Abstract
The innate immune system acts rapidly in an identical and nonspecific way every time the body is exposed to pathogens. As such, it cannot build and maintain immunological memory to help prevent reinfection. Researchers contend that trained immunity is influenced by intracellular metabolic pathways and epigenetic remodeling. The purpose of this review was to explore the topic of trained innate immunity based on the results of relevant previous studies. This systematic review entailed identifying articles related to trained innate immunity. The sources were obtained from PubMed using different search terms that included “trained innate immunity,” “trained immunity,” “trained,” “innate,” “immunity,” and “immune system.” Boolean operators were used to combine terms and phrases. A review of previous study results revealed that little is currently known about the molecular and cellular processes that mediate or induce a trained immune response in animals. However, it is believed that alterations in the phenotypes of cell populations and the numbers of specific cells may play a critical role in mediating the trained immune response. Increasing evidence shows that the protective processes and actions that occur during a secondary infection are not entirely linked to the adaptive immune system. Instead, these events also involve heightened activation of innate immune cells. While trained innate immune cells may have a shorter memory, they assist in the fight against pathogens and provide cross-protection. Identification of the mechanisms and molecules that underlie trained innate immunity has highlighted important features of the human immune response. Such advances continue to open doors for future research on how the body responds to disease-causing pathogens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The immune response is a complex process that involves a wide range of humoral and cellular components capable of detecting nonself-structures and providing protection against pathogens [1]. This system is critical to the growth and survival of multicellular organisms. Traditionally, the immune response has been categorized into innate and adaptative components [2, 3]. In adaptive immunity, B and T cells act as effectors to protect the body against foreign invasion by pathogens. Importantly, the adaptive immune system develops a few weeks after birth and involves targeting specific pathogens and generating immunological memory [4, 5]. In contrast, the innate immune system acts rapidly in an identical and nonspecific way every time the body is exposed to a pathogen. Furthermore, it lacks the ability to build and maintain immunological memory to help prevent reinfection [6,7,8].
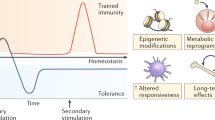
In recent years, the idea that the innate immune response lacks immunological memory and acts in a nonspecific way has been challenged. Researchers have noted that the presence of pattern recognition receptors (PRRs) gives specificity to innate immunity [8,9,10]. In addition, there is evidence that the innate immune system may adapt its response after the first encounter with a pathogen. Moreover, several studies have revealed that the innate immune response is enhanced after reinfection in some organisms [11, 12]. These phenomena have been confirmed in both humans and other vertebrates and have led to the idea of trained immunity. Researchers contend that trained immunity is influenced by intracellular metabolic pathways and epigenetic remodeling [13,14,15]. In addition, trained immunity has been linked to the emergence of proinflammatory phenotypes and has been shown to increase the likelihood of cytokine responses. The outcomes of these studies have spurred interest in the concept of trained immunity. Thus, the purpose of this review was to explore the topic of trained innate immunity based on the results of relevant previous studies.
Methodology
This study entailed identifying articles on trained innate immunity in PubMed using different search terms that included “trained innate immunity,” “trained immunity,” “trained,” “innate,” “immunity,” and “immune system.” Boolean operators were used to combine terms and phrases. The search was limited to scholarly journal articles published between 2010 and 2020. Other records were obtained by examining the bibliographies of the identified reports. The abstracts of the selected sources were carefully evaluated to determine their relevance to the present study. The records that met the inclusion criteria were subjected to full-text review, which entailed checking the credibility of the authors and reviewing the objectives, methodologies, results, discussion, conclusions, and limitations of each study. After a careful review of every article, 67 eligible studies were identified, as indicated in the PRISMA diagram below (Fig. 1).
Results
The topic of trained innate immunity has been explored in both human and animal studies over the last decade [16, 17]. The studies used in this review revealed that trained immunity is considered to encompass defense mechanisms and immune responses that lead to an increased nonspecific response to heterologous and homologous pathogens as well as protection from these pathogens [18, 19]. As a result, innate immunity has specific roles in protecting the body from the adverse effects of pathogens. First, it stimulates the production of different chemical factors, such as cytokines, and recruits immune cells to infection sites [20]. Second, trained innate immunity facilitates the activation of complement cascades that help identify the pathogen. Furthermore, it assists in the removal of foreign substances from the blood, tissues, and organs [21]. In some cases, innate immunity functions as a chemical and physical barrier to various infectious agents through a wide range of mechanisms, such as clotting.
Mechanisms responsible for trained innate immunity
Previous cell population studies have included descriptions of immune cells that assist in the development of innate immunity. Moreover, previous research has identified a wide range of mechanisms and components, including PRRs recognizing flagellin, muramyl dipeptide, CpG-containing oligodeoxynucleotides, and β-glucan, that facilitate the innate immune response and protect the body from pathogens [22, 23]. A previous animal model study revealed that β-glucan-coated microbeads could protect mice from the adverse effects of Escherichia coli [24, 25]. In addition, β-glucan could protect mice from Vibrio salmonicida infection. The Bacillus Calmette-Guérin (BCG) vaccine, on the other hand, induced T cell-independent, nonspecific protection against Candida albicans and Schistosoma mansoni infection [26]. In more recent studies, the concept of trained innate immunity has been linked to T and B cell-independent processes. Researchers contend that the development of trained immunity involves the functional programming and reprogramming of monocytes, macrophages, and NK cells and encompasses the induction of recognition receptor pathways, such as the intracellular MAP kinase-dependent signaling pathway [27]. These events may cause epigenetic changes that lead to the development of innate immunity.
Netea et al. noted that even though a wide range of cell populations have been associated with trained innate immunity and memory, most researchers have focused on the roles of monocytes, macrophages, and NK cells [4]. This focused attention does not imply that these three cell types are more amenable to innate training than other cell types; however, it does reflect the reported connection between lipopolysaccharide (LPS)-induced tolerance and innate memory [28, 29]. Early studies showed that macrophages have memory-like features that affect LPS-induced tolerance. For instance, it has been reported that macrophages can be primed by LPS to become more or less responsive to future activation signals [30]. In addition, research has revealed that when macrophages and monocytes are exposed to C. albicans or β-glucan, they show enhanced stimulatory capability during future pathogenic attacks [31]. The immune training process is also accompanied by the modulation of chromatin and enhanced protection against parasitic and viral pathogens.
In addition to the memory-like features found in macrophages, group 2 innate lymphoid cells (ILC2s) have also been observed to possess memory-like properties attributed to allergen exposure. Martinez-Gonzalez et al. showed that ILC2s in the lungs become stimulated after the inhalation of allergens [32]. These cells do not recognize allergens directly but become stimulated by cytokines such as interleukin (IL)-33 stemming from the damaged epithelium. ILC2s respond to allergens by producing T helper 2 cell cytokines that induce allergic lung inflammation through a T cell-independent mechanism. The point worth noting here is that allergen-experienced ILC2s respond to different allergens more potently than do naïve ILC2s, thereby exhibiting memory-like attributes that explain why some patients, for instance, asthmatic individuals, are sensitive to particular allergens [33].
The activation of ILC2s by IL-33 in the neonatal period has also been observed to train ILC2 seeding in the lung after birth, which helps a person respond quite effectively to challenges that happen later in life. Steer et al. used mice to show that ILC2s can be activated with the help of the epithelium-derived alarmin IL-33 [34]. Activated ILC2s multiply and produce IL-13 and IL-5, which respond to allergic reactions. Notably, however, the activation of ILC2s in the neonatal lungs occurs spontaneously with a reliance on IL-33, which continues to increase as the ILC2s expand [35]. As a result, the activation of ILC2s by endogenous IL-33 has been noted to significantly impact the functionality of ILC2s among adults.
Another worthwhile observation of the memory features of NK cells/ILC1s is the apparent antigen specificity, which can be seen when mice are rechallenged. This is quite a surprising observation for the innate cells described by Paust et al., as they posit that the antigen-specific attribute is common in mice deficient in T cells and B cells [36, 37]. Researchers have reported that nonnaïve or nonsplenic NK cells can develop a particular memory for vaccines comprising antigens from various viruses, such as influenza, HIV-1, or vesicular stomatitis virus. As a result, the adoptive transfer of a virus-sensitized form of NK cells into naïve mice has been noted to enhance the survival of these animas after a lethal challenge with a sensitizing virus [36]. In other words, the administration of virus-sensitized NK cells mediates innate protection against secondary challenge with different viruses, such as vaccinia virus [38]. This result seems to oppose the existing research in which immunological memory has often been linked with T and B lymphocytes.
Another group of researchers has focused on the manner in which monocytes contribute to the development of trained innate immunity [39]. Notably, monocytes have a short life in the circulation. However, clinical observations and cellular studies have shown that trained monocytes can persist in BCG-vaccinated persons for up to 3 months after vaccination [31, 39]. These results suggest that training entails the reprogramming of monocytes at the progenitor cell stage. More recently, researchers reported that innate memory could be successfully transferred through hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (HPCs) but not via the bone marrow [40, 41]. Additionally, NK cell progenitors, known as killer cell lectin-like receptor G1 (KLRG1)-negative progenitors, can also become memory NK cells [42]. Hematopoietic stem cell- and progenitor cell-derived macrophages develop tolerance to pathogens when they are exposed to Toll-like receptor 2 (TLR2) ligand. Moreover, studies in animal models have shown that exposing these cells to ultraviolet radiation can cause immunosuppression and epigenetic reprogramming [43, 44]. The findings of these studies support the possible involvement of monocytes in the development of trained innate immunity. However, further investigations are needed to determine whether vaccines such as BCG are capable of automatically stimulating trained innate immunity and memory or instead induce similar effects through the reprogramming of progenitor cells alone [45].
Emerging research evidence indicates that NK cells may respond more vigorously to subsequent pathogen exposure than to the initial attack. Furthermore, NK cell memory is believed to be affected by cytokines, such as IL-12, IL-15, and IL-18 [43]. In addition, NK cells have been reported to be capable of undergoing significant expansion after viral infection, such as influenza A infection. Under such circumstances, the activation and expansion of NK cells may lead to the development of immune protection against reinfection through the production and rapid accumulation of cytokines [44]. In this case, the immune response is independent of T cells. More recently, experimental studies and animal models have revealed that NK cells produce protective immunological responses after vaccination against unrelated bacterial and viral pathogens [6, 45]. While explaining the involvement of NK cells in trained innate immunity, researchers have referenced various mechanisms that include activation of the costimulatory molecule DNAM-1, mitophagy, and Atg3-dependent pathways.
Another important area that has attracted the attention of researchers involves pathways and processes responsible for the persistence and maintenance of NK cell memory. Research has shown that NK cell memory and the ability to exert protective effects against viruses may depend on chemokine receptors such as CXCR6 [46,47,48]. Recent investigations indicate that the development of NK cell memory involves epigenetic changes [31]. Accordingly, a recent study reported that the DNA methylation patterns in cytotoxic T cells and NK cells were similar to each other but different from those in canonical NK cells. DNA methylation is modulated by the transcription factor promyelocytic leukemia zinc finger protein (PLZF) and effects on gene promoters [31]. Taken together, the evidence from these studies indicates the complex nature of trained innate immunity and the possible effects of epigenetic reprogramming and antigen-dependent actions on the entire process.
Preliminary investigations have also revealed that the characteristics associated with monocytes, macrophages, and NK cells may also be found in other populations of cells, such as polymorphonuclear leukocytes. Unlike lymphocytes, cells responsible for innate immunity, such as polymorphonuclear leukocytes, do not express the machinery for rearranging antigen receptor genes [20, 21]. Instead, they express receptors such as PRRs that help recognize and respond to endogenous signals of danger and pathogenic invasion. While the immune response may not be specific to antigen receptors, research results have shown that it is accompanied by the expression of particular families of PRRs, such as RIG-I-like receptors and C-type lectin receptors [49, 50]. In addition, the immune response may trigger signaling pathways that initiate immune actions designed to deal with a specific type of pathogen.
Both in vivo and in vitro studies have reported that trained immunity is often induced by danger-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs). The results from animal studies showed that administration of C. albicans, for instance, could protect mice from Staphylococcus aureus reinfection [51, 52]. In vitro experiments, on the other hand, showed that β-glucan could induce epigenetic remodeling and alter the functional reprogramming process via a dectin-1/Raf1-dependent mechanism [53]. Human studies have shown that the BCG vaccine can lead to the upregulation of cytokine production and protect the body from pathogens. However, this protective effect depends on a wide range of factors that include histone methylation, autophagy, and NOD2 signaling [54, 55]. More recent investigations have revealed that oxidized low-density lipoprotein (oxLDL), an atherogenic lipid, can lead to changes in monocyte activity and cytokine expression [56]. In this case, the protective effect may be influenced by TLR4 and histone methylation.
Induction of trained innate immune memory
Trained innate immunity causes a stronger transcriptional response than untrained immunity while fighting disease-causing pathogens or other danger signals [57]. The enhanced activation is correlated with different molecular events, such as inflammatory gene expression and persistent miRNA induction [56]. A review of previous study results showed that little is currently known about the molecular and cellular processes that mediate or induce a trained immune response in animals [58, 59]. However, it is believed that alterations in the phenotypes of cell populations and the numbers of specific cells may play a critical role in mediating the trained immune response. Research has shown that immune cells, such as neutrophils, have a high turnover rate, which makes it difficult for them to maintain trained immune memory [4, 60, 61]. In comparison, innate immune cells with low rates of turnover, such as NK cells, are more likely to develop the characteristics that help them fight pathogens. In macrophages, the mechanisms that induce innate training may include changes in pentose phosphate pathway (PPP) expression and phenotypic processes, such as cytokine production and phagocytosis [62]. The underlying molecular pathways that shift cells towards phenotypes with memory-like capabilities have not been conclusively studied or identified [63]. Recent research, however, indicates that epigenetic changes can cause transcriptional profile reprogramming and enable innate cells to acquire immune memory traits [63]. In some cases, chromatin modification facilitates the storage of critical immune information that facilitates the fight against pathogens.
In other studies, researchers have noted that vaccines such as the BCG vaccine may play an important role in inducing innate immune cell memory [64]. Vaccinated persons showed an increased number of inflammatory mediators produced by monocytes compared to their nonvaccinated counterparts. In addition, innate memory induction involves histamine modification as a result of gene activation. In macrophages and monocytes, the BCG vaccine leads to epigenetic reprogramming through reduced PLZF and SYK tyrosine kinase expression [40, 64]. Importantly, BCG vaccine-related innate cell memory may involve IFNγ priming within NK cells. Such epigenetic processes enable innate cells to acquire memory and fight subsequent infection.
A final category of studies has shown that miRNAs may mediate the induction of innate cell memory [65]. This conjecture is based on research evidence that miRNAs are long-living molecules with minimal effects on the proliferation of myeloid cell subpopulations. For example, miRNAs may persist within the immune system indefinitely after the primary pathogen or stimulus has been eliminated. Recent research has revealed that miR-155 is central to the induction of innate cell memory because it hyperactivates myeloid cells by upregulating and promoting responses to different inflammatory cues, such as microbial pathogens [66, 67]. In addition, miR-155 can regulate signaling pathways and ensure that myeloid cells remain primed even in the absence of the primary pathogen.
Discussion
Evidence from previous studies has demonstrated that trained immune cells are a key component of the defense system and immune response [40, 53, 63]. In addition, there is a consensus among researchers that trained immunity differs significantly from other immunological responses. The cells involved in trained immunity are not specific, and their induction and action involve reprogramming and epigenetic changes to subpopulations of immune cells, such as myeloid and NK cells [40, 55, 57, 58]. Increasing evidence indicates that the protective processes during a secondary infection may not be entirely linked to the adaptive immune system; instead, they may involve the heightened activation of innate immune cells. Although trained innate immune cells may have a shorter memory, they assist in the fight against pathogens and provide cross-protection.
Further investigations are needed in the future to help researchers and practitioners understand the topic of trained innate immunity and its underlying molecular pathways [53]. Among the areas of focus for researchers is the evaluation of the mechanisms that mediate trained innate immunity at the cell-type level [63]. Additionally, it is imperative to explore the possible epigenetic and metabolic alterations and outcomes that may affect the memory duration of trained innate immune cells. In such investigations, researchers will need to evaluate how the duration of innate immune memory determines the way the body responds to different pathogens. In the future, researchers may also focus on the identification of new cell subpopulations that possess features of innate immunity [65,66,67]. Such studies may be aided by novel technologies and techniques, such as epigenomics analysis and genome-wide sequencing. The outcomes will assist researchers and healthcare practitioners in understanding the immunological process and identifying new therapeutic targets to treat diseases and improve the host defense system.
Conclusion
Research has revealed that exposure to disease-causing pathogens leads to epigenetic changes that affect the function of innate immune cell populations. These changes make the cells more responsive to a secondary infection. Furthermore, these alterations must increase the production of inflammatory mediators while also increasing the capacity of innate immune cells to eliminate an infection. Elucidation of the mechanisms and molecules that underlie trained innate immunity has revealed important features of the human immune response, and advances continue to open doors for future research on how the body responds to pathogens. In addition, future findings will provide novel ideas for treating and managing immune paralysis or other diseases caused by immunodeficiency or autoinflammation.
References
Thompson RC, Allam AH, Lombardi GP, Wann LS, Sutherland ML, Sutherland JD, et al. Atherosclerosis across 4000 years of human history: the Horus study of four ancient populations. Lancet Lond Engl. 2013;381(9873):1211–22.
Pothineni NVK, Subramany S, Kuriakose K, Shirazi LF, Romeo F, Shah PK, et al. Infections, atherosclerosis, and coronary heart disease. Eur Heart J. 2017;38(43):3195–201.
Corrales-Medina VF, Musher DM, Shachkina S, Chirinos JA. Acute pneumonia and the cardiovascular system. Lancet Lond Engl. 2013;381(9865):496–505.
Netea MG, Joosten LAB, Latz E, Mills KHG, Natoli G, Stunnenberg HG, et al. Trained immunity: a program of innate immune memory in health and disease. Science. 2016;352(6284):aaf1098.
Wang H, Peng G, Bai J, He B, Huang K, Hu X, et al. Cytomegalovirus infection and relative risk of cardiovascular disease (ischemic heart disease, stroke, and cardiovascular death): a meta-analysis of prospective studies up to 2016. J Am Heart Assoc. 2017;6(7).
Kleinnijenhuis J, Quintin J, Preijers F, Joosten LAB, Ifrim DC, Saeed S, et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A. 2012;109(43):17537–42.
van der Valk FM, Bekkering S, Kroon J, Yeang C, Van den Bossche J, van Buul JD, et al. Oxidized phospholipids on lipoprotein(a) elicit arterial wall inflammation and an inflammatory monocyte response in humans. Circulation. 2016;134(8):611–24.
Bekkering S, Quintin J, Joosten LAB, van der Meer JWM, Netea MG, Riksen NP. Oxidized low-density lipoprotein induces long-term proinflammatory cytokine production and foam cell formation via epigenetic reprogramming of monocytes. Arterioscler Thromb Vasc Biol. 2014;34(8):1731–8.
Arts RJW, Novakovic B, Ter Horst R, Carvalho A, Bekkering S, Lachmandas E, et al. Glutaminolysis and fumarate accumulation integrate immunometabolic and epigenetic programs in trained immunity. Cell Metab. 2016;24(6):807–19.
Bekkering S, Arts RJW, Novakovic B, Kourtzelis I, van der Heijden CDCC, Li Y, et al. Metabolic induction of trained immunity through the mevalonate pathway. Cell. 2018;172(1–2):135–146.e9.
Arts RJW, Carvalho A, La Rocca C, Palma C, Rodrigues F, Silvestre R, et al. Immunometabolic pathways in BCG-induced trained immunity. Cell Rep. 2016;17(10):2562–71.
Mitroulis I, Ruppova K, Wang B. Trained immunity orchestrates enhanced myelopoiesis through tailored and sustained transcriptional and metabolic adaptations in hematopoietic progenitor cells. Cell. 2018;172:147–61.
Wang M, Subramanian M, Abramowicz S, Murphy AJ, Gonen A, Witztum J, et al. Interleukin-3/granulocyte macrophage colony-stimulating factor receptor promotes stem cell expansion, monocytosis, and atheroma macrophage burden in mice with hematopoietic ApoE deficiency. Arterioscler Thromb Vasc Biol. 2014;34(5):976–84.
van Splunter M, van Osch TLJ, Brugman S, Savelkoul HFJ, Joosten LAB, Netea MG, et al. Induction of trained innate immunity in human monocytes by bovine milk and milk-derived immunoglobulin G. Nutrients. 2018;10(10).
Vetvicka V, Vannucci L, Sima P. The effects of β - glucan on fish immunity. North Am J Med Sci. 2013;5(10):580–8.
Petit J, Wiegertjes GF. Long-lived effects of administering β-glucans: indications for trained immunity in fish. Dev Comp Immunol. 2016;64:93–102.
Boraschi D, Italiani P. Innate immune memory: time for adopting a correct terminology. Front Immunol. 2018;9:799.
Arts RJW, Joosten LAB, Netea MG. The potential role of trained immunity in autoimmune and autoinflammatory disorders. Front Immunol. 2018;9:298.
Rusek P, Wala M, Druszczyńska M, Fol M. Infectious agents as stimuli of trained innate immunity. Int J Mol Sci. 2018;19(2).
Bauer M, Weis S, Netea MG, Wetzker R. Remembering pathogen dose: long-term adaptation in innate immunity. Trends Immunol. 2018;39(6):438–45.
Chang MX, Zhang J. Alternative Pre-mRNA splicing in mammals and teleost fish: a effective strategy for the regulation of immune responses against pathogen infection. Int J Mol Sci. 2017;18(7).
Camilli G, Tabouret G, Quintin J. The complexity of fungal β-glucan in health and disease: effects on the mononuclear phagocyte system. Front Immunol. 2018;9:673.
Vetvicka V, Vetvickova J. Glucans and cancer: comparison of commercially available β-glucans - Part IV. Anticancer Res. 2018;38(3):1327–33.
Synytsya A, Novak M. Structural analysis of glucans. Ann Transl Med. 2014;2(2):17.
Bashir KMI, Choi J-S. Clinical and physiological perspectives of β-Glucans: the past, present, and future. Int J Mol Sci. 2017;18(9).
Nie L, Cai S-Y, Shao J-Z, Chen J. Toll-like receptors, associated biological roles, and signaling networks in non-mammals. Front Immunol. 2018;9:1523.
Solbakken MH, Tørresen OK, Nederbragt AJ, Seppola M, Gregers TF, Jakobsen KS, et al. Evolutionary redesign of the Atlantic cod (Gadus morhua L.) Toll-like receptor repertoire by gene losses and expansions. Sci Rep. 2016;6:25211.
You X, Bian C, Zan Q, Xu X, Liu X, Chen J, et al. Mudskipper genomes provide insights into the terrestrial adaptation of amphibious fishes. Nat Commun. 2014;5:5594.
Brubaker SW, Bonham KS, Zanoni I, Kagan JC. Innate immune pattern recognition: a cell biological perspective. Annu Rev Immunol. 2015;33:257–90.
Chen SN, Zou PF, Nie P. Retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs) in fish: current knowledge and future perspectives. Immunology. 2017;151(1):16–25.
Hong M, Sandalova E, Low D, Gehring AJ, Fieni S, Amadei B, et al. Trained immunity in newborn infants of HBV-infected mothers. Nat Commun. 2015;6:6588.
Martinez-Gonzalez I, Mathä L, Steer CA, Ghaedi M, Poon GFT, Takei F. Allergen-experienced group 2 innate lymphoid cells acquire memory-like properties and enhance allergic lung inflammation. Immunity. 2016;45(1):198–208.
Gold MJ, Antignano F, Halim TYF, Hirota JA, Blanchet M-R, Zaph C, et al. Group 2 innate lymphoid cells facilitate sensitization to local, but not systemic, TH2-inducing allergen exposures. J Allergy Clin Immunol. 2014;133(4):1142–8.
Steer CA, Mathä L, Shim H, Takei F. Lung group 2 innate lymphoid cells are trained by endogenous IL-33 in the neonatal period. JCI Insight. 2020;5(14).
Steer CA, Martinez-Gonzalez I, Ghaedi M, Allinger P, Mathä L, Takei F. Group 2 innate lymphoid cell activation in the neonatal lung drives type 2 immunity and allergen sensitization. J Allergy Clin Immunol. 2017;140(2):593–595.e3.
Paust S, Gill HS, Wang B-Z, Flynn MP, Moseman EA, Senman B, et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol. 2010;11(12):1127–35.
Wang X, Peng H, Cong J, Wang X, Lian Z, Wei H, et al. Memory formation and long-term maintenance of IL-7Rα+ ILC1s via a lymph node-liver axis. Nat Commun. 2018;9(1):4854.
Gillard GO, Bivas-Benita M, Hovav A-H, Grandpre LE, Panas MW, Seaman MS, et al. Thy1+ NK [corrected] cells from vaccinia virus-primed mice confer protection against vaccinia virus challenge in the absence of adaptive lymphocytes. PLoS Pathog. 2011;7(8):e1002141.
Green TJ, Helbig K, Speck P, Raftos DA. Primed for success: oyster parents treated with poly(I:C) produce offspring with enhanced protection against ostreid herpesvirus type I infection. Mol Immunol. 2016;78:113–20.
Nabekura T, Girard J-P, Lanier LL. IL-33 receptor ST2 amplifies the expansion of NK cells and enhances host defense during mouse cytomegalovirus infection. J Immunol Baltim Md 1950. 2015;194(12):5948–52.
Peng H, Jiang X, Chen Y, Sojka DK, Wei H, Gao X, et al. Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. J Clin Invest. 2013;123(4):1444–56.
Kamimura Y, Lanier LL. Homeostatic control of memory cell progenitors in the natural killer cell lineage. Cell Rep. 2015;10(2):280–91.
Schlums H, Cichocki F, Tesi B, Theorell J, Beziat V, Holmes TD, et al. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity. 2015;42(3):443–56.
Askenase MH, Han S-J, Byrd AL, Morais da Fonseca D, Bouladoux N, Wilhelm C, et al. Bone-marrow-resident nk cells prime monocytes for regulatory function during infection. Immunity. 2015;42(6):1130–42.
Benn CS, Netea MG, Selin LK, Aaby P. A small jab - a big effect: nonspecific immunomodulation by vaccines. Trends Immunol. 2013;34(9):431–9.
Jensen KJ, Larsen N, Biering-Sørensen S, Andersen A, Eriksen HB, Monteiro I, et al. Heterologous immunological effects of early BCG vaccination in low-birth-weight infants in Guinea-Bissau: a randomized-controlled trial. J Infect Dis. 2015;211(6):956–67.
Peng H, Wisse E, Tian Z. Liver natural killer cells: subsets and roles in liver immunity. Cell Mol Immunol. 2016;13(3):328–36.
Peng H, Tian Z. Natural killer cell memory: progress and implications. Front Immunol. 2017;8:1143.
Redelman-Sidi G, Glickman MS, Bochner BH. The mechanism of action of BCG therapy for bladder cancer--a current perspective. Nat Rev Urol. 2014;11(3):153–62.
Buffen K, Oosting M, Quintin J, Ng A, Kleinnijenhuis J, Kumar V, et al. Autophagy controls BCG-induced trained immunity and the response to intravesical BCG therapy for bladder cancer. PLoS Pathog. 2014;10(10):e1004485.
Yona S, Kim K-W, Wolf Y, Mildner A, Varol D, Breker M, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38(1):79–91.
Yáñez A, Hassanzadeh-Kiabi N, Ng MY, Megías J, Subramanian A, Liu GY, et al. Detection of a TLR2 agonist by hematopoietic stem and progenitor cells impacts the function of the macrophages they produce. Eur J Immunol. 2013;43(8):2114–25.
Beemelmanns A, Roth O. Grandparental immune priming in the pipefish Syngnathus typhle. BMC Evol Biol. 2017;17(1):44.
Levy O, Wynn JL. A prime time for trained immunity: innate immune memory in newborns and infants. Neonatology. 2014;105(2):136–41.
Morelli SS, Mandal M, Goldsmith LT, Kashani BN, Ponzio NM. The maternal immune system during pregnancy and its influence on fetal development. Res Rep Biol. 2015;6:171–89.
Savva A, Roger T. Targeting toll-like receptors: promising therapeutic strategies for the management of sepsis-associated pathology and infectious diseases. Front Immunol. 2013;4:387.
Goodridge HS, Ahmed SS, Curtis N, Kollmann TR, Levy O, Netea MG, et al. Harnessing the beneficial heterologous effects of vaccination. Nat Rev Immunol. 2016;16(6):392–400.
Sun JC, Ugolini S, Vivier E. Immunological memory within the innate immune system. EMBO J. 2014;33(12):1295–303.
Gourbal B, Pinaud S, Beckers GJM, Van Der Meer JWM, Conrath U, Netea MG. Innate immune memory: an evolutionary perspective. Immunol Rev. 2018;283(1):21–40.
Domínguez-Andrés J, Joosten LA, Netea MG. Induction of innate immune memory: the role of cellular metabolism. Curr Opin Immunol. 2019;56:10–6.
Faure F, Jouve M, Lebhar-Peguillet I, Sadaka C, Sepulveda F, Lantz O, et al. Blood monocytes sample MelanA/MART1 antigen for long-lasting cross-presentation to CD8+ T cells after differentiation into dendritic cells. Int J Cancer. 2018;142(1):133–44.
Breyne K, Steenbrugge J, Demeyere K, Vanden Berghe T, Meyer E. Preconditioning with lipopolysaccharide or lipoteichoic acid protects against Staphylococcus aureus mammary infection in mice. Front Immunol. 2017;8:833.
van der Heijden CDCC, Noz MP, Joosten LAB, Netea MG, Riksen NP, Keating ST. Epigenetics and trained immunity. Antioxid Redox Signal. 2018;29(11):1023–40.
Álvarez-Errico D, Vento-Tormo R, Sieweke M, Ballestar E. Epigenetic control of myeloid cell differentiation, identity and function. Nat Rev Immunol. 2015;15(1):7–17.
Bekkering S, Blok BA, Joosten LAB, Riksen NP, van Crevel R, Netea MG. In vitro experimental model of trained innate immunity in human primary monocytes. Clin Vaccine Immunol CVI. 2016;23(12):926–33.
Cheng S-C, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, et al. mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345(6204):1250684.
Bekkering S, van den Munckhof I, Nielen T, Lamfers E, Dinarello C, Rutten J, et al. Innate immune cell activation and epigenetic remodeling in symptomatic and asymptomatic atherosclerosis in humans in vivo. Atherosclerosis. 2016;254:228–36.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arneth, B. Trained innate immunity. Immunol Res 69, 1–7 (2021). https://doi.org/10.1007/s12026-021-09170-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-021-09170-y