The prediction of ICOS-associated miRNAs in GD patients. a qRT-PCR analysis of ICOS mRNA expression in the PBMCs from 30 GD patients and 30 controls. b The correlation between ICOS mRNA expression and the percentage of CD4+CXCR5+ T cells in 30 GD patients. c The correlation between the transcript levels of ICOS and IL-21 mRNA expression in 30 GD patients. Hierarchical clustering heatmap (d) and scatter plots (e) showing the dysregulated expression of miRNAs between 5 GD patients and 5 controls. f Statistical analysis of dysregulated miRNAs. Green represents differentially expressed miRNAs (490 upregulation vs. 820 downregulation) and red represents significantly dysregulated miRNAs (fold change >1.5, p <0.05) (3 upregulation vs. 28 downregulation). g The Venn diagram revealed the intersection of miRNAs targeting ICOS by prediction software and downregulated miRNAs from sequencing data. Each data point represents an individual subject, and the horizontal lines show the mean. ***p < 0.001
ICOS is a functional target of miR-29a-3p
We detected miR-29a-3p levels by expanding the sample size of GD and controls, and found that miR-29a-3p expression was significantly attenuated in the PBMCs from GD patients (Fig. 3a). Moreover, an inverse correlation between the levels of ICOS and the levels of miR-29a-3p was indicated in GD patients (r = −0.4456; p = 0.0136) (Fig. 3b). ICOS 3′UTR has a matching site with miR-29a-3p (Fig. 3c). To determine whether ICOS was a direct target of miR-29a-3p, miR-29a-3p mimics were co-transfected with WT luciferase reporter and mutant luciferase reporter into HEK293T cells, respectively. Our data indicated that miR-29a-3p reduced the luciferase activity, but did not influence the luciferase activity of mutant reporter that is unable to bind miR-29a-3p (Fig. 3d). To further investigate the interaction between miR-29a-3p and ICOS, we transfected miR-29a-3p mimics and miR-NC in the PBMCs. After examination by qRT-PCR and flow cytometry analysis, our results indicated that transfection with miR-29a-3p (Fig. 3e) reduced ICOS mRNA (Fig. 3f) and protein levels (Fig. 3g, h) in the PBMCs. Together, these data showed that miR-29a-3p can directly suppress ICOS expression.
ICOS is a functional target of miR-29a-3p. a qRT-PCR analysis for the transcript levels of miR-29a-3p in the PBMCs from 30 GD patients and 30 controls. b The correlation between miR-29a-3p expression and the transcript levels of ICOS in the PBMCs from 30 GD patients. c The binding sites of miR-29a-3p on the sequence of ICOS 3′UTR contained complementarities to miR-29a-3p seed regions (wild-type (WT)), and mutant sequence was unable to bind miR-29a-3p. d Luciferase activity of ICOS-WT and ICOS-mutant cotransfected with miR-29a-3p mimics and miR-NC in HEK293T cells. e qRT-PCR analysis showing miR-29a-3p expression in the PBMCs after transfection with miR-29a-3p mimics and miR-NC. f The relative expression of ICOS mRNA in the PBMCs transfected with miR-29a-3p mimics and miR-NC. g, h Flow cytometry analysis showing the MFI of ICOS in the PBMCs transfected with the miR-29a-3p mimics and miR-NC. Data represent mean ± SD from independent experiments. Each data point represents an individual subject. The results are shown as the mean ± SD of three independent experiments, and the horizontal lines show the mean. **p < 0.01, ***p < 0.001. MFI, mean fluorescence intensity
Regulatory effect of miR-29a-3p on Tfh memory cells
To further investigate the relationship between miR-29a-3p and Tfh memory cells, we analyzed the relationship between miR-29a-3p and CD4+CXCR5+ T cells. A dramatically inverse correlation between miR-29a-3p expression and the proportion of CD4+CXCR5+ T cells was observed in GD patients (r = −0.4798; p = 0.0073) (Fig. 4a). We further found that overexpression of miR-29a-3p significantly reduced the proportion of CD4+CXCR5+ T cells in vitro (Fig. 4b, c). These results indicated that miR-29a-3p contributes to circulating Tfh memory cells in GD.
Regulatory effect of miR-29a-3p on Tfh memory cells. a The correlation between miR-29a-3p expression and the percentage of circulating Tfh memory cells in 30 GD patients. b, c Flow cytometry analysis showing the proportion of circulating Tfh memory cells in the PBMCs transfected with the miR-29a-3p mimics and miR-NC. Each data point represents an individual subject. The results are shown as the mean ± SD of three independent experiments, and the horizontal lines show the mean. **p < 0.01
MiR-29a-3p expression was associated with the serum levels of TRAb in GD patients
We analyzed the relationship between miR-29a-3p and thyroid autoantibodies, and found that miR-29a-3p expression inversely correlated with the serum levels of TRAb (r = −0.3996; p = 0.0287) (Fig. 5a), but not with the serum concentrations of TgAb (r = −0.0401; p = 0.8334) (Fig. 5b) and TPOAb (r = −0.1179; p = 0.5348) (Fig. 5c). These data showed that miR-29a-3p is closely related to GD disease.
Discussion
In contrast to the extensive studies of Tfh cells in secondary lymphoid organs, the current understanding of human circulating CD3+CD4+CXCR5+ cells in peripheral blood has changed dramatically in the past years [34]. These cells have been defined as circulating memory Tfh cells, which belonged to Tfh lineage and shared functional properties with Tfh cells [18, 35, 36]. There is total consensus on the cell surface markers to define human circulating Tfh memory cells and their subsets, which have been broadly applied in various human autoimmune diseases [37]. GD is directly caused by thyroid-stimulating antibodies, which bind to thyroid follicular cells and cause TSH receptors to be activated, ultimately leading to hyperthyroidism and goiter [38]. Tfh cells, as a subset of helper B cells’ maturation and differentiation, are increased in peripheral blood and thyroid tissues of GD [21, 39], and upregulated Tfh-related factor IL-21 is correlated with the serum levels of thyroid hormones and thyroid autoantibodies in GD [40]. Our results showed that the proportion of circulating Tfh memory cells was increased and positively correlated with the serum levels of TRAb in the peripheral blood from GD patients. Moreover, the transcript levels of IL-21 in the PBMCs and the plasma concentrations of IL-21 were significantly increased in GD patients. Zhu et al. found that there was no change in the frequencies of CD4+CXCR5+ T cells between GD patients and healthy controls [41]. However, another report indicated that the frequencies of circulating CD4+CXCR5+CD45RA− Tfh cells were significantly increased in GD patients [21]. One possible explanation for the difference is the small sample size included in the study (10 GD patients in Zhu et al. study). Another possible explanation is that the combination of markers among laboratories tends to be different. The underlying reasons remain to be further studied. Collectively, these observations indicate that the increased proportion of circulating Tfh memory cells was associated with GD.
To investigate the underlying mechanisms of increased circulating Tfh memory cells in GD patients, miRNAs have come into the spotlight with the special regulation pattern. MiR-346 has been found to regulate Bcl-6 in GD patients [8]. miR-4443 induced CD4+ T-cell dysfunction by targeting TNFR-associated factor (TRAF) 4 in GD [42]. Although dysregulation of miRNAs has been reported in GD [8, 43], no prior study has investigated their functions to ICOS in GD patients. ICOS is known as a costimulatory molecule and a migration receptor for Tfh cells [12, 44]. Phosphoinositide 3-kinase (PI3k), activated by ICOS signaling, is critical for Tfh cells [24, 45]. Here, our data first determined that upregulated ICOS expression was significantly correlated with circulating Tfh memory cells in GD patients. To investigate the potential miRNAs that might regulate ICOS expression, miRNA sequencing was subsequently performed to identify downregulated miRNAs. The intersection of miRNAs predicted by prediction software and miRNA sequencing were miR-29a-3p, miR-29b-3p, and miR-98-5p. MiR-29a-3p was finally screened by pretesting of selected miRNAs. The luciferase assays showed that miR-29a-3p can directly regulate ICOS expression. In OVA-induced asthmatic mice, miR-29b levels were decreased and reversed the imbalance of Th1/Th2 response by targeting ICOS [46]. However, the expression of miR-29b in GD tended to be upregulated in our sequencing data. A previous study showed that miR-146a represses Tfh cell numbers by targeting multiple Tfh-related molecules, prominently ICOS in mice [47]. Intriguingly, there are differences in the expression of miR-146a in autoimmune diseases, such as low expression in SLE and high expression in RA [48, 49]. However, our sequencing data showed that there was no difference in miR-146a expression between GD and controls (fold change <1.5 and p >0.05). The possible reason is the heterogeneity of miRNAs in different diseases. As a member of miR-29 family (miR-29a/b/c), miR-29a-3p expression was decreased in myasthenia gravis (MG), which contributed to the increased expression of IFN-β and the emergence of Th17 cells [50]. However, the authors did not identify the target genes of miR-29a-3p. A significant correlation between DICER expression and miR-29a in MG thymuses suggests a pathway to find the mechanism of low expression of miR-29a-3p in GD.
Notably, we found that downregulated miR-29a-3p expression was negatively associated with the proportion of circulating Tfh memory cells and the transcript levels of ICOS in GD patients. Meanwhile, transfection with miR-29a-3p mimics could dampen ICOS expression, which consequently caused the reduction of the percentage of circulating Tfh memory cells in vitro. These data suggested that miR-29a-3p regulates circulating Tfh memory cells by targeting ICOS in GD patients. Clinically, TRAb test is used to diagnose GD [51]. TgAb and TPOAb are also markers of thyroid autoimmunity, and they can be measured in part in GD patients [52]. MiR-29a-3p was found to be inversely correlated with the serum TRAb levels in GD patients. This is the first study to explore the clinical relevance of miR-29a-3p expression in GD patients. However, a major limitation of the present study is small sample size. Another limitation is lack of animal model experiments, which will be further investigated.
Conclusion
In summary, we provide the first line of evidence that miR-29a-3p is an important molecule in GD. MiR-29a-3p exerts a critical role in contributing to the pathogenic role of the circulating Tfh memory cells’ response in GD patients by targeting ICOS.
Data availability
The sequencing datasets of miRNAs can be found in the GEO/GSE183576, https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE183576. The data that support the findings of this study are available from the corresponding author on reasonable request.
References
Weetman AP. Autoimmune thyroid disease: propagation and progression. Eur J Endocrinol. 2003;148:1–9.
Subekti I, Pramono LA. Current diagnosis and management of Graves' disease. Acta Med Indones. 2018;50:177–82.
Smith TJ, Hegedus L. Graves' disease. N Engl J Med. 2016;375:1552–65.
Weetman AP. Graves' disease. N Engl J Med. 2000;343:1236–48.
Bartalena L. Diagnosis and management of Graves disease: a global overview. Nat Rev Endocrinol. 2013;9:724–34.
Burch HB, Cooper DS. Management of Graves disease: a review. JAMA. 2015;314:2544–54.
Barbesino G, Tomer Y. Clinical review: clinical utility of TSH receptor antibodies. J Clin Endocrinol Metab. 2013;98:2247–55.
Chen J, Tian J, Tang X, Rui K, Ma J, Mao C, Liu Y, Lu L, Xu H, Wang S. MiR-346 regulates CD4(+)CXCR5(+) T cells in the pathogenesis of Graves' disease. Endocrine. 2015;49:752–60.
Tomer Y. Mechanisms of autoimmune thyroid diseases: from genetics to epigenetics. Annu Rev Pathol. 2014;9:147–56.
Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, Forster R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192:1545–52.
Yu D, Walker LSK, Liu Z, Linterman MA, Li Z. Targeting TFH cells in human diseases and vaccination: rationale and practice. Nat Immunol. 2022;23:1157–68.
Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–6.
Haynes NM, Allen CD, Lesley R, Ansel KM, Killeen N, Cyster JG. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J Immunol. 2007;179:5099–108.
Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, Crotty S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–10.
Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, Wang YH, Dong C. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–5.
Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, Srivastava M, Linterman M, Zheng L, Simpson N, Ellyard JI, Parish IA, Ma CS, Li QJ, Parish CR, Mackay CR, Vinuesa CG. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31:457–68.
Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS, Mackay CR. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol. 2004;173:68–78.
Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, Foucat E, Dullaers M, Oh S, Sabzghabaei N, Lavecchio EM, Punaro M, Pascual V, Banchereau J, Ueno H. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34:108–21.
Lu J, Wu J, Xia X, Peng H, Wang S. Follicular helper T cells: potential therapeutic targets in rheumatoid arthritis. Cell Mol Life Sci. 2021;78:5095–106.
Simpson N, Gatenby PA, Wilson A, Malik S, Fulcher DA, Tangye SG, Manku H, Vyse TJ, Roncador G, Huttley GA, Goodnow CC, Vinuesa CG, Cook MC. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 2010;62:234–44.
Liu Y, Yuan X, Li X, Cui D, Xie J. Constitutive changes in circulating follicular helper T cells and their subsets in patients with Graves' disease. J Immunol Res. 2018;2018:8972572.
Dong C, Nurieva RI. Regulation of immune and autoimmune responses by ICOS. J Autoimmun. 2003;21:255–60.
Swallow MM, Wallin JJ, Sha WC. B7h, a novel costimulatory homolog of B7.1 and B7.2, is induced by TNFalpha. Immunity. 1999;11:423–32.
Weber JP, Fuhrmann F, Feist RK, Lahmann A, Al Baz MS, Gentz LJ, Vu Van D, Mages HW, Haftmann C, Riedel R, Grun JR, Schuh W, Kroczek RA, Radbruch A, Mashreghi MF, Hutloff A. ICOS maintains the T follicular helper cell phenotype by down-regulating Kruppel-like factor 2. J Exp Med. 2015;212:217–33.
Wang F, Yan T, Chen L, Chen X, Liu T, Shen S, Li T, Gao L, Wang T, Sun J, Liu C, Wu H, Zhang X, Chen L. Involvement of inducible costimulator ligand (ICOSL) expression in thyroid tissue in hyperthyroidism of Graves' disease patients. J Clin Immunol. 2012;32:1253–61.
Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–54.
Maul J, Alterauge D, Baumjohann D. MicroRNA-mediated regulation of T follicular helper and T follicular regulatory cell identity. Immunol Rev. 2019;288:97–111.
Jafarzadeh A, Naseri A, Shojaie L, Nemati M, Jafarzadeh S, Bannazadeh Baghi H, Hamblin MR, Akhlagh SA, Mirzaei H. MicroRNA-155 and antiviral immune responses. Int Immunopharmacol. 2021;101:108188.
Bartel DP. Metazoan MicroRNAs. Cell. 2018;173:20–51.
Martinez-Hernandez R, Serrano-Somavilla A, Ramos-Levi A, Sampedro-Nunez M, Lens-Pardo A, Munoz De Nova JL, Trivino JC, Gonzalez MU, Torne L, Casares-Arias J, Martin-Cofreces NB, Sanchez-Madrid F, Marazuela M. Integrated miRNA and mRNA expression profiling identifies novel targets and pathological mechanisms in autoimmune thyroid diseases. EBioMedicine. 2019;50:329–42.
Yin L, Zeng C, Yao J, Shen J. Emerging roles for noncoding RNAs in autoimmune thyroid disease. Endocrinology. 2020;161.
Yao Q, Wang X, He W, Song Z, Wang B, Zhang J, Qin Q. Circulating microRNA-144-3p and miR-762 are novel biomarkers of Graves' disease. Endocrine. 2019;65:102–9.
Liu Y, Ding X, Xiong S, Wang X, Tang X, Wang L, Wang S, Peng H. Circulating microRNA expression profiling identifies miR-125a-5p promoting T helper 1 cells response in the pathogenesis of Hashimoto's thyroiditis. Front Immunol. 2020;11:1195.
Schmitt N, Bentebibel SE, Ueno H. Phenotype and functions of memory Tfh cells in human blood. Trends Immunol. 2014;35:436–42.
Luthje K, Kallies A, Shimohakamada Y, Belz GT, Light A, Tarlinton DM, Nutt SL. The development and fate of follicular helper T cells defined by an IL-21 reporter mouse. Nat Immunol. 2012;13:491–8.
Weber JP, Fuhrmann F, Hutloff A. T-follicular helper cells survive as long-term memory cells. Eur J Immunol. 2012;42:1981–8.
Ueno H. T follicular helper cells in human autoimmunity. Curr Opin Immunol. 2016;43:24–31.
Rapoport B, McLachlan SM. Graves' hyperthyroidism is antibody-mediated but is predominantly a Th1-type cytokine disease. J Clin Endocrinol Metab. 2014;99:4060–1.
Cai Y, Wang Z, Liu X, Wei L, Li S, Zheng X, Yang T, Xu X. The frequency of intrathyroidal follicular helper T cells varies with the progression of Graves' disease and Hashimoto's thyroiditis. J Immunol Res. 2022;2022:4075522.
Zhang J, Ren M, Zeng H, Guo Y, Zhuang Z, Feng Z, Yan H, Xu M, Liang W, Yang C, Cheng H, Ding H, Yan L. Elevated follicular helper T cells and expression of IL-21 in thyroid tissues are involved in the pathogenesis of Graves' disease. Immunol Res. 2015;62:163–74.
Zhu C, Ma J, Liu Y, Tong J, Tian J, Chen J, Tang X, Xu H, Lu L, Wang S. Increased frequency of follicular helper T cells in patients with autoimmune thyroid disease. J Clin Endocrinol Metab. 2012;97:943–50.
Qi Y, Zhou Y, Chen X, Ye L, Zhang Q, Huang F, Cui B, Lin D, Ning G, Wang W, Wang S. MicroRNA-4443 causes CD4+ T cells dysfunction by targeting TNFR-associated factor 4 in Graves' disease. Front Immunol. 2017;8:1440.
Sun Y, Wang W, Tang Y, Wang D, Li L, Na M, Jiang G, Li Q, Chen S, Zhou J. Microarray profiling and functional analysis of differentially expressed plasma exosomal circular RNAs in Graves' disease. Biol Res. 2020;53:32.
Xu H, Li X, Liu D, Li J, Zhang X, Chen X, Hou S, Peng L, Xu C, Liu W, Zhang L, Qi H. Follicular T-helper cell recruitment governed by bystander B cells and ICOS-driven motility. Nature. 2013;496:523–7.
Gigoux M, Shang J, Pak Y, Xu M, Choe J, Mak TW, Suh WK. Inducible costimulator promotes helper T-cell differentiation through phosphoinositide 3-kinase. Proc Natl Acad Sci U S A. 2009;106:20371–6.
Yan J, Zhang X, Sun S, Yang T, Yang J, Wu G, Qiu Y, Yin Y, Xu W. miR-29b Reverses T helper 1 cells/T helper 2 cells imbalance and alleviates airway eosinophils recruitment in OVA-induced murine asthma by targeting inducible co-stimulator. Int Arch Allergy Immunol. 2019;180:182–94.
Pratama A, Srivastava M, Williams NJ, Papa I, Lee SK, Dinh XT, Hutloff A, Jordan MA, Zhao JL, Casellas R, Athanasopoulos V, Vinuesa CG. MicroRNA-146a regulates ICOS-ICOSL signalling to limit accumulation of T follicular helper cells and germinal centres. Nat Commun. 2015;6:6436.
Li J, Wan Y, Guo Q, Zou L, Zhang J, Fang Y, Zhang J, Zhang J, Fu X, Liu H, Lu L, Wu Y. Altered microRNA expression profile with miR-146a upregulation in CD4+ T cells from patients with rheumatoid arthritis. Arthritis Res Ther. 2010;12:R81.
Wang G, Tam LS, Li EK, Kwan BC, Chow KM, Luk CC, Li PK, Szeto CC. Serum and urinary cell-free MiR-146a and MiR-155 in patients with systemic lupus erythematosus. J Rheumatol. 2010;37:2516–22.
Cron MA, Payet CA, Fayet OM, Maillard S, Truffault F, Fadel E, Guihaire J, Berrih-Aknin S, Liston A, Le Panse R. Decreased expression of miR-29 family associated with autoimmune myasthenia gravis. J Neuroinflammation. 2020;17:294.
Soh SB, Aw TC. Laboratory testing in thyroid conditions – pitfalls and clinical utility. Ann Lab Med. 2019;39:3–14.
Weetman AP. Autoimmune thyroid disease. Autoimmunity. 2004;37:337–40.
Funding
This work was supported by National Natural Science Foundation of China (Grant No. 81800698), Zhenjiang Sixth Phase 169 Project Training Fund Support Project (No. 28 of academic hard-core personnel research project), and Zhenjiang Science and Technology Planning Project (Grant Nos. SH2021026, SH2021059).
Author information
Authors and Affiliations
Contributions
Y.L., X.W., and W.L. designed the methodology, performed the experiments, and analyzed or synthesized study data. Y.L. wrote the original draft. J.Z. provided study materials, reagents, and materials and maintained research data. J.X. participated in the collection of specimens. S.W. and H.P. were responsible for the conceptualization, funding acquisition, project administration, and supervision for the research activity. All authors participated in the discussion and finalization of this manuscript.
Corresponding authors
Ethics declarations
The study was reviewed and approved by the Ethics Committee of the Affiliated People’s Hospital of Jiangsu University and the ethics approval number is K-20200012-Y. The participants provided their written informed consent to participate in the study. All operations in this study adhered to standard biosecurity and institutional safety procedures.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information

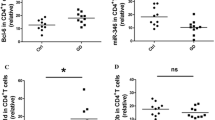
Fig. S1
The relative expression of intersected miRNAs in the PBMCs from 10 GD patients and 10 healthy controls was verified in the preliminary study. The transcript levels of miR-29a-3p (a), miR-29b-3p (b), and miR-98-5p (c) are shown. Each data point represents an individual subject, and the horizontal lines show the mean. ***p< 0.001, NS: no significance (PNG 82 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Y., Wang, X., Luan, W. et al. MiR-29a-3p negatively regulates circulating Tfh memory cells in patients with Graves’ disease by targeting ICOS. Immunol Res 71, 173–184 (2023). https://doi.org/10.1007/s12026-022-09333-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-022-09333-5