Abstract
Loss of intestinal epithelial barrier function including typical tight junction changes and epithelial cell apoptosis plays an important role in Crohn’s disease. SEW2871, a selective sphingosine-1-phosphate type-1 receptor agonist, has been proven to be efficient in protecting against colitis in IL-10−/− mice in our previous study. Here we performed additional studies to investigate whether treatment with SEW2871 was associated with an improved epithelial barrier function in IL-10−/− mice. SEW2871 was administered by gavage at a dose of 20 mg/kg/day for 2 weeks to IL-10−/− mice. Severity of colitis, CD4+ T cells in colon lamina propria and proinflammatory cytokine productions were evaluated. Furthermore, intestinal permeability, tight junction (occludin and ZO-1) expressions and distributions, as well as epithelial cell apoptosis, were also assessed. SEW2871 treatment attenuated established colitis associated with decreased CD4+ T cells in colon lamina propria and reduced TNF-α and IFN-γ levels. Moreover, enhanced barrier function, which resulted from ameliorated tight junction (occludin and ZO-1) expressions and suppressed epithelial cell apoptosis, was found to contribute to the therapeutic effects. SEW2871 treatment protects from colitis in IL-10−/− mice through reduced epithelial cell apoptosis and improved barrier function. Thus, targeting sphingosine-1-phosphate may represent a new therapeutic approach in Crohn’s disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Crohn’s disease (CD) is a chronic relapsing inflammatory disorder, mainly affecting the gastrointestinal tract, and is generally considered as a result from the interactions between environmental factors and inappropriate immune responses in genetically susceptible individuals [1, 2]. Although the underlying causes of CD still remain unclear, the fundamental defect involves loss of intestinal epithelial barrier function and a consequent increase in intestinal permeability [3, 4]. Since many microorganisms normally reside in the gut lumen, the intestinal epithelium effectively forms a barrier to prevent bacteria from entering the lamina propria (LP) [5]. Barrier function in the intestine is maintained via a number of structures. Epithelial cells comprise the largest amount of surface area and are sealed at paracellular interfaces by tight junctions [6, 7]. When the epithelium is intact, intestinal barrier function is largely defined by permeability characteristics of the epithelial tight junction. Moreover, epithelial cell death can also cause barrier loss, regardless of tight junction function [8].
A variety of animal models of both acute and chronic intestinal inflammation has been developed to investigate disease pathogenesis and novel treatment modalities of CD [9]. Among them, the interleukin-10 gene-deficient (IL-10−/−) mice are considered as the most appropriate model of human CD when maintained under specific pathogen-free (SPF) conditions [10, 11]. IL-10−/− mice spontaneously develop chronic colitis characterized by a CD4+ T cell response with secreting numerous inflammatory mediators such as tumor necrosis factor (TNF)-α, IL-1β and interferon (IFN)-γ [12, 13]. Previous studies have showed that IL-10−/− mice exhibited elevations in colonic permeability when compared to wild type controls, and this preceded colitis development [4, 14].
Sphingosine-1-phosphate (S1P) is a potent bioactive sphingolipid metabolite that regulates diverse cellular processes that are important for inflammation and immune responses [15]. Most of the functions of S1P have been attributed to its activation of the cell surface G protein-coupled receptors (S1P receptor 1 (S1P1)–S1P5) [16]. Especially, the trafficking and migration of lymphocytes is controlled by S1P via the S1P1 [17]. S1P receptor agonists (such as FTY720, KRP-203 and W-061) have already been found to be effective in ameliorating acute or chronic inflammation in murine colitis models [18–21]. SEW2871, another selective S1P1 agonist, has also been proven to be efficient in protecting against colitis in IL-10−/− mice in our previous study [22]. However, the changes of intestinal barrier function after SEW2871 treatment still remain unknown. In this study, we investigated whether treatment with SEW2871 is associated with an improved epithelial barrier function in IL-10−/− mice.
Materials and methods
Animals
Both wild type (WT) and IL-10−/− mice on a C57BL/6 background were obtained from Jackson Laboratory (Bar Harbor, ME, USA). The mice were bred and maintained under specific pathogen-free (SPF) conditions at the Model Animal Research Center of Nanjing University (Nanjing, China). 20–24-week-old IL-10−/− mice were used in our study as previously mentioned [22]. All mice received humane care in accordance with the law concerning the protection and control of animals in China.
Drug treatment of mice
SEW2871 (Cayman Chemical) was dissolved in 100 % dimethyl sulfoxide and diluted with 50 % Tween 20 for use. We still used a dose of 20 mg/kg/day for 2 weeks by gavage for the treatment. As mentioned in a study by Lien et al. [22, 23] and our previous report, we found this dose was well tolerated in mice, and no obvious macroscopic adverse effects were observed. In brief, the mice were divided into three groups: (1) treatment group: IL-10−/− mice received 20 mg/kg/day SEW2871 by gavage; (2) control group: IL-10−/− mice that received equal volume of distilled water; (3) WT group: WT mice also received equal volume of distilled water. Four weeks after the administration, the mice were killed by excessive anesthesia with pentobarbital sodium, and colons were collected for experiments.
Histological evaluation
After mice were killed, samples were obtained from proximal colon. The samples were fixed in 10 % formalin for 24 h and paraffin embedded. Fixed tissues then were sectioned at a 6 µm thick and stained with hematoxylin and eosin (H&E). Histological score of H&E-stained samples of the proximal colon was determined by two independent pathologists in a blind manner according to the method described by Singh et al. [24].
Flow cytometric analysis
For isolation of LP lymphocytes, we used the method reported in our previous study [22]. For immunofluorescent staining, cells isolated from colon tissues were counted and approximately one million cells transferred to each flow test tube. The cells were stained with fluorescein isothiocyanate-conjugated anti-CD4 (RM4-5; BD Biosciences) or an appropriate negative control. Then the stained cells were incubated at room temperature for 30 min in the dark, washed twice with 2 ml RPMI-1640 at room temperature and suspended in 500 µl RPMI-1640. Data were acquired on a FACS Calibur (BD Biosciences), followed by analysis using FlowJo software (version 7.6.1, Tree Star).
Mucosal cytokine measurements
Colonic organ cultures were prepared from mice in three groups. Briefly, colon tissue was removed, flushed with PBS and cut into pieces. Then tissues were cultured in RPMI 1640 supplemented with 10 % heat inactivated fetal calf serum (FCS), 100 U/ml penicillin and 100 U/ml streptomycin at 37 °C in 5 % CO2. After 24-h culture, supernatants were harvested, centrifuged and stored at −80 °C until assay. TNF-α and IFN-γ levels were measured using enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems) according to the manufacturer’s instructions, and data were expressed as picograms per milligram tissue (pg/mg tissue).
Ussing chamber assay
After mice were killed, segments of colon were immediately removed for assessment of intestinal permeability. We used the method previously reported by Arrieta et al. [25]. Briefly, the mucosa was mounted in Lucite chambers exposing mucosal and serosal surfaces to 10 ml of oxygenated Krebs buffer (115 mmol/l NaCl, 8 mmol/l KCl, 1.25 mmol/l CaCl2, 1.2 mmol/l MgCl2, 2 mmol/l KH2PO4 and 225 mmol/l NaHCO3; pH 7.35), which was maintained at 37 °C by a heated water jacket and gassed with 5 % CO2/95 % O2. Fructose (10 mmol/l) was added to the serosal and mucosa sides. For measurement of basal mannitol fluxes, 1 mmol/l of mannitol with 370 KBOr of 3H was added to the mucosal side. The spontaneous transepithelial potential difference (PD) was determined, and the tissue was clamped at zero voltage by continuously introducing an appropriate short-circuit current with an automatic voltage clamp (DVC 1000 World Precision Instruments). Tissue ion resistance was calculated from the potential difference and short-circuit current according to Ohm’s law and expressed as ohms times centimeters squared (Ω cm2).
Immunofluorescence
Immunofluorescence staining was also performed to determine the integrity of the tight junction in each group using previously described methods [26]. Briefly, 5–6-µm frozen sections of proximal colon samples were collected on coated slides and fixed in 1 % paraformaldehyde and washed three times with PBS, and nonspecific binding was blocked with 5 % normal goat serum in PBS. After incubation with primary antibodies against occludin (Abcam) and ZO-1 (Abcam) in PBS with 1 % goat serum overnight at 4 °C, sections were washed and incubated with Alexa 488—conjugated secondary antibody for 60 min and counterstained with Hoechst 33342 for detection of nucleus. Images were visualized using a confocal microscopy (OLYPUS).
Western blot analysis
Western blotting of TJ protein expressions was performed as previously described [27]. The primary antibodies against occludin and ZO-1 were purchased from Abcam. Relative changes in protein expression were estimated from the pixel density using UN-SCAN-IT version 6.1, normalized to β-actin and calculated as target protein expression/β-actin expression ratios.
Quantification of epithelial apoptosis by terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) staining
Epithelial apoptosis was quantified by TUNEL technology with the in situ cell death detection kit (Roche) according to the manufacturer’s instructions. The sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (Sigma) and then mounted. Slides were examined using a confocal microscope (OLYPUS).
Statistical analysis
Statistical analyses were performed using SPSS 17.0 software (SPSS Inc.). Data were expressed as mean ± standard error of the mean (SEM). The measurements were subjected to one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. A probability level of p < 0.05 was considered statistically significant.
Results
SEW2871 treatment ameliorated chronic colitis in IL-10−/− mice
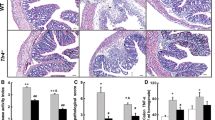
After mice were killed, we first collected the proximal colon tissues to test the effects of SEW2871 on colitis through HE-stained microscopic study. As shown in Fig. 1, HE staining revealed obvious disruptions in crypt architecture and a large amount of inflammatory cell infiltration in the LP of IL-10−/− mice compared with WT mice (Fig. 1a, b), and the inflammatory score was significantly higher (Fig. 1d). By contrast, IL-10−/− mice after SEW2871 treatment had only mild inflammatory cell infiltration and a reduced inflammatory score (Fig. 1c, d).
Histological features and scores of the proximal colons from mice in three groups. Representative HE-stained sections from three groups (×200 magnification) are shown. a Normal WT group. b Water-treated IL-10−/− control group. c SEW2871 treatment group. Arrows indicate infiltration of inflammatory cells. d Histological scores of all three groups. Data are presented as mean ± SEM (n = 6–8 for each group, * p < 0.05 versus water-treated IL-10−/− controls)
Flow cytometric analysis was next performed to observe the changes of CD4+ T lymphocytes in the colon LP. As shown in Fig. 2, we observed a significant increase of the percentage of CD4+ T cells in IL-10−/− mice compared with WT mice (p < 0.05). When IL-10−/− mice were treated with SEW2871, a significant decrease was found (p < 0.05).
SEW2871 treatment suppressed proinflammatory cytokine expressions in IL-10−/− mice
We subsequently evaluated the expressions of typical proinflammatory cytokines from colonic tissues. As shown in Fig. 3, TNF-α and IFN-γ levels were significantly increased in IL-10−/− mice. However, SEW2871 treatment significantly reduced the cytokine levels.
SEW2871 treatment prevented colonic permeability in IL-10−/− mice
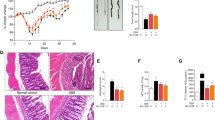
To investigate the effects of SEW2871 on colon function, we measured colonic permeability using Ussing chamber. As shown in Fig. 4, colonic permeability to mannitol was significantly increased in IL-10−/− mice with a corresponding decrease in electrical resistance compared with WT mice. In the SEW2871-treated IL-10−/− mice, these changes were largely prevented (Fig. 4).
SEW2871 treatment altered tight junction protein expressions and distributions in IL-10−/− mice
To identify whether there were changes in apical junction protein expressions and distributions after SEW2871 treatment, immunofluorescence and western blotting of occludin and ZO-1 were performed on the colon tissues. Immunofluorescence staining showed that occludin and ZO-1 were continuously distributed with bright spots along the membrane of the cells in WT mice (Figs. 5, 6). In water-treated IL-10−/− group, the fluorescence were faint and distributed dispersedly (Figs. 5, 6). By contrast, the intensity of fluorescence and distributions were significantly improved by SEW2871 treatment. Similarly, western blotting showed that the protein levels of occludin and ZO-1 were reduced in IL-10−/− mice when compared to WT mice (Fig. 7, p < 0.05), and SEW2871 treatment restored the protein levels of occludin and ZO-1 (Fig. 7, p < 0.05). These data suggest that SEW2871 treatment promotes typical tight junction protein expressions and distributions.
Representative images of occludin (green) and nuclei (blue) as obtained by immunofluorescence analysis of proximal colon tissues in three groups. Occludin was continuously distributed with bright spots along the membrane of the cells in WT mice while the fluorescence was faint and distributed dispersedly in water-treated IL-10−/− group. By contrast, the intensity of fluorescence and distributions were improved by SEW2871 treatment (Color figure online)
Representative images of ZO-1 (green) and nuclei (blue) as obtained by immunofluorescence analysis of proximal colon tissues in three groups. ZO-1 was continuously distributed with bright spots along the membrane of the cells in WT mice while the fluorescence was faint and distributed dispersedly in water-treated IL-10−/− group. By contrast, the intensity of fluorescence and distributions were improved by SEW2871 treatment (Color figure online)
Western blot analysis of occludin and ZO-1 expressions in the colon tissues. a The expressions of occludin and ZO-1 were statistically analyzed relative to β-actin expression by densitometry. b, c SEW2871 treatment improved the expressions of occludin and ZO-1, respectively. Data are presented as mean ± SEM (n = 4–6 for each group, * p < 0.05 versus water-treated IL-10−/− controls)
SEW2871 treatment reduced epithelial cell apoptosis in IL-10−/− mice
To further explore the therapeutic effects of SEW2871, we investigated intestinal epithelial cell apoptosis with the use of TUNEL staining. TUNEL assay of proximal colon tissue sections obtained from water-treated IL-10−/− mice revealed increased TUNEL staining in epithelial cells when compared to WT mice (Fig. 8a, b; p < 0.05). By contrast, SEW2871 treatment resulted in significant fewer TUNEL-positive cells (Fig. 8c; p < 0.05).
Epithelial apoptosis were visualized by the TUNEL assay. TUNEL-positive cells were stained with red. Representative images are shown (×200 magnification). a Wild type; b IL-10−/−; c SEW2871 treatment; d number of TUNEL-positive cells per 100 crypts. Data are presented as mean ± SEM (n = 5–6 for each group, * p < 0.05 versus water-treated IL-10−/−controls) (Color figure online)
Discussion
Since the discovery of S1P as a bioactive signaling molecule about 20 years ago [28], its functions have been extensively identified in a wide range of diseases, including atherosclerosis, diabetes, respiratory distress, and more importantly, cancer and inflammatory disorders [15, 16, 29]. Pharmacological agents targeting the S1P axis have shown promising therapeutic benefit in inflammatory and autoimmune disorders [30]. Of notice, fingolimod (also known as FTY720), an oral S1P receptor modulator, has been clinically approved for the treatment of relapsing and remitting multiple sclerosis [31]. S1P1 agonists seem attractive because, unlike conventional immunosuppressants, they induce lymphopenia through a reversible homing of lymphocytes from peripheral blood to secondary lymphoid tissues [18]. Our previous study showed that SEW2871, another selective S1P1 agonist, ameliorated established experimental colitis in IL-10−/− mice [22]. Here we performed additional experiments indicating that SEW2871 protected from Crohn’s colitis through reducing epithelial cell apoptosis and improving barrier function.
In the first series of our study, we found that treatment with SEW2871 in IL-10−/− mice resulted in a remarkable amelioration of colitis accompanying with improved microscopic histological changes, reduced CD4+ T cells in colon LP and less proinflammatory cytokine (TNF-α and INF-γ) expressions.
Disturbed intestinal barrier function is a key feature in inflammatory bowel disease (IBD), including CD and ulcerative colitis (UC). The intestinal epithelium at the interface between the intestinal microbiome and the lymphoid tissue plays a critical role in shaping the mucosal immune response [32]. The barrier defects are attributed to enhanced activity of proinflammatory cytokines like TNF-α and INF-γ, which are highly expressed in the chronically inflamed intestine [33]. Increased intestinal permeability has been seen as the sign of a break in intestinal barrier function [25]. In our study, colonic permeability to mannitol was significantly reduced with a corresponding increase in electrical resistance in IL-10−/− mice after SEW2871 treatment by Ussing chamber assay, meaning that SEW2871 restored the damaged barrier function in IL-10−/− mice.
Furthermore, epithelial tight junctions maintain the intestinal barrier while regulating permeability of ions, nutrients and water [7]. In active CD, changes in typical tight junction proteins like occludin and ZO-1 have been detected to be downregulated [34]. Moreover, altered tight junction proteins were also found in murine CD models [6, 35]. Previous studies have demonstrated that TNF, which is central to CD pathogenesis, causes tight junction barrier dysfunction via a process that requires myosin light chain kinase (MLCK) activation [36]. To investigate whether an altered change in apical junction protein expressions and distributions could be found after SEW2871 treatment, immunofluorescence and western blotting of occludin and ZO-1 were performed in this study. Our results showed a significant improvement of occludin and ZO-1 after treatment. With consideration of the relationship between tight junction proteins and the intestinal barrier function, restored tight junction expressions and distributions may contributed to the improved colonic barrier function in IL-10−/− mice after SEW2871 treatment.
Beyond tight junction changes, increased epithelial apoptosis can also contribute to intestinal barrier dysfunction [37]. In the physiologic state, intestinal epithelial cells have a relatively short life span, and their programed cell death through apoptosis is a carefully controlled process that is critical for the maintenance of normal barrier function [3]. However, in chronic IBD, the frequency of epithelial apoptosis is considerably increased, which is thought to contribute to the impairment of intestinal barrier function [38]. As an important proinflammatory cytokine in CD, TNF-α has a central role in inducing epithelial apoptosis [37, 39]. Moreover, induction of apoptosis by TNF-α is accompanied by alteration of TJ structure [38]. Therapeutically, TNF-α antibodies (infliximab) are reported to be able to restore barrier function in CD by downregulating epithelial apoptosis [40]. In this study, as shown in Fig. 8, our results indicated that an important therapeutic effect of SEW2871 was associated with a reduction of epithelial apoptosis in IL-10−/− colitis.
Taken together, our findings provided evidence that oral treatment with SEW2871 protected from experimental colitis through reduced epithelial cell apoptosis and improved TJ-dependent barrier function. Targeting the S1P axis might be a new therapeutic approach in the treatment of CD.
References
Salim SY, Soderholm JD. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17:362–81.
Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380:1590–605.
Tambuwala MM, Cummins EP, Lenihan CR, Kiss J, Stauch M, Scholz CC, Fraisl P, Lasitschka F, Mollenhauer M, Saunders SP, Maxwell PH, Carmeliet P, Fallon PG, Schneider M, Taylor CT. Loss of prolyl hydroxylase-1 protects against colitis through reduced epithelial cell apoptosis and increased barrier function. Gastroenterology. 2010;139:2093–101.
Lennon EM, Maharshak N, Elloumi H, Borst L, Plevy SE, Moeser AJ. Early life stress triggers persistent colonic barrier dysfunction and exacerbates colitis in adult IL-10−/− mice. Inflamm Bowel Dis. 2013;19:712–9.
Shen L. Tight junctions on the move: molecular mechanisms for epithelial barrier regulation. Ann N Y Acad Sci. 2012;1258:9–18.
Ewaschuk JB, Diaz H, Meddings L, Diederichs B, Dmytrash A, Backer J, Looijer-van Langen M, Madsen KL. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1025–34.
Turner JR. Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application. Am J Pathol. 2006;169:1901–9.
Su L, Nalle SC, Shen L, Turner ES, Singh G, Breskin LA, Khramtsova EA, Khramtsova G, Tsai PY, Fu YX, Abraham C, Turner JR. TNFR2 activates MLCK-dependent tight junction dysregulation to cause apoptosis-mediated barrier loss and experimental colitis. Gastroenterology. 2013;145:407–15.
Pizarro TT, Arseneau KO, Bamias G, Cominelli F. Mouse models for the study of Crohn’s disease. Trends Mol Med. 2003;9:218–22.
Scheinin T, Butler DM, Salway F, Scallon B, Feldmann M. Validation of the interleukin-10 knockout mouse model of colitis: antitumour necrosis factor-antibodies suppress the progression of colitis. Clin Exp Immunol. 2003;133:38–43.
Goettel JA, Scott Algood HM, Olivares-Villagomez D, Washington MK, Chaturvedi R, Wilson KT, Van Kaer L, Polk DB. KSR1 protects from interleukin-10 deficiency-induced colitis in mice by suppressing T-lymphocyte interferon-gamma production. Gastroenterology. 2011;140:265–74.
Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–74.
Gomes-Santos AC, Moreira TG, Castro-Junior AB, Horta BC, Lemos L, Cruz DN, Guimaraes MA, Cara DC, McCafferty DM, Faria AM. New insights into the immunological changes in IL-10-deficient mice during the course of spontaneous inflammation in the gut mucosa. Clin Dev Immunol. 2012;2012:560817.
Madsen KL, Malfair D, Gray D, Doyle JS, Jewell LD, Fedorak RN. Interleukin-10 gene-deficient mice develop a primary intestinal permeability defect in response to enteric microflora. Inflamm Bowel Dis. 1999;5:262–70.
Spiegel S, Milstien S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat Rev Immunol. 2011;11:403–15.
Kunkel GT, Maceyka M, Milstien S, Spiegel S. Targeting the sphingosine-1-phosphate axis in cancer, inflammation and beyond. Nat Rev Drug Discov. 2013;12:688–702.
Galicia-Rosas G, Pikor N, Schwartz JA, Rojas O, Jian A, Summers-Deluca L, Ostrowski M, Nuesslein-Hildesheim B, Gommerman JL. A sphingosine-1-phosphate receptor 1-directed agonist reduces central nervous system inflammation in a plasmacytoid dendritic cell-dependent manner. J Immunol. 2012;189:3700–6.
Mizushima T, Ito T, Kishi D, Kai Y, Tamagawa H, Nezu R, Kiyono H, Matsuda H. Therapeutic effects of a new lymphocyte homing reagent FTY720 in interleukin-10 gene-deficient mice with colitis. Inflamm Bowel Dis. 2004;10:182–92.
Daniel C, Sartory N, Zahn N, Geisslinger G, Radeke HH, Stein JM. FTY720 ameliorates Th1-mediated colitis in mice by directly affecting the functional activity of CD4+ CD25+ regulatory T cells. J Immunol. 2007;178:2458–68.
Song J, Matsuda C, Kai Y, Nishida T, Nakajima K, Mizushima T, Kinoshita M, Yasue T, Sawa Y, Ito T. A novel sphingosine 1-phosphate receptor agonist, 2-amino-2-propanediol hydrochloride (KRP-203), regulates chronic colitis in interleukin-10 gene-deficient mice. J Pharmacol Exp Ther. 2008;324:276–83.
Sanada Y, Mizushima T, Kai Y, Nishimura J, Hagiya H, Kurata H, Mizuno H, Uejima E, Ito T. Therapeutic effects of novel sphingosine-1-phosphate receptor agonist W-061 in murine DSS colitis. PLoS One. 2011;6:e23933.
Dong J, Wang H, Wu G, Zhao J, Zhang L, Zuo L, Zhu W, Gong J, Li Y, Gu L, Li J. Oral treatment with SEW2871, a sphingosine-1-phosphate type 1 receptor agonist, ameliorates experimental colitis in interleukin-10 gene deficient mice. Clin Exp Immunol. 2014;177:94–101.
Lien YH, Yong KC, Cho C, Igarashi S, Lai LW. S1P(1)-selective agonist, SEW2871, ameliorates ischemic acute renal failure. Kidney Int. 2006;69:1601–8.
Singh UP, Singh S, Taub DD, Lillard JW Jr. Inhibition of IFN-gamma-inducible protein-10 abrogates colitis in IL-10−/− mice. J Immunol. 2003;171:1401–6.
Arrieta MC, Madsen K, Doyle J, Meddings J. Reducing small intestinal permeability attenuates colitis in the IL10 gene-deficient mouse. Gut. 2009;58:41–8.
Clayburgh DR, Barrett TA, Tang Y, Meddings JB, Van Eldik LJ, Watterson DM, Clarke LL, Mrsny RJ, Turner JR. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. J Clin Invest. 2005;115:2702–15.
Wang H, Zhang W, Zuo L, Zhu W, Wang B, Li Q, Li J. Bifidobacteria may be beneficial to intestinal microbiota and reduction of bacterial translocation in mice following ischaemia and reperfusion injury. Br J Nutr. 2013;109:1990–8.
Zhang H, Desai NN, Olivera A, Seki T, Brooker G, Spiegel S. Sphingosine-1-phosphate, a novel lipid, involved in cellular proliferation. J Cell Biol. 1991;114:155–67.
Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nat Rev Cancer. 2010;10:489–503.
Fyrst H, Saba JD. An update on sphingosine-1-phosphate and other sphingolipid mediators. Nat Chem Biol. 2010;6:489–97.
Kappos L, Antel J, Comi G, Montalban X, O’Connor P, Polman CH, Haas T, Korn AA, Karlsson G, Radue EW, Group FDS. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med. 2006;355:1124–40.
Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–78.
Hering NA, Fromm M, Schulzke JD. Determinants of colonic barrier function in inflammatory bowel disease and potential therapeutics. J Physiol. 2012;590:1035–44.
Zeissig S, Burgel N, Gunzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 2007;56:61–72.
Liu Z, Zhang P, Ma Y, Chen H, Zhou Y, Zhang M, Chu Z, Qin H. Lactobacillus plantarum prevents the development of colitis in IL-10-deficient mouse by reducing the intestinal permeability. Mol Biol Rep. 2011;38:1353–61.
Weber CR, Raleigh DR, Su L, Shen L, Sullivan EA, Wang Y, Turner JR. Epithelial myosin light chain kinase activation induces mucosal interleukin-13 expression to alter tight junction ion selectivity. J Biol Chem. 2010;285:12037–46.
Gitter AH, Bendfeldt K, Schulzke JD, Fromm M. Leaks in the epithelial barrier caused by spontaneous and TNF-alpha-induced single-cell apoptosis. FASEB J: Off Publ Fed Am Soc Exp Biol. 2000;14:1749–53.
Schulzke JD, Bojarski C, Zeissig S, Heller F, Gitter AH, Fromm M. Disrupted barrier function through epithelial cell apoptosis. Ann N Y Acad Sci. 2006;1072:288–99.
Marini M, Bamias G, Rivera-Nieves J, Moskaluk CA, Hoang SB, Ross WG, Pizarro TT, Cominelli F. TNF-alpha neutralization ameliorates the severity of murine Crohn’s-like ileitis by abrogation of intestinal epithelial cell apoptosis. Proc Natl Acad Sci USA. 2003;100:8366–71.
Zeissig S, Bojarski C, Buergel N, Mankertz J, Zeitz M, Fromm M, Schulzke JD. Downregulation of epithelial apoptosis and barrier repair in active Crohn’s disease by tumour necrosis factor alpha antibody treatment. Gut. 2004;53:1295–302.
Acknowledgments
The present study was supported in part by funding from the National Natural Science Foundation of China (Grants 81200263, 81270006 and 81170365) and Jiangsu Provincial Special Program of Medical Sciences, China (BL2012006). This study was also partly supported by the Model Animal Research Center, Nanjing University (Nanjing, China). The authors also want to thank Professor Xiang Gao and Peiliang Shi (Model Animal Research Center of Nanjing University, China) for their excellent technical assistances.
Conflict of interest
The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jianning Dong, Honggang Wang, and Jie Zhao are contributed equally to this work.
Rights and permissions
About this article
Cite this article
Dong, J., Wang, H., Zhao, J. et al. SEW2871 protects from experimental colitis through reduced epithelial cell apoptosis and improved barrier function in interleukin-10 gene-deficient mice. Immunol Res 61, 303–311 (2015). https://doi.org/10.1007/s12026-015-8625-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-015-8625-5