Abstract
Multiple sclerosis (MS) is an autoimmune disease which is characterized by neuroaxonal degeneration in the central nervous system. Impaired function of regulatory T cells (Tregs) is believed to be an underlying pathogenic mechanism in MS. Tregs is able to release exosomes, which contain a considerable amount of protein and RNA. Exosomes are capable of transporting their content to other cells where the released content exerts biological functions. Here, we investigated whether Tregs exosomes of RRMS patients or healthy controls might regulate the proliferation or survival of T lymphocytes. Regulatory T cells derived from MS patients or healthy controls were cultured for 3 days and exosomes were purified from supernatants. Treg-derived exosomes were co-cultured with conventional T cells (Tconv). The percentages of Tconv proliferation and apoptosis were measured. Our findings showed that the percentage of proliferation suppression induced by exosomes in patients compared to healthy controls was 8.04 ± 1.17 and 12.5 ± 1.22, respectively, p value = 0.035. Moreover, the rate of Tconv apoptosis induced by exosome of MS patient was less than healthy controls (0.68 ± 0.12 vs. 1.29 ± 0.13; p value = 0.015). Overall, Treg-derived exosomes from MS patients and healthy controls suppressed the proliferation and induced apoptosis in Tconv. However, the effect of MS-derived exosomes was significantly less than healthy controls. Our results point to an alternative Treg inhibitory mechanism which might be important in immunopathogenesis of MS. Although, the cause of the exosomal defect in MS patients is unclear, manipulation of patients’ Treg-derived exosomes to restore their suppressive activity might be considered as a potential therapeutic approach.

ᅟ
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is a multifactorial autoimmune disease, in which chronic inflammation of the central nervous system (CNS) leads to demyelination and neural cell injury in both white and gray matter [1,2,3]. Different clinical patterns have been reported for MS with relapsing-remitting MS (RRMS) being the most common clinical form of the disease with several periods of relapses and remissions [4, 5].
Numerous studies performed on MS patients or its animal models have pointed to CD4+ and CD8+ T cells as crucial players in the pathogenesis of disease [6, 7]. It is known that infiltration of CNS with myelin-reactive T cells with either a Th1 or Th17 phenotype is one of the determining pathogenic events in RRMS [1, 8, 9]. Various mechanisms have been proposed to explain this pathogenic activation and infiltration of autoreactive T cells. One of the proposed explanations which have considerable empirical evidence is insufficient control and suppression of autoreactive T cells due to defective functioning of CD4+ CD25High regulatory T cells [10, 11]. Indeed, some immunomodulatory drugs that are used for the treatment of MS are known to be able to restore or enhance Treg function [12, 13].
Regulatory T cells have been shown to exert their suppressive activity by a variety of molecular mechanisms such as production of immunomodulatory cytokines, expression of inhibitory receptors, and direct cytotoxic killing of target cells [14, 15]. Recent evidence has indicated that, similar to most other cells, Tregs are capable of releasing small, membrane-bound vesicles called exosomes [16,17,18,19]. These nano-vesicles encapsulate cytosolic contents including specific RNA and protein species, traverse the intercellular space, merge with and release their contents into other cells influencing target cell’s biology [20, 21]. In 2007, Valadi and colleagues showed that mRNA- and miRNA-containing exosomes are secreted by mast cells and delivered to other cells. Interestingly, these exosomes were functional in the new location [20]. Another study has shown that miRNA-containing exosomes could travel from T cells to dendritic cells and inhibit the expression of target genes [22].
Studies on Treg-derived exosomes have demonstrated that these particular exosomes can have immunomodulatory effects in different contexts, providing Tregs with yet another mechanism to exert their regulatory function [23, 24]. Indeed, investigating the molecular contents of Treg-derived exosomes has illustrated that their molecular repertoire is specific and distinct from other T cells including cells with a Th1 or Th2 phenotype. Of note, Okoye et al. have demonstrated that Treg-derived exosomes can suppress the proliferation and cytokine production by Th1 cells in a mouse model of colitis [25].
It is conceivable that diseases involving immune system might affect the content and hence the function of leukocyte-derived exosomes, including exosomes produced by Tregs. Studies on CNS-related diseases such as Alzheimer and multiple sclerosis showed that the biomolecules contents of secreted exosomes are dysregulated compared to normal individuals [26,27,28]. In the current study, we asked the question as to whether insufficient activity of Tregs in MS patients might be associated with altered functioning of Treg-derived exosomes. We isolated exosomes from MS or healthy control-derived Treg cells and investigated and compared their effects on conventional T cells. Experiments were performed to examine the effect of Treg-derived exosomes on proliferative capacity as well as survival of T CD4+ cells from patients with RRMS.
Material and methods
Subjects
This study was performed using cells from 10 patients with relapsing-remitting MS (mean age 35.2 ± 6.7) diagnosed according to McDonald’s criteria [29]. All of patients were in relapse phase and none of them were under immunosuppressive and or immunomodulatory therapy at least 3 months before obtaining the samples. Disability was assessed by an experienced neurologist using expanded disability status scale (EDSS). All of the patients had EDSS scores below 3. After confirmation of the disease and the patient’s desire, 25–30 cm3 peripheral blood was taken, and then the patient’s treatment was started. Ten sex-/age-matched individuals, who had no history of MS or other autoimmune and inflammatory diseases in their families, were signed up as healthy controls. All patients and controls were Iranian origin. The study was approved by ethics committee of Tehran University of Medical Sciences (IR.TUMS.REC.1394.1551) and written informed consent was obtained from each study subject prior to the study. All of the patients were referred to Iranian Center of Neurological Research in Imam Khomeini General Hospital, Tehran University of Medical Sciences, Tehran, Iran.
Purification of T cell subsets
Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood by Ficoll density gradient centrifugation using Lymphodex (Innotrain, Germany). The CD4+CD25− conventional T cells (Tconv) and CD4+CD25+ regulatory T cells (Treg) were isolated using Dynabead Regulatory CD4+CD25+ T cell kit, according to manufacturer’s instructions (Invitrogen Dynal Carlsbad, CA). Isolated cell subsets were stained by CD4-APC and CD25-PE monoclonal antibodies (BioLegend, USA), and the purity of cells was assessed using flow cytometry.
Culture of regulatory T cells
Purified Tregs were cultured in RPMI 1640 (Biosera, France) supplemented with penicillin (100 IU/ml) and streptomycin (100 μg/ml), 1% l-glutamine 100 mM, 1% sodium pyruvate 1 M, and 10% exosome-depleted fetal bovine serum (SBI System Bioscience, USA) for 3 days. To stimulate polyclonal activation of cells, soluble anti-CD3 (MabTech, Sweden) and soluble anti-CD28 (MabTech, Sweden) were added at 1 and 5 μg/ml concentrations, respectively.
Isolation and characterization of exosomes from cell culture supernatant
Exosomes were purified from the supernatant of Treg cultures using Total Exosome Isolation kit (Invitrogen, Carlsbad, CA) according to manufacturer’s guidelines. Briefly, cell supernatant was centrifuged at 2000g for 30 min to remove dead cells and cell debris. Cell-free media was transferred to a new tube and Total Exosome Isolation reagent was added. After 16–18-h incubation at 4 °C, the samples were centrifuged at 10000g for 1 h at 4 °C to pellet exosomes. Exosomes were quantified using a CD63 ELISA kit (SBI System Bioscience, USA). A standard curve generated from known CD63+ exosomes.
Proliferation assay
Proliferation assay was performed in 96 well flat bottom plates. 1 × 105 Tconv cells were pre-labeled with 2.5 μM Cell Trace CFSE (Invitrogen, Carlsbad, CA) according to manufacturer’s guidelines and cultured for 3 days in the presence or absence of Treg-derived exosomes. To stimulate polyclonal activation of cells, soluble anti-CD3 and anti-CD28 antibodies were added at 1 and 5 μg/ml concentrations, respectively. Percentage of suppression (S) was calculated as follows [30]:
where a is the percentage of proliferation in the absence of Treg-derived exosomes and b is the percentage of proliferation in the presence of Treg-derived exosomes.
Analysis of apoptosis
To examine the effect of exosomes on T cell apoptosis, 1 × 105 Tconv cells were cultured for 3 days in the presence or absence of Treg-derived exosomes. Apoptosis was analyzed using AnnexinV-Apoptosis Detection Kit APC (eBioscience, USA) following manufacturer’s instructions. The results were analyzed on a BD FACS Calibur. The rate of apoptosis induced by Treg-derived exosomes was quantified by calculating the difference between the percentage of Annexin V+ /PI + Tconv cells in presence and absence of Treg-derived exosomes. FlowJo software (FlowJo, Ashland, OR) was used for all flow cytometry analyses.
Statistics
Statistical analyses were performed using Prism Software (GraphPad). Mann-Whitney U test was used to evaluate the statistical significance between MS and control groups. Data are presented as mean ± SEM and p values below 0.05 are considered significant.
Results
Ten MS patients and 10 healthy controls were examined in this study. Demographic and clinical characteristics of patients are given in Table 1. Eighty percent of the patients exert lesion in brain periventricular region. On the other hand, nearly 70% of patients exert the sensory symptoms as the first clinical sign. Disease duration was from 1 month to 7 years.
Exosomes were purified from Tregs
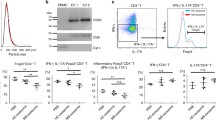
The purity of isolated CD4+CD25− (Tconv) and CD4+CD25+ (Treg) T cell subsets was 95 and 98% respectively as determined by flow cytometry analysis (Fig. 1). The purified exosomes were quantified using a CD63 ELISA kit. CD63 is a well-characterized marker to detection of exosomes. On average, 3 × 108 CD63+ exosomes were extracted from Tregs.
Purity of isolated cell subsets. Conventional and regulatory T cells were isolated from PBMC using Dynabead Regulatory CD4+CD25+ T cell kit; the purity of isolated T cell subsets was assessed by flow cytometry. The purity of isolated cells was ≥ 90%. (a) FSC/SSC gate on lymphocytes, (b) unstained cells, (c) conventional T cells (CD4+ CD25−), and (d) regulatory T cells (CD4+ CD25+)
Treg-derived exosomes from MS patients are less effective in suppressing conventional T cell proliferation
Cell proliferation after the antigen recognition is the first sign of cell activation. In order to evaluate the immunomodulatory effect of Treg-derived exosomes on the proliferation of Tconv, 1 × 105 CFSE-labeled Tconv cells were cultured for 3 days in the presence or absence of Treg-derived exosomes, as described in the “Methods” section. As shown in Fig. 2, the mean percentage of proliferation suppression in MS patient and healthy control groups was 8.04 ± 1.17 and 12.5 ± 1.22, respectively. This difference was statistically significant (p value = 0.035). These findings showed that the suppressive function of Treg-derived exosomes is declined in MS patients.
Treg-derived exosomes from patients suppress conventional T cell proliferation less than exosomes from healthy controls. 1 × 105 CFSE-labeled Tconv cells were cultured for 3 days in the presence or absence of Treg-derived exosomes and the proliferation of Tconv cells was measured using CFSE dilution method. (a) Unlabeled cells, (b) day 0 of CFSE labeling, (c) representative graph showing the proliferation rate in Tconv cells alone (gray line) or with Treg-mediated exosomes (red line) in comparison to day 0 (dashed line), and (d) bar graph shows mean ± SEM of suppression of Tconv proliferation in MS patients and healthy controls. Mann-Whitney U test was used to analyze differences between the groups. *p value < 0.05
Treg-derived exosomes from patients induce conventional T cell apoptosis less than healthy controls
Survival of activated T cells is an important factor in determining the magnitude and duration of T cell responses both in physiological and pathological settings. To examine the effect of Treg-derived exosomes on the survival of Tconv cells, 1 × 105 Tconv cells were cultured for 3 days in the presence or absence of Treg-derived exosomes. We then evaluated the rate of early (AnnexinV+ cells) and late (PI+/AnnexinV+ cells) apoptosis induced by Treg-derived exosomes. Interestingly, as shown in Fig. 3, the frequency of cells in late stage of apoptosis was significantly lower in T cells receiving MS Treg-derived exosomes compared with cells treated with exosomes from healthy controls (0.68 ± 0.12 vs 1.29 ± 0.13; p value = 0.015). However, there was no significant difference in early apoptosis between groups (p value = 0.07). Therefore, it seems that the strength of Treg-derived exosomes to induce apoptosis in Tconv cells were less in MS patient compared to healthy controls.
Treg-derived exosomes from patients induce conventional T cell apoptosis less than exosomes from healthy controls. 1 × 105 Tconv cells were cultured for 3 days in the presence or absence of Treg-derived exosomes and the rate of apoptosis in Tconv cells was measured using Annexin V− Apoptosis Detection Kit. (a) Unstained cells, (b) cultured conventional T cells alone (untreated-Tconv), (c) cultured conventional T cells with Treg-exosomes (exosome-treated-Tconv), and (d) bar graph shows mean ± SEM of apoptosis of conventional T cells treated with exosomes from MS patients or healthy controls. Mann-Whitney U test was used to analyze differences between the groups. *p value < 0.05
Discussion
Regulatory T cells (Treg) are able to exert their immunosuppressive properties through various mechanisms such as immunomodulatory cytokines (e.g., IL-10 and TGF-β), expression of inhibitory receptors including cytotoxic T lymphocyte-associated protein 4 (CTLA-4) and glucocorticoid-induced TNFR-related protein (GITR) and cytolysis of target cells mediated by granzyme-perforin [11, 15]. Additionally, a number of microRNAs such as miR-10a and miR-146a express in Treg cells more than other T cell subtypes which have been documented as a critical player of Treg cell development and functions. miR-10a increases Foxp3 expression and stability of Tregs. miR-146a could suppress activation of Th1 through targeting Signal Transducer and Activator of Transcription 1 (STAT1) [31,32,33].
One recently reported mechanism of Treg activity is the release of exosomes, membrane-bound vesicles that contain different biomolecules particularly micrcoRNAs, originating from the cytoplasm of the mother cell [16,17,18]. In 2014, Okoye and colleagues showed that Treg cells were able to produce exosomes carrying unique set of microRNAs which reduced T cell proliferation and production of pro-inflammatory cytokines in vitro and in vivo. Any defect in the pathway of exosomes production/secretion would impair their immunomodulatory function. They also showed that Let-7d was overexpressed in Treg-derived exosomes, and Let7d deficient exosomes were compromised to suppress the inflammation in the animal model of colitis [25] As well, in a study by Yu et al., researchers showed that CD4+ CD25+ T cell-derived exosomes from transplant donors had a suppressive role in acute rejection and inhibited T cell proliferation [34]. Exosomes released by natural CD8+ CD25+ regulatory T cells could also diminish antitumor CD8+ T cell responses [35].
In the current study, we aimed to evaluate the immunomodulatory functions of Treg-derived exosomes from MS patients compared to healthy individuals. To this end, we isolated CD63+ exosomes from Treg cells of either healthy or diseased group and co-cultured them with conventional T cells (CD4+ CD25−). To our knowledge, for the first time, we showed that there was a deficiency in function of exosomes in MS patients. Treg-derived exosomes suppressed Tconv proliferation in MS patients less than healthy controls (8.04 vs. 12.5%). Moreover, Treg-derived exosomes from MS patients induced apoptosis in Tconv less than the control group (0.68 vs. 1.29%).
Defective immunomodulatory function of CD4+ CD25high Tregs has been previously reported in patients with MS [10, 36, 37]. However, the main causes of Treg cell defects are not completely understood. Various studies have shown that dysregulation of suppressive markers, e.g., CTLA-4 on Tregs and decreased expression of Foxp3, IL-10, TGF-β, and IL-35 could contribute to the pathogenesis and progression of the disease [38, 39]. Since transferring of biomolecules by exosomes known as one of the inhibitory mechanisms of Treg cells, hence the defect in function of these nano-vesicles in MS patients may be related to defect in expression of exosomal immunomodulatory molecules.
Alternatively, the dysfunction of MS patients’ exosomes to suppress Tconv cells may also be attributed to the resistance of pathogenic T cells in MS patients. Schneider et al. have shown that enhanced IL-6 signaling through the phosphorylation of signal transducer and activator of transcription 3 (pSTAT3) increased the resistance of effector T cells to regulation by Tregs in RRMS [40]. Another study has also demonstrated that, effector CD4+ T cells are refractory to Treg induced proliferation suppression [41].
The exact molecular changes in production or functions of MS patients’ exosomes need to be investigated. Intriguingly, Verderio et al. demonstrated that level of circulating exosomes was increased in experimental autoimmune encephalomyelitis (EAE); they also showed that pro-inflammatory cytokines stimulate immune cells to secret pro-inflammatory molecules containing exosomes [42]. In 2017, Kimura et al. have demonstrated that the plasma exosomes of MS patients transferred let-7i, an inflammatory microRNA, to CD4+ T cells. The transferred let-7i targeting the insulin like growth factor 1 receptor (IGF1R) and transforming growth factor β receptor 1 (TGFBR1), lead to the inhibition of Treg cell differentiation. These investigations indicate that exosomes might expand inflammation in MS patients [43]. Thus, it is conceivable that patients’ exosomes could be manipulated to correct their molecular content and the modified exosomes could be used as a therapeutic vehicle for the treatment of patients. Indeed, manipulated exosomes have been previously used in the context of cancer. In a study in 2016, Rivoltini and colleagues produced TRAIL+ exosomes by transducing K562 cells with lentiviral vectors encoding human TRAIL. These armed exosomes could induce a higher degree of apoptosis in lymphoma and melanoma cells [44]. In another study, Mu et al. enhanced inhibitory function of CD8+25+ Tregs by arming them with ovalbumin (OVA)-specific exosomal peptid-MHC complexes [23]. Similar modifications on Treg-derived exosomes from patients affected by MS or other neuroinflammatory diseases might open up new therapeutic windows for these disorders.
Conclusion
Altogether, we found that Treg-derived exosomes in MS patients were defective in modulating the proliferation and survival of conventional T cells; however, those of healthy subject could suppress the proliferation and survival of conventional T cells. The cause of the exosomal defect in immune regulation is remained to investigate. Manipulation of patients’ Treg-derived exosomes to restore their suppressive activity might be considered as a potential therapeutic approach for autoimmune diseases.
References
Frischer JM, Bramow S, Dal-Bianco A, Lucchinetti CF, Rauschka H, Schmidbauer M, et al. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain. 2009;132(5):1175–89.
Geurts JJ, Barkhof F. Grey matter pathology in multiple sclerosis. Lancet Neurol. 2008;7(9):841–51.
Ghabaee M, Bayati A, Saroukolaei SA, Sahraian MA, Sanaati MH, Karimi P, et al. Analysis of HLA DR2&DQ6 (DRB1* 1501, DQA1* 0102, DQB1* 0602) haplotypes in Iranian patients with multiple sclerosis. Cell Mol Neurobiol. 2009;29(1):109–14.
Harris VK, Sadiq SA. Biomarkers of therapeutic response in multiple sclerosis: current status. Molecular Diagnosis & Therapy. 2014;18(6):605–17. https://doi.org/10.1007/s40291-014-0117-0.
Lassmann H, van Horssen J, Mahad D. Progressive multiple sclerosis: pathology and pathogenesis. Nat Rev Neurol. 2012;8(11):647–56.
Popescu BFG, Lucchinetti CF. Pathology of demyelinating diseases. Annual Review of Pathology: Mechanisms of Disease. 2012;7:185–217.
Salehi Z, Doosti R, Beheshti M, Janzamin E, Sahraian MA, Izad M. Differential frequency of CD8+ T cell subsets in multiple sclerosis patients with various clinical patterns. PLoS One. 2016;11(7):e0159565.
Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM, et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol. 2008;172(1):146–55.
Frisullo G, Nociti V, Iorio R, Patanella AK, Marti A, Caggiula M, et al. IL17 and IFNγ production by peripheral blood mononuclear cells from clinically isolated syndrome to secondary progressive multiple sclerosis. Cytokine. 2008;44(1):22–5.
Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+ CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199(7):971–9.
Kitz A, Singer E, Hafler D. Regulatory T cells: from discovery to autoimmunity. Cold Spring Harbor Perspectives in Medicine. 2018:a029041.
Haas J, Korporal M, Balint B, Fritzsching B, Schwarz A, Wildemann B. Glatiramer acetate improves regulatory T-cell function by expansion of naive CD4+ CD25+ FOXP3+ CD31+ T-cells in patients with multiple sclerosis. J Neuroimmunol. 2009;216(1):113–7.
Namdar A, Nikbin B, Ghabaee M, Bayati A, Izad M. Effect of IFN-beta therapy on the frequency and function of CD4(+)CD25(+) regulatory T cells and Foxp3 gene expression in relapsing-remitting multiple sclerosis (RRMS): a preliminary study. J Neuroimmunol. 2010;218(1–2):120–4. https://doi.org/10.1016/j.jneuroim.2009.10.013.
Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30(5):636–45. https://doi.org/10.1016/j.immuni.2009.04.010.
Azimi M, Aslani S, Mortezagholi S, Salek A, Javan MR, Rezaiemanesh A, et al. Identification, isolation, and functional assay of regulatory T cells. Immunol Investig. 2016;45(7):584–602. https://doi.org/10.1080/08820139.2016.1193869.
Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–79.
Bobrie A, Colombo M, Raposo G, Théry C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12(12):1659–68.
Gutiérrez-Vázquez C, Villarroya-Beltri C, Mittelbrunn M, Sánchez-Madrid F. Transfer of extracellular vesicles during immune cell-cell interactions. Immunol Rev. 2013;251(1):125–42.
Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–89.
Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–9. https://doi.org/10.1038/ncb1596.
Ekstrom K, Valadi H, Sjostrand M, Malmhall C, Bossios A, Eldh M, et al. Characterization of mRNA and microRNA in human mast cell-derived exosomes and their transfer to other mast cells and blood CD34 progenitor cells. J Extracell Vesicles. 2012;1 https://doi.org/10.3402/jev.v1i0.18389.
Mittelbrunn M, Gutiérrez-Vázquez C, Villarroya-Beltri C, González S, Sánchez-Cabo F, González MÁ, et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2:282.
Mu C, Zhang X, Wang L, Xu A, Ahmed KA, Pang X, et al. Enhanced suppression of polyclonal CD8+ 25+ regulatory T cells via exosomal arming of antigen-specific peptide/MHC complexes. J Leukoc Biol. 2017;101(5):1221–31.
Smyth LA, Ratnasothy K, Tsang JY, Boardman D, Warley A, Lechler R, et al. CD73 expression on extracellular vesicles derived from CD4+ CD25+ Foxp3+ T cells contributes to their regulatory function. Eur J Immunol. 2013;43(9):2430–40. https://doi.org/10.1002/eji.201242909.
Okoye Isobel S, Coomes Stephanie M, Pelly Victoria S, Czieso S, Papayannopoulos V, Tolmachova T, et al. MicroRNA-containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity. 2014;41(1):89–103. https://doi.org/10.1016/j.immuni.2014.05.019.
Goetzl EJ, Boxer A, Schwartz JB, Abner EL, Petersen RC, Miller BL, et al. Altered lysosomal proteins in neural-derived plasma exosomes in preclinical Alzheimer disease. Neurology. 2015;85(1):40–7.
Manterola L, Guruceaga E, Gallego Perez-Larraya J, Gonzalez-Huarriz M, Jauregui P, Tejada S, et al. A small noncoding RNA signature found in exosomes of GBM patient serum as a diagnostic tool. Neuro-Oncology. 2014;16(4):520–7. https://doi.org/10.1093/neuonc/not218.
Kanninen KM, Bister N, Koistinaho J, Malm T. Exosomes as new diagnostic tools in CNS diseases. Biochim Biophys Acta. 2016;1862(3):403–10. https://doi.org/10.1016/j.bbadis.2015.09.020.
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302. https://doi.org/10.1002/ana.22366.
Long AE, Tatum M, Mikacenic C, Buckner JH. A novel and rapid method to quantify Treg mediated suppression of CD4 T cells. J Immunol Methods. 2017;449:15–22. https://doi.org/10.1016/j.jim.2017.06.009.
Lu L-F, Boldin MP, Chaudhry A, Lin L-L, Taganov KD, Hanada T, et al. Function of miR-146a in controlling Treg cell-mediated regulation of Th1 responses. Cell. 2010;142(6):914–29. https://doi.org/10.1016/j.cell.2010.08.012.
Jeker LT, Bluestone JA. MicroRNA regulation of T-cell differentiation and function. Immunol Rev. 2013;253(1):65–81. https://doi.org/10.1111/imr.12061.
Jeker LT, Zhou X, Gershberg K, de Kouchkovsky D, Morar MM, Stadthagen G, et al. MicroRNA 10a marks regulatory T cells. PLoS One. 2012;7(5):e36684. https://doi.org/10.1371/journal.pone.0036684.
Yu X, Huang C, Song B, Xiao Y, Fang M, Feng J, et al. CD4+CD25+ regulatory T cells-derived exosomes prolonged kidney allograft survival in a rat model. Cell Immunol. 2013;285(1–2):62–8. https://doi.org/10.1016/j.cellimm.2013.06.010.
Xie Y, Zhang X, Zhao T, Li W, Xiang J. Natural CD8+ 25+ regulatory T cell-secreted exosomes capable of suppressing cytotoxic T lymphocyte-mediated immunity against B16 melanoma. Biochem Biophys Res Commun. 2013;438(1):152–5.
Venken K, Hellings N, Broekmans T, Hensen K, Rummens J-L, Stinissen P. Natural naive CD4+ CD25+ CD127low regulatory T cell (Treg) development and function are disturbed in multiple sclerosis patients: recovery of memory Treg homeostasis during disease progression. J Immunol. 2008;180(9):6411–20.
Venken K, Hellings N, Liblau R, Stinissen P. Disturbed regulatory T cell homeostasis in multiple sclerosis. Trends Mol Med. 2010;16(2):58–68.
Buckner JH. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat Rev Immunol. 2010;10(12):849–59. https://doi.org/10.1038/nri2889.
Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8(7):523–32. https://doi.org/10.1038/nri2343.
Schneider A, Long SA, Cerosaletti K, Ni CT, Samuels P, Kita M, et al. In active relapsing-remitting multiple sclerosis, effector T cell resistance to adaptive Tregs involves IL-6–mediated signaling. Sci Transl Med. 2013;5(170):170ra15.
Bhela S, Kempsell C, Manohar M, Dominguez-Villar M, Griffin R, Bhatt P, et al. Nonapoptotic and extracellular activity of granzyme B mediates resistance to regulatory T cell (Treg) suppression by HLA-DR-CD25hiCD127lo Tregs in multiple sclerosis and in response to IL-6. J Immunol. 2015;194(5):2180–9. https://doi.org/10.4049/jimmunol.1303257.
Verderio C, Muzio L, Turola E, Bergami A, Novellino L, Ruffini F, et al. Myeloid microvesicles are a marker and therapeutic target for neuroinflammation. Ann Neurol. 2012;72(4):610–24. https://doi.org/10.1002/ana.23627.
Kimura K, Hohjoh H, Fukuoka M, Sato W, Oki S, Tomi C, et al. Circulating exosomes suppress the induction of regulatory T cells via let-7i in multiple sclerosis. Nat Commun. 2018;9(1):17. https://doi.org/10.1038/s41467-017-02406-2.
Rivoltini L, Chiodoni C, Squarcina P, Tortoreto M, Villa A, Vergani B, et al. TNF-related apoptosis-inducing ligand (TRAIL)–armed exosomes deliver proapoptotic signals to tumor site. Clin Cancer Res. 2016;22(14):3499–512.
Funding
This study was supported by Tehran University of Medical Sciences grant No.9403-30-30075. Authors appreciate technical help from technicians at Department of Immunology at Tehran University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by ethics committee of Tehran University of Medical Sciences (IR.TUMS.REC.1394.1551) and written informed consent was obtained from each study subject prior to the study.
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Azimi, M., Ghabaee, M., Moghadasi, A.N. et al. Immunomodulatory function of Treg-derived exosomes is impaired in patients with relapsing-remitting multiple sclerosis. Immunol Res 66, 513–520 (2018). https://doi.org/10.1007/s12026-018-9008-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-018-9008-5