Abstract
Neuroendocrine tumors of the thyroid gland are generally considered to derive from parafollicular endocrine cells (C cells) and are generally referred to as medullary thyroid carcinomas (MTC). Calcitonin secretion is almost always detected in MTC and a prerequisite for both clinical and pathological diagnosis. Thyroid neuroendocrine tumors without any apparent calcitonin secretion reflect a diagnostic dilemma because non-calcitonin-producing MTCs have virtually not been characterized. Here, we report a case of primary thyroid neuroendocrine tumors lacking calcitonin secretion or expression. The tumor cells expressed cytokeratins, chromogranin A, and synaptophysin, all of which were consistent with epithelial and neuroendocrine differentiation. Thyroid transcription factor-1 paired box gene 8, and carcinoembryonic antigen were also immunohistochemically detected, consistent with its thyroid origin. However, the tumor was negative for calcitonin both by immunohistochemistry and in situ hybridization, hence, not meeting the definition of MTC. Despite the loss of calcitonin expression, immunoreactivity for the calcitonin-gene-related peptide was detected in the tumor. Somatic gene mutations of RET, H-RAS, K-RAS, or BRAF were not detected in this case. A limited number of calcitonin non-producing thyroid neuroendocrine tumors are available in the scientific literature available in English, and its etiology and clinical manifestations remain largely unknown. Our case, along with the rare, previously reported cases, suggests that calcitonin non-producing neuroendocrine tumors of the thyroid gland are most likely derived from C cells, but should be differentiated from ordinary MTCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thyroid neuroendocrine tumors present as medullary thyroid carcinomas (MTC). An MTC is defined as a neuroendocrine tumor derived from thyroid parafollicular cells, also called “C cells.” The clinical and pathological diagnosis of MTC requires the evidence of the C cell origin of the tumor cells, which is demonstrated by calcitonin production. Increased plasma calcitonin concentration is a significant clinical finding in MTC. Calcitonin immunoreactivity of the tumor cells further confirms the diagnosis of MTC; however, there have been a number of reports on thyroid neuroendocrine neoplasms morphologically identical to MTC for which there has been no evidence of calcitonin production [1–4]. In some of these previously reported cases, the term “atypical MTC” was applied to neuroendocrine tumors without definitive evidence of calcitonin production. Together with the expression of TTF-1 and PAX8, the expression of the calcitonin gene-related peptide (CGRP, which is encoded by an identical gene of calcitonin and stores C cell secretory granules) is considered to be associated with its C cell tumor. Here, we report a case of calcitonin non-producing primary thyroid neuroendocrine tumor. We assume this is a C cell-derived tumor because of its CGRP expression. This type of tumor is indeed very rare; however, primary calcitonin non-producing neuroendocrine tumors of the thyroid gland should be recognized as a distinct tumor entity different from MTC since both clinical and pathological characteristics do not meet the criteria of MTC. We also reviewed the previously reported similar cases for further discussion.
Clinical Summary
A mass was detected in the left lobe of the thyroid gland in a 48-year-old Japanese woman during a routine ultrasound examination. She had past medical histories of cholecystolithiasis, anal prolapse, and uterine leiomyoma. Her family history was not contributory. There was no evidence of thyroid hormonal abnormalities (thyroglobulin, 28.5 ng/mL; free T3, 3.1 pg/mL; free T4, 1.07 ng/dL, hTSH, 1.3 μIU/mL). Plasma calcitonin (29 pg/mL) and carcinoembryonic antigen (CEA, 1.3 ng/mL) were within the normal range. No metastases or masses were clinically detected in other organs. Due to the discrepancies observed between the serum calcitonin/CEA levels and cytological findings described below, a left hemithyroidectomy with lymph node dissection was selected for initial surgery. The surgery was free of complications, and her postoperative course was unremarkable.
Pathological Findings
Fine-Needle Aspiration Cytology Examination
Tumor cells displayed oval to round nuclei with coarse chromatin patterns. Nucleoli were indistinct. Mild to moderate cell adhesion and thin-walled vessel structures were frequently detected. The amyloid-like material was not detected. Based on the characteristic cytological features of the tumor cells, a diagnosis of MTC was strongly suspected (Fig. 1a).
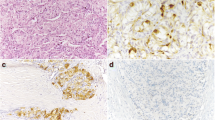
Representative illustrations of tumor pathology. a Fine-needle aspiration cytology. Tumor cells were loosely clustered. Oval to round nuclei with coarse nuclear chromatin. Nucleoli were indistinct. b A macroscopic image of the tumor. The tumor was well demarcated, and its cut surface appeared gray to slightly yellowish. c A microscopic image of the tumor at a low magnification. The tumor was encapsulated and composed of polygonal- to spindle-shaped cells. Tumor cells were arranged in middle-sized nests separated by thin-walled blood vessels. d A microscopic image of the tumor at a high magnification. Tumor cell nuclei were round to oval with mild to moderate atypia, slightly coarse chromatin pattern, and inconspicuous nucleoli
Macroscopic and Microscopic Findings
Macroscopically, the tumor was well-demarcated, 28 × 18 × 18 mm in size, and its cut surface appeared gray to slightly yellowish (Fig. 1b). Histologically, the tumor was encapsulated and composed of polygonal- to spindle-shaped cells with indistinct cellular borders. The tumor cells were arranged as mid-sized nests separated by thin-walled blood vessels (Fig. 1c). The nuclei of the tumor cells were round to oval, and the nuclear atypia was mild to moderate. A coarse chromatin pattern and inconspicuous nucleoli suggested neuroendocrine differentiation of this particular tumor (Fig. 1d). Amyloid deposition was not detected. Mitotic figures were rarely identified; only one in 50 high-power fields. Necrosis was not present. No foci of vascular invasion or capsular invasion were detected. No foci of metastases were detected in the dissected lymph nodes.
Immunohistochemistry
Immunohistochemical studies were performed on 3-μm-thick paraffin-embedded sections (Table 1). Peroxidase-conjugated avidin (Nichirei Bioscience, Tokyo, Japan) was employed for the colorimetric reaction of the secondary antibody for cytokeratins (CK) AE1/AE3, CK7, CK8, CK18, S100, parathyroid hormone (PTH), and Ki-67. EnVision system HRP (Dako, Glostrup, Denmark) was used for thyroid transcription factor-1 (TTF-1), thyroid transcription factor-2 (TTF-2, FOXE1), paired box gene 8 (PAX8), CEA, chromogranin A, synaptophysin, calcitonin, and CGRP. The antigen-antibody complex was visualized by 3,3′-diaminobenzidine tetrachloride (DAB) for all antibodies. CKAE1/AE3, CK18, chromogranin A, synaptophysin, and vimentin were seen diffusely in the cytoplasm of the tumor cells (Fig. 2a, AE1/AE3; Fig. 2b, chromogranin A). Diffuse nuclear expression of TTF-1 and PAX8 was observed (Fig. 2c, TTF-1; Fig. 2d, PAX8). No immunoreactivity was detected for CK7, CK8, CK19, thyroglobulin, thyroperoxidase, HBME1, S100, or PTH. TTF-2 was not detected in the nuclei but expressed in the cytoplasm. CEA was detected in a small portion of the tumor, corresponding to less than 5 %. Calcitonin immunoreactivity was not detected in any of the tumor cells (Fig. 2e). Despite its lack of calcitonin immunoreactivity, CGRP immunoreactivity was detected in approximately 70 % of the tumor cells (Fig. 2f). Ki-67 labeling index was very low, 0.3 %.
Immunohistochemical features of the tumor cells. a CKAE1/AE3, diffuse immunoreaction toward pan-cytokeratin. b chromogranin A, diffuse and strong expression of chromogranin A. c TTF-1, nuclear immunoreactivity of thyroid transcription factor 1. d PAX8, paired box gene 8. e calcitonin, no immunoreactivity of calcitonin was detected in any of the tumor cells. A case of medullary carcinoma as a positive control (inset image). f CGRP, the calcitonin-gene-related peptide was expressed in approximately 70 % of tumor cells
mRNA In Situ Hybridization
mRNA in situ hybridization for calcitonin and thyroglobulin was further performed on 3-μm-thick paraffin sections using single-strand DNA commercial probes (CAM-0017, Histosonda Calcitonin [Cenbimo, Lugo, Spain] and CAM-0016, Histosonda Thyroglobulin [Cenbimo]), following the manufacturer’s protocol. Tumor cells were negative for calcitonin and thyroglobulin mRNA (Fig. 3a, calcitonin; Fig. 3b, thyroglobulin).
Gene Mutation Analysis
For molecular analysis, genomic DNA was extracted from the paraffin-embedded tumor tissue using the QIAamp DNA Mini Kit (QIAGEN, Hilden, Germany), following the manufacturer’s instructions. Specific polymerase chain reaction (PCR) amplification of exons 8, 10, 11, 13, 14, 15, and 16 of RET protooncogene followed by direct Sanger sequencing was performed, the mutations in these exons accounting for about 95 % of RET mutations in familial MTC. Due to the fact that H-RAS (codons 12, 13, and 61) and K-RAS (codon 61) gene mutations were identified in RET-negative sporadic MT [5], we investigated H-RAS (codons 12, 13, 58, 59, 61, 117, and 146) and K-RAS (codons 12, 13, 58, 59, 61, 117, 118, and 146) somatic mutations using pyrosequencing (PyroMark Q24 System, QIAGEN, Germany). We also used a real-time PCR technology system (Cobas 4800 System, v2.0; Roche Diagnostics, San Cugat del Vallés, Spain) for the detection of the V600E (1799 T > A) BRAF gene mutation (Cobas 4800 BRAF V600 Mutation Test, Roche Diagnostics) because of their prevalence in the frame of thyroid tumors. No RET, H-RAS, K-RAS, or BRAF gene somatic mutations were detected.
Discussion
An elevated plasma calcitonin level is regarded as a highly sensitive and specific indication of underlying MTC, but its diagnostic relevance is limited in a subset of MTC [6] and related conditions, such as small-sized MTC or cases of C cell hyperplasia [7]. Therefore, detection of calcitonin expression in tumor cells is generally required for the diagnosis of MTC [8]. Schmid et al. previously studied 142 cases of primary thyroid neuroendocrine tumors; in three cases (2.1 %), calcitonin immunoreactivity was detected only in very few tumor cells, and in one case (0.7 %), calcitonin expression was absent [1]. The authors referred to these exceptional cases as “atypical MTC.” However, due to insufficient evidence of their C cell origin or calcitonin production, these cases—especially the last case in the series completely lacking calcitonin production—do not necessarily meet the criteria of MTC. To the best of our knowledge, primary thyroid neuroendocrine tumors characterized by a complete lack of calcitonin production, both clinically and histopathologically, are extremely rare and so far reported only in four cases in the scientific literature available in English [1–4] (Table 2). The present case, along with a case reported by Nakazawa et al., lacked calcitonin expression as evidenced by both immunohistochemistry and mRNA in situ hybridization but exhibited CGRP in the tumor cells [4] (Table 2). CGRP, expressed both in MTC and in non-neoplastic C cells, is a member of the calcitonin family of neuropeptides, which is generated from the alternative RNA splicing of the CALCA gene [4, 9]. CGRP is also produced in other organs; therefore, CGPR expression alone does not necessarily indicate the origin of the tumor cells [10]. However, together with the expression of cytokeratin, TTF-1, and PAX-8, the presence of CGRP expression is consistent with C cell origin [4]. On the other hand, the cases studied by Schmid et al. and Sobol et al. did not appear to express CGRP [1, 2], and CGRP was not tested for in a case reported by Chernyavsky et al. [3].
TTF-1 is a transcriptional factor mainly regulating thyroid-specific gene expression in the thyroid gland, including thyroglobulin and thyroperoxidase [11]. TTF-1 mRNA was also identified in C cells with a critical role with regard to regulating extracellular Ca2+ homeostasis [12]. MTC as well as non-neoplastic C cells express TTF-1 [11]. TTF-1 expression in the current case suggested thyroid origin and is a common feature of MTC. TTF-1 expression in a calcitonin non-producing neuroendocrine tumor of the thyroid gland was also reported by Nakazawa et al. [4]. PAX8 and TTF-2 are also other important transcription factors maintaining thyroid differentiation in the embryonic stage [13]. PAX8 and TTF-2 are diffusely expressed in most cases of papillary carcinoma and follicular neoplasms in the thyroid, but their expressions in MTC and C cell hyperplasia vary, being rather indistinct [14].
An increased level of plasma CEA is useful for detection and post-surgical follow-up of MTC. In the current case, plasma CEA was not increased, but its immunoreactivity was focally detected. The CEA expression of calcitonin non-producing neuroendocrine tumors varied among the previously reported cases (Table 2). In addition, there was no correlation between CEA production and tumor progression in these cases, while, in MTC, CEA production is generally increased in progressive diseases [15].
Some other tumors should be considered as a differential diagnosis of calcitonin non-producing neuroendocrine tumors. In particular, thyroid primary paraganglioma, although extremely rare, could be an important differential diagnosis because its morphology is similar to calcitonin non-producing neuroendocrine tumor of the thyroid gland, exemplified by the solid nesting or organoid patterns with capillary vessel networks. Both tumors do not produce calcitonin; however, paraganglioma differs from calcitonin non-producing neuroendocrine tumor in terms of the absence of cytokeratin, TTF-1, and thyroglobulin [16]. The other differential diagnosis of calcitonin non-producing neuroendocrine tumor of the thyroid gland is metastatic neuroendocrine neoplasm originating from other organs. Approximately 30 cases of such neoplasms have been reported [17–20]. Neuroendocrine neoplasms originating from bronchopulmonary neuroendocrine tumors, e.g., typical and atypical carcinoids or small-cell carcinomas, express TTF-1, but not thyroglobulin. However, among the cases reported as calcitonin non-producing neuroendocrine tumor in the thyroid, expression of TTF-1 and thyroglobulin was not necessarily reported in all the cases. In addition, calcitonin production is not necessarily a specific feature of MTC; it has also been identified in a minor population of neuroendocrine tumors observed in other sites [21]. In addition, pancreatic neuroendocrine tumors metastasizing to the thyroid gland could potentially express PAX-8 [18, 22]. Therefore, radiological examination with a view to identifying the possibility of metastatic disease is very important when establishing the diagnosis of calcitonin non-producing neuroendocrine tumor of the thyroid gland.
No association with hereditary neoplastic syndromes such as multiple endocrine neoplasia type 2A (MEN2A) has been reported in any of these cases. C cell hyperplasia, which is considered a precursor condition of MTC and associated with MEN2A, was not detected in the surrounding thyroid tissues. Molecular analyses did not divulge any somatic mutations frequently identified in cases of familial MTC or in RET-negative sporadic MTCs.
The prognosis of calcitonin non-producing neuroendocrine tumor of the thyroid gland remains unclear. The clinical course was indolent in three cases among those reported in the literature. These tumors exhibited “well-differentiated” morphology with low Ki-67 labeling index [4] equivalent to G1 of gastroenteropancreatic neuroendocrine tumors [23]. One patient reported by Sobol et al. died 23 months after the initial diagnosis due to multiple metastases. Further information is required to clarify the clinical course of this rare tumor.
In summary, we report a rare calcitonin non-producing neuroendocrine tumor of the thyroid gland. The expressions of TTF-1 and PAX8 were consistent with its thyroid origin, and the observation of CGPR expression further suggested its C cell derivation. Despite the shared origin, we propose that this condition is distinct from MTC since the clinical and pathological findings differ from the characteristic features of MTC.
References
Schmid KW, Ensinger C (1998) “Atypical” medullary thyroid carcinoma with little or no calcitonin expression. Virchows Archiv 433:209–215
Sobol RE, Memoli V, Deftos LJ (1989) Hormone-negative, chromogranin A-positive endocrine tumors. N Engl J 320 (7):444–447. doi:10.1056/NEJM198902163200707
Chernyavsky VS, Farghani S, Davidov T, Ma L, Barnard N, Amorosa LF, Trooskin SZ (2011) Calcitonin-negative neuroendocrine tumor of the thyroid: a distinct clinical entity. Thyroid 21:193–196. doi:10.1089/thy.2010.0299
Nakazawa T, Cameselle-Teijeiro J, Vinagre J, Soares P, Rousseau E, Eloy C, Sobrinho-Simoes M (2014) C-Cell-Derived Calcitonin-Free Neuroendocrine Carcinoma of the Thyroid: The Diagnostic Importance of CGRP Immunoreactivity. Int J Surg Pathol 22:530–535 doi:10.1177/1066896914525228
Moura MM, Cavaco BM, Pinto AE, Leite V (2011) High prevalence of RAS mutations in RET-negative sporadic medullary thyroid carcinomas. J Clin Endocrinol Metab 96:E863–868. doi:10.1210/jc.2010-1921
Brutsaert EF, Gersten AJ, Tassler AB, Surks MI (2015) Medullary thyroid cancer with undetectable serum calcitonin. J Clin Endocrinol Metab 100:337–341. doi:10.1210/jc.2014-3095
Rink T, Truong PN, Schroth HJ, Diener J, Zimny M, Grunwald F (2009) Calculation and validation of a plasma calcitonin limit for early detection of medullary thyroid carcinoma in nodular thyroid disease. Thyroid 19:327–332. doi:10.1089/thy.2008.0102
Zajac JD, Penschow J, Mason T, Tregear G, Coghlan J, Martin TJ (1986) Identification of calcitonin and calcitonin gene-related peptide messenger ribonucleic acid in medullary thyroid carcinomas by hybridization histochemistry. J Clin Endocrinol Metab 62:1037–1043. doi:10.1210/jcem-62-5-1037
Williams ED, Ponder BJ, Craig RK (1987) Immunohistochemical study of calcitonin gene-related peptide in human medullary carcinoma and C cell hyperplasia. Clin endocrinol 27:107–114
Yu LC, Hou JF, Fu FH, Zhang YX (2009) Roles of calcitonin gene-related peptide and its receptors in pain-related behavioral responses in the central nervous system. Neurosci Biobehav Rev 33:1185–1191. doi:10.1016/j.neubiorev.2009.03.009
Katoh R, Miyagi E, Nakamura N, Li X, Suzuki K, Kakudo K, Kobayashi M, Kawaoi A (2000) Expression of thyroid transcription factor-1 (TTF-1) in human C cells and medullary thyroid carcinomas. Hum Pathol 31:386–393
Suzuki K, Kobayashi Y, Katoh R, Kohn LD, Kawaoi A (1998) Identification of thyroid transcription factor-1 in C cells and parathyroid cells. Endocrinol 139:3014–3017. doi:10.1210/endo.139.6.6126
Trueba SS, Auge J, Mattei G, Etchevers H, Martinovic J, Czernichow P, Vekemans M, Polak M, Attie-Bitach T (2005) PAX8, TITF1, and FOXE1 gene expression patterns during human development: new insights into human thyroid development and thyroid dysgenesis-associated malformations. J Clin Endocrinol Metab 90:455–462. doi:10.1210/jc.2004-1358
Nonaka D, Tang Y, Chiriboga L, Rivera M, Ghossein R (2008) Diagnostic utility of thyroid transcription factors Pax8 and TTF-2 (FoxE1) in thyroid epithelial neoplasms. Mod Pathol 21:192–200. doi:10.1038/modpathol.3801002
Mendelsohn G, Wells SA, Jr., Baylin SB (1984) Relationship of tissue carcinoembryonic antigen and calcitonin to tumor virulence in medullary thyroid carcinoma. An immunohistochemical study in early, localized, and virulent disseminated stages of disease. Cancer 54:657–662
Yu BH, Sheng WQ, Wang J (2013) Primary paraganglioma of thyroid gland: a clinicopathologic and immunohistochemical analysis of three cases with a review of the literature. Head Neck Pathol 7:373–380. doi:10.1007/s12105-013-0467-7
Matias-Guiu X, LaGuette J, Puras-Gil AM, Rosai J (1997) Metastatic neuroendocrine tumors to the thyroid gland mimicking medullary carcinoma: a pathologic and immunohistochemical study of six cases. Am J Surg Pathol 21:754–762
Leboulleux S, Baudin E, Young J, Caillou B, Lazar V, Pellegriti G, Ducreux M, Schaison G, Schlumberger M (1999) Gastroenteropancreatic neuroendocrine tumor metastases to the thyroid gland: differential diagnosis with medullary thyroid carcinoma. Eur J Endocrinol 140:187–191
Hirsch MS, Faquin WC, Krane JF (2004) Thyroid transcription factor-1, but not p53, is helpful in distinguishing moderately differentiated neuroendocrine carcinoma of the larynx from medullary carcinoma of the thyroid. Mod Pathol 17:631–636. doi:10.1038/modpathol.3800105
Sivrikoz E, Ozbey NC, Kaya B, Erbil Y, Kaya S, Yilmazbayhan D, Firat P, Kapran Y (2012) Neuroendocrine tumors presenting with thyroid gland metastasis: a case series. J Med Case Rep 6:73. doi:10.1186/1752-1947-6-73
Sano T, Saito H, Yamasaki R, Hamaguchi K, Ooiwa K, Shimoda T, Hosoi E, Saito S, Hizawa K (1986) Immunoreactive somatostatin and calcitonin in pulmonary neuroendocrine tumor. Cancer 57:64–68
Koo J, Mertens RB, Mirocha JM, Wang HL, Dhall D (2012) Value of Islet 1 and PAX8 in identifying metastatic neuroendocrine tumors of pancreatic origin. Mod Pathol 25:893–901. doi:10.1038/modpathol.2012.34
Bosman F, Camerio F, Hruban R, Theise N (2010) WHO classification of tumours of the digestive system. vol 4th ed. IARC Press, Lyon
Acknowledgments
The authors would like to acknowledge Ms. Yayoi Takahashi for her excellent technical support. This work was supported in part by the Grand-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (to A.K.) and by grant PI12/00749-European Regional Development Fund (to J.M.C.-T) from the Instituto de Salud Carlos III, Ministry of Economy and Competitiveness, Spain. The authors would like to thank Enago (www.enago.jp) for the English language review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Kasajima, A., Cameselle-Teijeiro, J., Loidi, L. et al. A Calcitonin Non-producing Neuroendocrine Tumor of the Thyroid Gland. Endocr Pathol 27, 325–331 (2016). https://doi.org/10.1007/s12022-016-9416-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-016-9416-9