Abstract
Dysregulation of the immune system and impairment in the function and number of patient-derived regulatory T cells (Treg) have an important role in multiple sclerosis (MS) pathogenesis. MS patients still receive different medications to overcome the relapses and to slow the disease progression. However, the benefits of these therapies are limited and are accompanied by different side effects. The immunoregulatory effects of Silymarin as a plant-derived flavonoid have shown in studies. In the present study, regulatory T cells (Tregs) were isolated from MS patients who diagnosed as new cases and IFN-β-treated RRMS patients. Isolated Treg cells were cultured in the presence of different concentrations of Silymarin (50, 100, 150 μM) for 48, 72, and 120 h. Proliferation and activation of Treg cells were assessed by flow cytometry. Also, FOXP3, JAK3, and STAT5 gene expression, IL-10, and TGF-β production by Tregs were evaluated by real-time PCR and ELISA respectively. The results showed that Silymarin promoted Treg proliferation at 100 μM concentration after 72 h. Additionally, IL-10, TGF-β levels, and FOXP3, JAK3, and STAT5 gene expression enhanced by Silymarin dose and time dependently. Our preliminary results suggest that the induction and activation of Tregs could be an underlying mechanism of the ancient used herbal medicine Silymarin, providing effective means against autoimmune and inflammatory diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Multiple sclerosis (MS) is a neurodegenerative disease in which autoreactive immune responses to autoantigens in the central nervous system (CNS) affect the neuromuscular junction (NMJ). In addition to the inflammatory response in CNS, immune dysregulation has indicated in these patients [1]. Migration and infiltration of autoreactive T cells especially Th1 and Th17 cells from the periphery into the CNS is accompanied with autoimmune responses [2]. Additionally, impairment in the regulatory function of regulatory T cells (Tregs) plays the strategic role in MS pathogenesis [3]. Accordingly, an imbalance between T cells and Treg cells has demonstrated in autoimmune disorders such as MS [4]. In fact, Tregs (CD4+, CD25+, FOXP3+) are the main players in the suppression of autoimmune responses [5]. Interestingly, transdifferentiation of Tregs into inflammatory cells (exTreg) such as Th17 cells has indicated in autoimmune condition [6]. Tregs dysfunction and/or Tregs differentiation into exTreg is associated with autoimmune diseases, such as systemic lupus erythematosus (SLE), multiple sclerosis (MS), and ankylosing spondylitis (AS) [7]. In Tregs activation trough IL-2 receptor, JAK3/STAT5 pathway plays a crucial role in the conservation and induction of Foxp3 expression in Treg cells as a specific transcription factor [8]. Kinases of the Jak (“Janus kinase”) family and transcription factors (TFs) of the STAT (“signal transducer and activator of transcription”) family comprise a fast signaling module that affects the different phases of the immune system [9].The increase of STAT5 expression is accompanied by the high frequency of Tregs and the decrease of Th17 cells [10]. Also, it is suggested that STAT5 activation is needed for both the development and function of Tregs [11]. Treg cells exert their functions via cell-cell interactions and/or the production of anti-inflammatory cytokines including interleukin-10 (IL-10) and transforming growth factor-beta (TGF-β) [12]. IL-10 is involved in the suppression of Th17 cells by Tregs and the role of interleukin-10 (IL-10) producing has indicated before [13]. In addition, TGF-β plays the main role in the induction of Foxp3+ T regulatory cells and inhibits inflammatory responses by suppressing of inflammatory immune cells and/or raising the function of Tregs [14]. Also, deficiency of TGF-β and its signaling has correlated with the onset of the autoimmune disease like MS [15, 16].
MS patients still receive different medications to overcome the relapses and to slow the disease progression including anti-inflammatory agents (corticosteroids), interferon and interferon beta, and monoclonal antibodies [17, 18]. However, the benefits of these therapies are limited and are accompanied with different side effects, like influenza-like syndrome and self-limited impression [19]. Silybum marianum or milk thistle is a plant-derive flavonoid with an ancient history. The milk thistle extracts are prepared from its fruits contain 30–65% Silymarin as an active component [20]. The antioxidant activity of Silymarin and its hepatoprotective effects have demonstrated in previous studies. Furthermore, Silymarin suppresses the inflammatory reactions and hepatic fibrogenesis. Also, its beneficial effect on the equilibrium of the cell viability and apoptosis has been shown [21]. Silymarin exerts both immunostimulatory and immunosuppression activities dose and time dependently. The anti-inflammatory impact of Silymarin exerts by the suppression of NF-κB transcription factor. Also, immunostimulatory and immunosuppression effects of Silymarin on T cells have indicated in high doses and low doses respectively [22]. Recently, in a study in our department, Shajanian et al. have found Treg cell population and Foxp3 gene expression increased in the presence of Silymarin compared to rapamycin [23]. In recent years, application of nanocarrier systems such as liposomes, cyclodextrin complexes, self-emulsifying, and nanoparticles for targeting treatments has been developed which improve bioavailability, biodistribution, and therapeutic potency of natural compounds [24,25,26]. Accordingly, in the present study, we investigated the impact of Silymarin on the proliferation and function of Treg cells isolated from MS patients by assessment the expression of FOXP3, JAK3, and STAT5 genes and measuring IL-10 and TGF-β release by these cells.
MATERIALS AND METHODS
Patients and Sample Collection
Seven new MS cases (5 females and 2 males) and 9 relapsing remitting (RR) MS patients (6 females and 3 males) who received IFN-β for the last 6 months were recruited into the study. Written informed consent was received from all patients and the research protocols were confirmed by the Ethics Committee of Isfahan University of Medical Sciences, Isfahan, Iran. MS patients with an age ranging between 29 and 48 years (mean 39.2 ± 6.7) diagnosed according to the revised McDonald criteria [27]. In IFN-β-treated group blood samples were accumulated 24–48 h after IFN-β injection. Also, patients treated with anti-inflammatory and other immunosuppressive drugs, pregnant patients, and patients with other inflammatory diseases were eliminated from the study. Twenty milliliters of peripheral blood samples was collected from all the subjects and peripheral blood mononuclear cells (PBMCs) were separated using the Ficoll-Hypaque isolation method.
Preparation of Silymarin
To prepare a 100 mM stock solution of Silymarin, its powder (Sigma, S0292, USA) was dissolved in 100% dimethyl sulphoxide (DMSO). The stock solution was divided into micro tubes and stored at − 20 °C for no longer than 30 days. Letter dilutions were prepared in RPMI medium in order to provide Silymarin with the 50, 100, and 150 μM concentrations.
Apoptosis Detection
The apoptosis-inducing effects of Silymarin on PBMCs were evaluated using the Annexin FITC/PI double staining apoptosis detection kit (BD Biosciences, Waltham, MA). The cells were cultured for 48, 72, and 120 h at the presence of 50, 100, and 150 μM Silymarin concentrations. Cells were collected, washed with PBS, and then suspended cells in binding buffer. The cells were stained with FITC Annexin V and PI (5 μl) and incubated 20 min at room temperature in a dark place. The analyze of cells was performed by a FACS Calibur flow cytometer (Becton Dickinson, San Jose, CA, USA).
Treg Cells Isolation, Stimulation, and Culture with Silymarin
Regulatory T cells (Tregs) were isolated from PBMCs using a CD4+CD25+CD127dim/− Regulatory T cell Immunomagnetic Isolation Kit (EasySep™) according to the manufacturer instructions. Briefly, in the first step, CD25+ cells were isolated by positive selection and then non-Treg cells were removed. Treg cells purification was determined by flow cytometry and the percentage of CD4+, CD25+, and Foxp3+ cells was > 93% (data not shown). After Treg isolation, 104 cells were labeled with CFSE and activated with plate-bound anti-CD3 monoclonal antibody (mAb) OKT-3 (eBioscience, San Diego, CA, USA) at 5 μg/ml and soluble anti-CD28 mAb (eBioscience) at 2 μg/ml in 96-well tissue culture plates. After 18 h, the cells were stimulated by IL-2 and simultaneously, cells were treated with various concentrations of Silymarin (50, 100, 150 μM) for different incubation times (48, 72, and 120 h) in RPMI 1640 supplemented with 10% FBS, penicillin (100 U/ml), and streptomycin (100 μg/ml) under standard culture conditions (37 °C, 95% humidified atmosphere with 5% CO2). The control of experiments had similar culture conditions and the cells were treated with DMSO alone instead of Silymarin. The final concentration of DMSO in all experiments was less than 1%. All experiments were repeated at least three times separately.
Flow Cytometry
For the analysis of Treg cell activation, the PE-conjugated anti-CD69 monoclonal antibody (eBioscience, USA) and matched isotype control (eBioscience, USA) were used. Also, as mentioned before for the proliferation assay, Treg cells (104/well) were stained with 1 μM CFSE (Invitrogen) before culture initiation. After 48, 72, and 120 h of culture, cells were collected, incubated with antibodies, and dilution of CFSE and CD69 expression were evaluated by flow cytometry.
Cytokine Assays
Supernatants of cultured cells were collected after 48, 72, and 120 h and stored at − 20 °C. The IL-10 and TGF-β concentrations were measured with the use of ELISA kit (PeproTech® EC Ltd., UK and R&D Systems, Minneapolis, MN, USA, respectively).
Real-time PCR
RT-PCR analysis of Foxp3, JAK3, and STAT5 was performed using the SYBR Green Master Mix protocol (Thermo Fisher Scientific). Total RNA extracted was extracted with Yekta tajhiz azma kit (Yekta tajhiz azma Co., Iran). Reverse transcription of RNA was carried out by the Biosystems kit. Real-time PCR was performed in duplicate on the7900HT fast real-time PCR system (Applied Biosystems) with 1 ng of cDNA. The reaction mixture of a 10-μl final volume was contained 1 μl of diluted cDNA, 0.2 μl of each pair oligonucleotide primers, 3.8 μl water, and 5 μl SYBR Green Master Mix (Thermo Fisher Scientific). Real-time PCR was performed with the following cycling conditions: 1 cycle of denaturation at 95 °C for 10 min, followed by a 40-cycle amplification consisting of denaturation at 95 °C for 15 s, and extension at 60 °C for 60 s. Following the last cycle, melting curves analysis was generated to verify qPCR product. Primers used to quantify FOXP3 mRNA were 5′-CAC CTG GAA GAA CGC CAT CC-3′ (forward) and 3′-CTC ATC CAC GGT CCA CAC AG-5′ (reverse), JAK3 mRNA were 5′-CAGCAGCACATGCAGGG-3′ (forward) and 3′-CCTTCGAAAGTCCAGGGTC-5′ (reverse); STAT5 mRNA were 5′-CCTCCAGAGACACCTGCTTC-3′ (forward) and 3′-CCAGTACCAGGAGAGCCTGA-5′ (reverse). The gene levels were normalized with those of GAPDH as an internal control, and the relative expression levels were assessed using the 2−ΔΔCt method.
Statistical Analysis
GraphPad Prism version 6.0 (GraphPad Software, Inc., San Diego, CA) and SPSS version 21.0 were used for statistical analyses. Statistical differences between control and experimental groups were assessed by one-way analysis of variance (ANOVA) and multivariate analysis of variance (MANOVA) with post-Dunnett’s multiple comparisons test. Data were accounted as mean ± SEM for replicate values.
RESULTS
The Effect of Silymarin on Cell Death in Peripheral Blood Mononuclear Cells
To explore the effect of Silymarin on PBMCs apoptosis, Annexin V/PI staining and flow cytometry were performed in our investigation. PBMCs were treated with Silymarin at 50, 100, and 150 μM concentrations for 48, 72, and 120 h. As shown in Fig. 1, there were no significant differences between values for apoptosis (early apoptosis plus late apoptosis) in treatment with different concentrations of Silymarin and DMSO control after 48, 72, and 120 h (P ≥ 0.05).
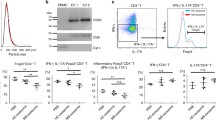
Effect of Silymarin on CD69 Expression on Treg Cells Isolated from Newly Diagnosed and IFN-β-Treated RRMS Patients
To identify the impact of Silymarin on Treg cell activation, CD69 expression as an early activation marker was assessed after treatment the cells with Silymarin at 50, 100, and 150 μM concentrations for 48, 72, and 120 h by flow cytometry (Fig. 2a). In new cases subjects, CD69 expression was higher compared to DMSO control at 50 and 150 μM Silymarin concentrations, but this increase was not statistically significant (P ≥ 0.05). Also, in IFN-β-treated RRMS patients, there was no statistically significant difference in expression of CD69 between different groups after 48-, 72-, and 120-h treatment with 50, 100, and 150 μM concentrations of Silymarin compared with the DMSO control (P ≥ 0.05) (Fig. 2b, c).
Silymarin promotes Treg cell activation in vitro. a Histogram plots of Treg cells following of the co-culture of these cells and Silymarin (50, 100, 150 μM). After 48, 72, and 120 h of culture, cells were harvested and stained with anti-CD69 monoclonal antibody and analyzed by flow cytometry. Graphical representation of the percentage of CD69+ Treg cells isolated from newly diagnosed (b) and IFN-β-treated RRMS patients (c). Data were pooled from three independent experiments and expressed as means ± SEM.
Effect of Silymarin on Treg Cells Proliferation in Newly Diagnosed and IFN-β-Treated RRMS Patients
To determine the effect of Silymarin on the proliferation of Treg cells, we treated the cells isolated from new cases and IFN-β-treated RRMS patients, with Silymarin at 50, 100, and 150 μM concentrations for 48, 72, and 120 h, then analyzed the impact on proliferation by CFSE assay through flow cytometry (Fig. 3a). In new cases MS patients, at the presence of 100 μM of Silymarin after 72 h, Treg cells proliferation was significantly increased compared to DMSO control (P = 0.0377). However, there was no significant difference between groups in the proliferation of Treg cells after 48 and 120 h (P ≥ 0.05) (Fig. 3b).
Silymarin effects on Treg cells proliferation isolated from MS patients. a CFSE histogram plots of Treg cells following of the co-culture of these cells and Silymarin (50, 100, 150 μM). Following 48, 72, and 120 h of culture, cells were harvested and analyzed by flow cytometry to identify proliferating cell populations. CFSE histograms depict the number of events (y-axis) and the fluorescence intensity (x-axis), with proliferating cells displaying a progressive twofold loss in fluorescence intensity following cell division, indicative of proliferating cells. Graphical representation of the proliferating Treg cells isolated from newly diagnosed (b) and IFN-β-treated RRMS patients (c). Treg cells following 48, 72, and 120 h of culture in the presence of DMSO (control) or in the presence of three concentrations of Silymarin (50, 100, 150 μM). Data were pooled from three independent experiments. *P < 0.05 indicates statistically significant differences between DMSO and Silymarin co-culture conditions, using a one-way analysis of variance (ANOVA).
In IFN-β-treated RRMS patients, there was no significant difference in proliferation of Treg cells between different groups after 48, 72, and 120 h (P ≥ 0.05) (Fig. 3c).
Effect of Silymarin on FOXP3 Gene Expression in Treg Cells Isolated from Newly Diagnosed and IFN-β-Treated RRMS Patients
FOXP3 transcript expression was assessed by real-time PCR technique. FOXP3 levels in new cases were increased significantly after 72-h treatment with 100 μM concentration of Silymarin compared to DMSO control (P = 0.0003), but FOXP3 expression was not significantly changed after 48 and 120 h of exposure with different concentrations of Silymarin (P ≥ 0.05) (Fig. 4a).
Silymarin effects on FOXP3 gene expression in Treg cells isolated from MS patients. Relative gene expression of FOXP3 gene was measured by RT-qPCR in Treg cells isolated from newly diagnosed (a) and IFN-β-treated RRMS patients (b) at different doses of Silymarin (50, 100, 150 μM) after 48, 72, and 120 h. Data were pooled from three independent experiments and expressed as means ± SEM. ***P < 0.001 was determined by one-way ANOVA.
Also, in IFN-β-treated RRMS patients, FOXP3 gene expression was increased in treatment with100 μM concentration of Silymarin in comparison with DMSO control but it was not statistically significant (P ≥ 0.05) (Fig. 4b).
Effect of Silymarin on JAK3 Gene Expression in Treg Cells Isolated from Newly Diagnosed and IFN-β-Treated RRMS Patients
We also assessed JAK3 gene expression as an important signaling molecule in Treg cell activation. The expression of JAK3 gene in the Treg cells after 48, 72, and 120 h was evaluated. JAK3 expression in new cases was increased significantly after 48- and 72-h treatment with 50, 100, and 150 μM concentrations of Silymarin respectively (P = 0.0213, P < 0.0001), but it was not significantly changed after 120 h of exposure with different concentrations of Silymarin (P ≥ 0.05) (Fig. 5a). Also, the significant increase in JAK3 gene expression was observed among IFN-β-treated RRMS patients with 150 μM concentration of Silymarin after 120 h (P = 0.0445) (Fig. 5b).
Silymarin effects on JAK3 gene expression in Treg cells isolated from MS patients. Relative gene expression of JAK3 gene was measured by RT-qPCR in Treg cells isolated from newly diagnosed (a) and IFN-β-treated RRMS patients (b) at different doses of Silymarin (50, 100, 150 μM) after 48, 72, and 120 h. Data were pooled from three independent experiments and expressed as means ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 were determined by one-way ANOVA.
Effect of Silymarin on STAT5 Gene Expression in Treg Cells Isolated from Newly Diagnosed and IFN-β-Treated RRMS Patients
STAT5 promotes Treg development so we also determined the effects of Silymarin on STAT5 gene expression. In new cases, there was no statistically significant difference in expression of STAT5 gene between different groups after 48 and 120 h (P ≥ 0.05), but STAT5 expression was significantly increased compared to DMSO control after 72 h of exposure with 100, 150 μM concentrations of Silymarin (P < 0.0001) (Fig. 6a).
Silymarin effects on STAT5 gene expression in Treg cells isolated from MS patients. Relative gene expression of STAT5 gene was measured by RT-qPCR in Treg cells isolated from newly diagnosed (a) and IFN-β-treated RRMS patients (b) at different doses of Silymarin (50, 100, 150 μM) after 48, 72, and 120 h. Data were pooled from three independent experiments and expressed as means ± SEM. *P < 0.05 and ***P < 0.001 were determined by one-way ANOVA.
Additionally, in IFN-β-treated RRMS patients, there was no significant difference in expression of STAT5 gene between different groups after 48 and 72 h (P ≥ 0.05); however, it was upregulated with different concentrations of Silymarin (50, 100, 150 μM) after 120 h (P = 0.0097) (Fig. 6b).
Effect of Silymarin on IL-10 Production by Treg Cells Isolated from Newly Diagnosed and IFN-β-Treated RRMS Patients
IL-10 as a suppressive cytokine is produced by Treg cells and we assessed IL-10 release by Treg cells after treatment with Silymarin. In new cases, after 48 h of Treg cells treated with concentrations of 50, 100, and 150 μM of Silymarin, the IL-10 concentration was significantly increased at 150 μM (P = 0.0206) compared to DMSO. However, after 72 and 120 h, Treg cells treatment with 50, 100, and 150 μM Silymarin, the level of IL-10 cytokine was not significantly different from that of the DMSO control (P ≥ 0.05) (Fig. 7a). In INF-β-treated subjects, after 48 h of Treg cells treatment with Silymarin, IL-10 concentration was significantly increased at 100 μM (P = 0.0241) compared to DMSO control, while after 72 and 120 h, Treg cells treatment with 50, 100, and 150 μM Silymarin, no significant differences were found between groups and the DMSO (P ≥ 0.05) (Fig. 7b).
Silymarin effects on IL-10 production by Treg cells isolated from newly diagnosed (a) and IFN-β-treated RRMS patients (b). ELISA measurements of IL10 concentrations in conditioned supernatants collected from co-cultures of Treg cells with different doses of Silymarin (50, 100, 150 μM) after 48, 72, and 120 h. Data were pooled from three independent experiments and calculated as the average cytokine concentration from duplicate wells and expressed as means ± SEM. *P < 0.05 was determined by one-way ANOVA.
Effect of Silymarin on TGF-β Production by Treg Cells Isolated from Newly Diagnosed and IFN-β-Treated RRMS Patients
In order to assess Silymarin impacts on TGF-β production in newly diagnosed and IFN-β-treated RRMS patients, we treated the Treg cells with Silymarin at 50, 100, and 150 μM concentrations for 48, 72, and 120 h and the TGF-β level was measured in the culture supernatants. In new cases, TGF-β level was significantly increased after 72-h treatment with 150 μM Silymarin compared to DMSO control (P = 0.0083) but after 48 and 120 h, TGF-β level was not significantly different in treatment with 50, 100, and 150 μM Silymarin in comparison to DMSO control (P ≥ 0.05) (Fig. 8a). Also, the TGF-β level was increased in IFN-β-treated RRMS patients after 72 and 120 h in treatment with Silymarin (100,150 and 50,150 μM respectively) (P = 0.0485, P = 0.015). Also, no significant difference of TGF-β concentration was found after 48-h treatment with different concentrations of Silymarin compared to DMSO (P ≥ 0.05) (Fig. 8b).
Silymarin effects on TGF-β release by Treg cells isolated from newly diagnosed (a) and IFN-β-treated RRMS patients (b). ELISA measurements of TGF-β concentrations in conditioned supernatants collected from co-cultures of Treg cells with different doses of Silymarin (50, 100, 150 μM) after 48, 72, and 120 h. Data were pooled from three independent experiments and calculated as the average cytokine concentration from duplicate wells and expressed as means ± SEM. *P < 0.05, **P < 0.01 was determined by one-way ANOVA.
DISCUSSION
In this study, we observed that Silymarin, a natural flavonoid extracted from Silybum marianum, could restore Treg cells function isolated from MS patients. Immunomodulatory effects of Silymarin and its impacts on different signaling pathways have been reported in previous studies [22]. Here, we found that CD69 expression was higher in the presence of 50 and 150 μM Silymarin concentrations after 72 h compared to DMSO control in newly diagnosed MS patients; however, this increase was not statistically significant. CD69 is a membrane receptor which usually considered as the earliest activation marker on leukocytes [28, 29]. Dual aspects of CD69 function have indicated in previous studies, some studies suggesting that CD69 is involved in pro-inflammatory responses while another study by Sancho et al. has shown CD69 may exert a regulatory function in immune responses [30, 31] [32]. Accordingly, CD69 deficiency has revealed severe autoreactive immune responses and also increased the induction of antitumor responses [32] [33]. Esplugues et al. have shown CD69 deficiency decreased TGF-β production, so they have suggested a close relation between CD69 and TGF-β production [33]. Also, Han et al. have indicated CD69+ T cells suppress T cells proliferation through the high expression of membrane-bound TGF-β1 [34]. Additionally, higher expression of inhibitory surface markers has indicated in FOXP3+CD69+ Treg cells in comparison to FOXP3+CD69− Treg cells [35].
Consistent with the findings above, we found that along with the increase in CD69 expression after 72-h treatment with Silymarin (50 and 150 μM), TGF-β production also increased. Accordingly, it seems that Silymarin increases the activity of regulatory T cells in newly diagnosed MS patients in vitro. However, additional experiments need to be performed to assess the mechanisms by Silymarin in Treg cell activation. Also, we observed in IFN-β-treated RRMS patients, CD69 expression was not significantly different among groups which treated with Silymarin. The previous report has indicated that in vitro and in vivo treatment with type I interferons (IFN-α/β) did not induce CD69 expression on T cells [36]. Consequently, it can be concluded that no significant difference in CD69 expression in this group may be related to the effects of IFN-β treatment.
Our experiments showed that Treg cells proliferation was significantly increased at the presence of 100 μM Silymarin concentration compared to DMSO control in newly diagnosed MS patients. Also, we found that the increase of CD69 expression was consistent with Treg cells proliferation in both groups. Almasi et al. have indicated T cells proliferation was decreased at the 100 μM and 200 μM concentrations of Silymarin after 5 days (120 h) [37]. However, we did not find the significant difference in Treg cells proliferation between groups after 120 h. This difference may be due to the use of pure Treg cell population in our study and their low frequency in peripheral blood. Therefore, according to our findings and previous studies, we can conclude that Silymarin exerts its immunoregulatory function dose and time dependently.
Our results highlight a previously effect of Silymarin on Treg cell activation to study the mechanisms by which Treg cells are induced and expanded in treatment with Silymarin. Shajanian et al. have reported Treg cell population and Foxp3 gene expression significantly increased in the culture of PBMCs isolated from healthy subjects with 100 μM Silymarin after 72 h and these increases were higher compared to rapamycin treatment [23]. Our study confirmed their findings and found Foxp3 gene expression was increased also in Treg cells isolated from MS patients after treatment with 100 μM Silymarin. Particularly, suppression of AKT and mTOR signaling pathways lead to Foxp3 expression and Treg cells expansion [38, 39]. Gharagozloo and her colleagues have shown Silymarin inhibits mTOR activity in activated T cells after 72 h [40]. These findings suggest that inhibition of mTOR signaling pathway by Silymarin is a mechanism for the increase of Foxp3 gene expression and Treg cell activation. In our study, we also found JAK3 and STAT5 gene expression were increased in Treg cells isolated from MS patients after treatment with Silymarin. Previous studies on mice have shown a deficiency of IL-2/CD122/JAK3/STAT5 signaling pathway is accompanied by the decrease in thymic and peripheral Treg cells [41, 42]. Goldstein et al. have suggested that Foxp3 turn over mostly related to JAK3 activity [8]. Also, studies have indicated the expression of a constitutively active form of STAT5 induces Foxp3 expression in Treg cells. In addition, interaction and the collaboration of mTOR and STAT5 pathways in Treg cells differentiation and expansion have been suggested. Accordingly, a study recently has indicated mTOR-deficient mice (T cell–specific TSC1-deficient mice and RICTOR-deficient mice) had a higher number of Treg cells which positively correlated with raise of STAT5 activation [43]. Also, rapamycin as a mTOR inhibitor enhances Treg cell population while STAT5 activity also is needed for Treg cells proliferation in rapamycin treatment [43]. Consequently, our results confirm the findings from previous studies and suggest that Silymarin probably exerts its effect on Foxp3 expression, Treg cell activation and, proliferation by regulation of two signaling pathways: (1) inhibition of mTOR and (2) activation of JAK3/STAT5 signaling pathways.
In contrast with Silymarin, type I IFN receptor activates mTOR pathways which induce IFN responses and consequently the biological effects of IFNs [44]. Therefore, no significant increase of Foxp3 gene expression in IFN-β-treated RRMS patients may be related to mTOR activation by IFN-β which compensates inhibitory effects of Silymarin. Also, the increase of JAK3 and STAT5 mRNA after 120 h may be due to the decrease of stimulatory effects of IFN-β on mTOR signaling. However, further researches are required to assess the complementary effects of Silymarin and IFN-β on Treg cell activation.
We also observed that Treg-associated anti-inflammatory cytokines (IL-10 and TGF-β) were increased in treatment with Silymarin dose and time dependently. Treg-dependent inhibitory effects might be established through a TGF-β-dependent mechanism [45]. TGF-β and IL-2 are necessary factors for the expression of Foxp3 and Treg cells induction by naïve T cells [46, 47]. Additionally, Chaudhry et al. have indicated IL-10 signaling in Treg cells contributes in the suppression of Th17 cell–mediated immune responses [13]. TGF-β levels have increased in MS patients after IFN-β1 therapy [16]. Therefore, the increase of TGF-β in IFN-β-treated RRMS patients in the present study probably induced by both Silymarin and IFN-β.
In our study, the high expression of Foxp3, JAK3/STAT5 signaling molecules, the increase of Treg proliferation, the activation, and anti-inflammatory cytokines production were observed in treatment with Silymarin which suggesting activating of JAK3/STAT5 signaling is another mechanism for Silymarin promoting Treg cells and suppressing inflammation. Different delivery systems have been used for increasing of bioavailability and dissolution rate of curcumin as a natural flavonoid [24, 48, 49]. Accordingly to the higher safety index of Silymarin and its beneficial therapeutic effects as an anti-inflammatory and immunomodulatory agent, we suggest using various delivery systems such as nanocarriers improves the therapeutic efficacy of Silymarin.
CONCLUSION
The crucial role of Treg cells in immune modulation has attracted great interest in immunology researches. The increase of Treg cells or delivery of Treg cells has been proved to be a potent therapeutic method for autoimmune and inflammatory diseases in different studies. Our findings suggest that Silymarin might serve as an achievable therapeutic agent for MS or other autoimmune disorders.
References
Danikowski, K.M., S. Jayaraman, and B.S. Prabhakar. 2017. Regulatory T cells in multiple sclerosis and myasthenia gravis. Journal of Neuroinflammation 14 (1): 117. https://doi.org/10.1186/s12974-017-0892-8.
Lee, M.J., J.H. Choi, S.J. Lee, and I.H. Cho. 2017. Oriental medicine Samhwangsasim-tang alleviates experimental autoimmune encephalomyelitis by suppressing Th1 cell responses and upregulating Treg cell responses. Frontiers in Pharmacology 8: 192. https://doi.org/10.3389/fphar.2017.00192.
Schloder, J., C. Berges, F. Luessi, and H. Jonuleit. 2017. Dimethyl fumarate therapy significantly improves the responsiveness of T cells in multiple sclerosis patients for immunoregulation by regulatory T cells. International Journal of Molecular Sciences 18 (2). https://doi.org/10.3390/ijms18020271.
George, M.M., K. Subramanian Vignesh, J.A. Landero Figueroa, J.A. Caruso, and G.S. Deepe Jr. 2016. Zinc induces dendritic cell tolerogenic phenotype and skews regulatory T cell-Th17 balance. Journal of Immunology 197 (5): 1864–1876. https://doi.org/10.4049/jimmunol.1600410.
Spence, A., J.E. Klementowicz, J.A. Bluestone, and Q. Tang. 2015. Targeting Treg signaling for the treatment of autoimmune diseases. Current Opinion in Immunology 37: 11–20. https://doi.org/10.1016/j.coi.2015.09.002.
Joller, N., and V.K. Kuchroo. 2014. Good guys gone bad: exTreg cells promote autoimmune arthritis. Nature Medicine 20 (1): 15–17. https://doi.org/10.1038/nm.3439.
Nie, J., Y.Y. Li, S.G. Zheng, A. Tsun, and B. Li. 2015. FOXP3(+) Treg cells and gender bias in autoimmune diseases. Frontiers in Immunology 6: 493. https://doi.org/10.3389/fimmu.2015.00493.
Goldstein, J.D., A. Burlion, B. Zaragoza, K. Sendeyo, J.K. Polansky, J. Huehn, E. Piaggio, B.L. Salomon, and G. Marodon. 2016. Inhibition of the JAK/STAT signaling pathway in regulatory T cells reveals a very dynamic regulation of Foxp3 expression. PLoS One 11 (4): e0153682. https://doi.org/10.1371/journal.pone.0153682.
Villarino, A.V., Y. Kanno, and J.J. O’Shea. 2017. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nature Immunology 18 (4): 374–384. https://doi.org/10.1038/ni.3691.
Zheng, Y., Z. Wang, L. Deng, G. Zhang, X. Yuan, L. Huang, W. Xu, and L. Shen. 2015. Modulation of STAT3 and STAT5 activity rectifies the imbalance of Th17 and Treg cells in patients with acute coronary syndrome. Clinical Immunology 157 (1): 65–77. https://doi.org/10.1016/j.clim.2014.12.012.
Owen, D.L., and M.A. Farrar. 2017. STAT5 and CD4 (+) T cell immunity. F1000Res 6: 32. https://doi.org/10.12688/f1000research.9838.1.
Levings, M.K., R. Bacchetta, U. Schulz, and M.G. Roncarolo. 2002. The role of IL-10 and TGF-beta in the differentiation and effector function of T regulatory cells. International Archives of Allergy and Immunology 129 (4): 263–276. https://doi.org/10.1159/000067596.
Chaudhry, A., R.M. Samstein, P. Treuting, Y. Liang, M.C. Pils, J.M. Heinrich, R.S. Jack, F.T. Wunderlich, J.C. Bruning, W. Muller, and A.Y. Rudensky. 2011. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity 34 (4): 566–578. https://doi.org/10.1016/j.immuni.2011.03.018.
Wan, Y.Y., and R.A. Flavell. 2007. Yin-Yang’ functions of transforming growth factor-beta and T regulatory cells in immune regulation. Immunological Reviews 220: 199–213. https://doi.org/10.1111/j.1600-065X.2007.00565.x.
Mokhtarian, F., Y. Shi, D. Shirazian, L. Morgante, A. Miller, and D. Grob. 1994. Defective production of anti-inflammatory cytokine, TGF-beta by T cell lines of patients with active multiple sclerosis. Journal of Immunology 152 (12): 6003–6010.
Mirshafiey, A., and M. Mohsenzadegan. 2009. TGF-beta as a promising option in the treatment of multiple sclerosis. Neuropharmacology 56 (6–7): 929–936. https://doi.org/10.1016/j.neuropharm.2009.02.007.
Mahdavian, S., U. Dike, A. Bryant, C. Davison, P. Ghazvini, and A. Hill. 2010. Multiple sclerosis: a supplement on the disease state, current therapies, and investigational treatments. Journal of Pharmacy Practice 23 (2): 91–100. https://doi.org/10.1177/0897190009360022.
Axisa, P.P., and D.A. Hafler. 2016. Multiple sclerosis: genetics, biomarkers, treatments. Current Opinion in Neurology 29 (3): 345–353. https://doi.org/10.1097/WCO.0000000000000319.
Landi, D., M. Albanese, F. Buttari, F. Monteleone, L. Boffa, S. Rossi, C. Motta, E. Puma, and D. Centonze. 2017. Management of flu-like syndrome with cetirizine in patients with relapsing-remitting multiple sclerosis during therapy with interferon beta: results of a randomized, cross-over, placebo-controlled pilot study. PLoS One 12 (7): e0165415. https://doi.org/10.1371/journal.pone.0165415.
Anthony, K.P., and M.A. Saleh. 2013. Free radical scavenging and antioxidant activities of silymarin components. Antioxidants (Basel) 2 (4): 398–407. https://doi.org/10.3390/antiox2040398.
Feher, J., and G. Lengyel. 2012. Silymarin in the prevention and treatment of liver diseases and primary liver cancer. Current Pharmaceutical Biotechnology 13 (1): 210–217.
Esmaeil, N., S.B. Anaraki, M. Gharagozloo, and B. Moayedi. 2017. Silymarin impacts on immune system as an immunomodulator: one key for many locks. International Immunopharmacology 50: 194–201. https://doi.org/10.1016/j.intimp.2017.06.030.
Sarineh Shajanian, M.G., Mazdak Ganjalikhani-Hakemi, and M. Rafiee. 2015. Comparing in-vitro effects of two immunosuppressive drugs on the expression of Foxp3 from naïve CD4+ T cells. Journal of Isfahan Medical School 33 (329): 457–466.
Sharma, M., S. Sharma, and J. Wadhwa. 2019. Improved uptake and therapeutic intervention of curcumin via designing binary lipid nanoparticulate formulation for oral delivery in inflammatory bowel disorder. Artifical Cells, Nanomedicine, Biotechnology 47 (1): 45–55. https://doi.org/10.1080/21691401.2018.1543191.
Manconi, M., M.L. Manca, D. Valenti, E. Escribano, H. Hillaireau, A.M. Fadda, and E. Fattal. 2017. Chitosan and hyaluronan coated liposomes for pulmonary administration of curcumin. International Journal of Pharmaceutics 525 (1): 203–210. https://doi.org/10.1016/j.ijpharm.2017.04.044.
Maria Letizia Manca, J.E.P., Virginia Melis, Donatella Valenti, Maria Cristina Cardia, Donatella Lattuada, Elvira Escribano-Ferrer, Anna Maria Fadda, and Maria Manconia. 2015. Nanoincorporation of curcumin in polymer-glycerosomes and evaluation of their in vitro–in vivo suitability as pulmonary delivery systems. RSC Advances 5 (127): 105149–105159. https://doi.org/10.1039/c5ra24032h.
Polman, C.H., S.C. Reingold, B. Banwell, M. Clanet, J.A. Cohen, M. Filippi, K. Fujihara, E. Havrdova, M. Hutchinson, L. Kappos, F.D. Lublin, X. Montalban, P. O’Connor, M. Sandberg-Wollheim, A.J. Thompson, E. Waubant, B. Weinshenker, and J.S. Wolinsky. 2011. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Annals of Neurology 69 (2): 292–302. https://doi.org/10.1002/ana.22366.
De Maria, R., M.G. Cifone, R. Trotta, M.R. Rippo, C. Festuccia, A. Santoni, and R. Testi. 1994. Triggering of human monocyte activation through CD69, a member of the natural killer cell gene complex family of signal transducing receptors. The Journal of Experimental Medicine 180 (5): 1999–2004.
Sancho, D., M. Gomez, and F. Sanchez-Madrid. 2005. CD69 is an immunoregulatory molecule induced following activation. Trends in Immunology 26 (3): 136–140. https://doi.org/10.1016/j.it.2004.12.006.
Cebrian, M., E. Yague, M. Rincon, M. Lopez-Botet, M.O. de Landazuri, and F. Sanchez-Madrid. 1988. Triggering of T cell proliferation through AIM, an activation inducer molecule expressed on activated human lymphocytes. The Journal of Experimental Medicine 168 (5): 1621–1637.
Santis, A.G., M.R. Campanero, J.L. Alonso, A. Tugores, M.A. Alonso, E. Yague, J.P. Pivel, and F. Sanchez-Madrid. 1992. Tumor necrosis factor-alpha production induced in T lymphocytes through the AIM/CD69 activation pathway. European Journal of Immunology 22 (5): 1253–1259. https://doi.org/10.1002/eji.1830220521.
Sancho, D., M. Gomez, F. Viedma, E. Esplugues, M. Gordon-Alonso, M.A. Garcia-Lopez, H. de la Fuente, A.C. Martinez, P. Lauzurica, and F. Sanchez-Madrid. 2003. CD69 downregulates autoimmune reactivity through active transforming growth factor-beta production in collagen-induced arthritis. The Journal of Clinical Investigation 112 (6): 872–882. https://doi.org/10.1172/JCI19112.
Esplugues, E., D. Sancho, J. Vega-Ramos, C. Martinez, U. Syrbe, A. Hamann, P. Engel, F. Sanchez-Madrid, and P. Lauzurica. 2003. Enhanced antitumor immunity in mice deficient in CD69. The Journal of Experimental Medicine 197 (9): 1093–1106. https://doi.org/10.1084/jem.20021337.
Han, Y., Y. Yang, Z. Chen, Z. Jiang, Y. Gu, Y. Liu, S. Xu, C. Lin, Z. Pan, W. Zhou, and X. Cao. 2014. Human hepatocellular carcinoma-infiltrating CD4(+)CD69(+)Foxp3(-) regulatory T cell suppresses T cell response via membrane-bound TGF-beta1. Journal of Molecular Medicine (Berlin) 92 (5): 539–550. https://doi.org/10.1007/s00109-014-1143-4.
Cortes, J.R., R. Sanchez-Diaz, E.R. Bovolenta, O. Barreiro, S. Lasarte, A. Matesanz-Marin, M.L. Toribio, F. Sanchez-Madrid, and P. Martin. 2014. Maintenance of immune tolerance by Foxp3+ regulatory T cells requires CD69 expression. Journal of Autoimmunity 55: 51–62. https://doi.org/10.1016/j.jaut.2014.05.007.
Kamphuis, E., T. Junt, Z. Waibler, R. Forster, and U. Kalinke. 2006. Type I interferons directly regulate lymphocyte recirculation and cause transient blood lymphopenia. Blood 108 (10): 3253–3261. https://doi.org/10.1182/blood-2006-06-027599.
Almasi, E., M. Gharagozloo, N. Eskandari, A. Almasi, and A.M. Sabzghabaee. 2017. Inhibition of apoptosis and proliferation in T cells by immunosuppressive silymarine. Iranian Journal of Allergy, Asthma, and Immunology 16 (2): 107–119.
Treadaway, K., G. Cutter, A. Salter, S. Lynch, J. Simsarian, J. Corboy, D. Jeffery, B. Cohen, K. Mankowski, J. Guarnaccia, L. Schaeffer, R. Kanter, D. Brandes, C. Kaufman, D. Duncan, E. Marder, A. Allen, J. Harney, J. Cooper, D. Woo, O. Stuve, M. Racke, and E.M. Frohman. 2009. Factors that influence adherence with disease-modifying therapy in MS. Journal of Neurology 256 (4): 568–576. https://doi.org/10.1007/s00415-009-0096-y.
Stromnes, I.M., and J.M. Goverman. 2006. Active induction of experimental allergic encephalomyelitis. Nature Protocols 1 (4): 1810–1819. https://doi.org/10.1038/nprot.2006.285.
Gharagozloo, M., E.N. Javid, A. Rezaei, and K. Mousavizadeh. 2013. Silymarin inhibits cell cycle progression and mTOR activity in activated human T cells: therapeutic implications for autoimmune diseases. Basic & Clinical Pharmacology & Toxicology 112 (4): 251–256. https://doi.org/10.1111/bcpt.12032.
Soper, D.M., D.J. Kasprowicz, and S.F. Ziegler. 2007. IL-2Rbeta links IL-2R signaling with Foxp3 expression. European Journal of Immunology 37 (7): 1817–1826. https://doi.org/10.1002/eji.200737101.
Zorn, E., E.A. Nelson, M. Mohseni, F. Porcheray, H. Kim, D. Litsa, R. Bellucci, E. Raderschall, C. Canning, R.J. Soiffer, D.A. Frank, and J. Ritz. 2006. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood 108 (5): 1571–1579. https://doi.org/10.1182/blood-2006-02-004747.
Chen, H., L. Zhang, H. Zhang, Y. Xiao, L. Shao, H. Li, H. Yin, R. Wang, G. Liu, D. Corley, Z. Yang, and Y. Zhao. 2013. Disruption of TSC1/2 signaling complex reveals a checkpoint governing thymic CD4+ CD25+ Foxp3+ regulatory T-cell development in mice. The FASEB Journal 27 (10): 3979–3990. https://doi.org/10.1096/fj.13-235408.
Kaur, S., L. Lal, A. Sassano, B. Majchrzak-Kita, M. Srikanth, D.P. Baker, E. Petroulakis, N. Hay, N. Sonenberg, E.N. Fish, and L.C. Platanias. 2007. Regulatory effects of mammalian target of rapamycin-activated pathways in type I and II interferon signaling. The Journal of Biological Chemistry 282 (3): 1757–1768. https://doi.org/10.1074/jbc.M607365200.
Wan, Y.Y., and R.A. Flavell. 2007. Regulatory T cells, transforming growth factor-beta, and immune suppression. Proceedings of the American Thoracic Society 4 (3): 271–276. https://doi.org/10.1513/pats.200701-020AW.
Zheng, S.G., J.D. Gray, K. Ohtsuka, S. Yamagiwa, and D.A. Horwitz. 2002. Generation ex vivo of TGF-beta-producing regulatory T cells from CD4+CD25- precursors. Journal of Immunology 169 (8): 4183–4189.
Chen, W., W. Jin, N. Hardegen, K.J. Lei, L. Li, N. Marinos, G. McGrady, and S.M. Wahl. 2003. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. The Journal of Experimental Medicine 198 (12): 1875–1886. https://doi.org/10.1084/jem.20030152.
Hou, Y., H. Wang, F. Zhang, F. Sun, M. Xin, M. Li, J. Li, and X. Wu. 2019. Novel self-nanomicellizing solid dispersion based on rebaudioside A: a potential nanoplatform for oral delivery of curcumin. International Journal of Nanomedicine 14: 557–571. https://doi.org/10.2147/IJN.S191337.
Uchiyama, H., Y. Wada, Y. Hatanaka, Y. Hirata, M. Taniguchi, K. Kadota, and Y. Tozuka. 2019. Solubility and permeability improvement of quercetin by an interaction between alpha-glucosyl stevia nano-aggregates and hydrophilic polymer. Journal of Pharmaceutical Sciences. https://doi.org/10.1016/j.xphs.2019.01.007.
Acknowledgments
We appreciate the collaboration of the study participants.
Funding
This work was financially supported by the Iran National Science Foundation (grant no. 94014666) and Isfahan University of Medical Sciences (grant no. 194136).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Written informed consent was received from all patients and the research protocols were confirmed by the Ethics Committee of Isfahan University of Medical Sciences, Isfahan, Iran.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shariati, M., Shaygannejad, V., Abbasirad, F. et al. Silymarin Restores Regulatory T Cells (Tregs) Function in Multiple Sclerosis (MS) Patients In Vitro. Inflammation 42, 1203–1214 (2019). https://doi.org/10.1007/s10753-019-00980-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-019-00980-9