Abstract
Objective
To explore outcomes of metformin (Met) as an antihyperglycemic agent in patients with type 2 diabetes mellitus (T2DM) combined with chronic heart failure (CHF).
Methods
This article employed a meta-analysis approach to systematically search several databases. Stata 15.1 software was employed for statistical analysis.
Results
This meta-analysis encompassed 15 randomized controlled trials, involving 20,595 patients with T2DM and CHF. The results revealed that in comparison to the non-Met group, the Met group exhibited a significantly reduced risk of all-cause mortality (RR = 0.72, 95%CI: 0.60–0.87) and a notably lower risk of cardiovascular mortality (RR = 0.52, 95%CI:0.29–0.92). However, there was no significant difference in the risk of hospitalization due to heart failure (RR = 0.85, 95%CI: 0.70–1.04). Furthermore, the Met group demonstrated significant improvements in NT-proBNP levels compared to the non-Met group (WMD = −132.91, 95%CI: −173.03, −92.79). Regarding the enhancement of Left Ventricular Ejection Fraction and Left Ventricular End-Diastolic Dimension levels, no statistically significant differences were observed between the two groups.
Conclusion
In individuals with T2DM and CHF, the use of Met is linked to a decreased likelihood of all-cause mortality and cardiovascular-related mortality. Furthermore, it can enhance cardiac function in CHF patients without elevating the risk of hospitalization due to heart failure, establishing its safety and potential benefits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The International Diabetes Federation Atlas reveals that as of 2021, an estimated 537 million individuals are currently afflicted by diabetes mellitus (DM), with projections indicating an increase to 643 million by 2030 and 783 million by 2045. Furthermore, as of 2021, approximately 541 million individuals exhibited impaired glucose tolerance, and over 6.7 million individuals between the ages of 20 and 79 succumbed to diabetes-related complications [1]. Among these complications, heart failure (HF) ranks as the leading cause of mortality, surpassing atherosclerotic cardiovascular disease. Research indicates a 12% prevalence of HF among individuals with T2DM [2].
Metformin (Met), a prominent biguanide oral antihyperglycemic agent, demonstrates notable advantages in efficacy, safety, and economic efficiency in managing hyperglycemia and insulin resistance, especially compared to other oral antihyperglycemic agents. The most recent guidelines for the diagnosis and treatment of DM, jointly issued in 2019 by The American Diabetes Association (ADA) and The European Association for the Study of Diabetes (EASD), continue to endorse Met as the primary pharmacological therapy for T2DM [3]. Simultaneously, the guidelines emphasize that, unless contraindications are present, Met should constitute the foundational pharmaceutical intervention upon establishing a T2DM diagnosis, alongside comprehensive lifestyle modifications [3]. In specific situations, such as in the presence of chronic kidney disease (CKD), cardiac insufficiency, hypoxic conditions, advanced age, or the use of contrast agents, Met administration may induce elevated blood lactate levels and, in severe cases, lead to lactic acidosis (LA). Consequently, HF has historically been contraindicated for Met use. A 1998 study encompassing the initial million patients treated with Met in the United States reported 47 cases of lactic acidosis, of which 43 cases were associated with kidney failure or CHF. Regrettably, 20 of these cases were fatal [4].
However, in 2006, the FDA rescinded the contraindication against Met’s use in patients with HF, a decision influenced by two observational studies [5]. Animal experiments have subsequently confirmed Met’s significant enhancement of left ventricular function and survival rates through the activation of AMP-activated protein kinase (AMPK) and its downstream effectors, endothelial nitric oxide synthase (eNOS) and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1alpha). No scientific evidence supports an increased risk of lactic acidosis associated with Met use [6, 7]. Met’s safety profile positions it as a primary choice for patients with diabetes and concomitant HF [8]. A substantial observational study focused on individuals with DM and CHF revealed that Met was linked to reduced mortality and fewer CHF-related hospitalizations in comparison to other antidiabetic medications (primarily insulin and sulfonylureas). Notably, the incidence of lactic acidosis was relatively low. Based on these findings, MacDonald and colleagues proposed that Met might offer a safe and effective treatment for patients with T2DM and coexisting HF [9].
The thoughtful selection of antidiabetic medications, considering their implications for cardiac and renal health, represents a pivotal aspect of T2DM management. Presently, the absence of high-quality clinical investigations on the clinical consequences of Met administration in T2DM patients concurrently experiencing CHF engenders a lack of consensus regarding the safety profile of Met in this patient subgroup. Urgent calls for high-quality clinical research to address this knowledge gap have arisen. The objective of this study is to employ meta-analysis techniques to synthesize findings from all pertinent randomized control trials (RCTs) and meticulously conducted observational cohort studies. The study aims to elucidate the clinical outcomes associated with Met in treating individuals affected by T2DM alongside CHF. By doing so, it seeks to establish a robust evidence-based foundation that can guide clinical decision-making.
Methods
Literature retrieval strategy
Utilizing the predefined search terms “Chronic heart failure,” “Type 2 Diabetes mellitus,” “Metformin,” “Randomized controlled trial,” and “Cohort studies” as subject headings, we conducted an extensive search across multiple bibliographic databases, including PubMed, Cochrane Library, Web of Science, CNKI, Wanfang, and others. The search scope encompassed all documents published from January 2003 to August 2023 without language restrictions. In addition, references from the included studies were scrutinized, and clinical trial registration websites were thoroughly explored to identify pertinent literature that may not have been retrieved through the initial search.
Inclusion criteria
The included studies adhered to the following eligibility criteria: (1) Study Participants: Adults (aged ≥18 years) diagnosed with T2DM, following the 1999 WHO diagnostic criteria for T2DM, and with a concurrent diagnosis of CHF, as outlined in the 2018 Chinese Heart Failure Diagnosis and Treatment Guidelines or the 2021 European Society of Cardiology Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. These criteria were applied uniformly across diverse demographic groups, encompassing variations in race, gender, and disease duration. (2) Intervention Protocols: The experimental group received Met either as monotherapy or with other antidiabetic agents. Dosage was not restricted. The control group was treated with non-Metformin regimens, which could include alternative antidiabetic medications, a placebo, or no specific pharmacological intervention. The duration of treatment was identical between the two groups. Both groups adhered to standard therapeutic regimens, which involved dietary and exercise protocols and included measures for kidney protection or heart failure management. (3) Outcome Measures: Primary outcome measures encompassed significant adverse cardiovascular events, notably cardiovascular mortality, non-fatal myocardial infarction, non-fatal stroke, all-cause mortality, or hospitalization due to heart failure. Secondary outcome measures involved metabolic acidosis, alterations in Left Ventricular Ejection Fraction (LVEF), Left Ventricular End-Diastolic Dimension (LVEDD), or levels of NT-proBNP. (4) The study design encompassed RCTs and observational cohort studies.
Exclusion criteria
The studies were excluded due to non-compliance with the following criteria: (1) Patients with a history of Met allergy, prediabetes, metabolic syndrome, gestational diabetes, acute heart failure, or the co-occurrence of T2DM and CHF. (2) Repetition of the original study in multiple publications. (3) Document types such as case-control studies, cross-sectional studies, case reports, animal experiments, reviews, conference abstracts, and similar categories. (4) Studies that were either incomplete or lacked valid outcome data, whether due to non-completion or the absence of pertinent results. (5) Studies characterized by a notably low quality of evidence.
Data extraction and quality assessment of included studies
Under the predetermined inclusion and exclusion criteria, two independent researchers conducted a rigorous screening of the primary literature. Both researchers performed information extraction and an assessment of the methodological quality of the studies. Subsequently, a comprehensive cross-verification process was undertaken. Any discrepancies were resolved through discussion or by seeking the judgment of a third party. The extracted data encompassed the following elements: (1) General particulars, such as the first author, publication year, study design, and sample size; (2) Particulars regarding the research, encompassing general demographics of the study participants, such as gender distribution, mean age, body mass index (BMI), levels of glycosylated hemoglobin (HbA1c), as well as details on the specific intervention strategies or exposure variables and the duration of intervention or follow-up; (3) Outcome measures.
The quality assessment of the included studies was conducted using the modified Jadad scale for RCTs and the Newcastle-Ottawa scale (NOS) for cohort studies. The Jadad scale assigns a total score of 7 points, where scores between 1 and 3 denote studies of low quality, and scores between 4 and 7 indicate studies of high quality. Conversely, the NOS scale utilizes a total score of 9 points, classifying studies with scores between 1 and 3 as low quality and those with scores of 4 or higher as medium to high quality [10].
Statistical analysis
In this meta-analysis, all data analysis was conducted using Stata 15.1 [11, 12]. Statistical heterogeneity analysis of the included literature was performed by combining the Q test method and I² test. Heterogeneity was not significant when P > 0.05 and I² < 50%, and in such cases, the fixed-effect model was employed. Conversely, when P ≤ 0.05 and I² ≥ 50%, there was substantial heterogeneity, and the random-effects model was utilized. Efforts were made to identify the source of heterogeneity through sensitivity or subgroup analysis. If the source of heterogeneity could be determined, subgroup analysis was performed based on this source. In cases where methodological heterogeneity could not be eliminated, caution was exercised when interpreting the combined analysis results. For continuous variables, the effect size was expressed using weighted mean difference (WMD) or standardized mean difference (SMD), comparing differences before and after treatment uniformly. For binary variables, the effect size was expressed as relative risks (RR). A 95% confidence interval (95%CI) was utilized for interval estimates, and statistical significance was defined as P < 0.05. When an outcome indicator included five or more documents, the Egger linear regression method was employed to examine the presence of publication bias. A P value greater than 0.05 signified the absence of publication bias, while a P value less than or equal to 0.05 indicated the presence of publication bias [13]. The sensitivity of the results was assessed using Duval and Tweedie’s trim and fill test. If the pooled effect size exhibited a substantial change before and after the test, the study results were deemed unreliable, necessitating further analysis of the combined studies [14].
Results
Document screening, basic characteristics and quality assessment of studies
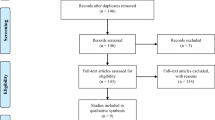
By conducting a comprehensive search across literature databases such as Pubmed, Cochrane Library, Web of Science, CNKI, and Wanfang, and clinical trial registration websites, a total of 917 relevant documents were initially retrieved. Utilizing Endnote 21 software for duplicate checking, 165 duplicate documents were identified and subsequently removed, leaving us with 752 unique documents. Subsequently, a meticulous examination of the abstracts and full texts was carried out following the inclusion and exclusion criteria. Documents that fail to meet these criteria, for example, documents concerning irrelevant topics, not satisfying inclusion criteria, case-control studies, cross-sectional studies, case reports, animal experiments, review articles, conference abstracts, or those of low quality and incomplete data, were excluded. Ultimately, 15 documents were selected, encompassing 20,595 patients with T2DM and CHF. These patients were distributed into the Met group (7,245 patients) and the non-Met group (13,350 patients) (Table 1). A detailed outline of the literature screening process is provided in Fig. 1.
The 15 documents incorporated in this analysis encompassed 3 RCTs, 5 retrospective cohort studies, and 7 prospective cohort studies. These studies spanned a research duration ranging from 3 months to 10 years. The experimental group received Met monotherapy or a combination of Met with other hypoglycemic drugs. In contrast, the control group was subjected to non-Met treatment, which included either a single placebo or no treatment. Both groups underwent conventional interventions such as dietary and exercise regimens, as well as HF management. Primary outcome indicators in studies concerning individuals with T2DM and CHF encompassed cardiovascular mortality, all-cause mortality, and hospitalization due to heart failure. Secondary outcome indicators included variations in LVEF, LVEDD, or NT-proBNP. For detailed baseline characteristics of these 15 documents, please refer to Table 2 [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29].
Meta-analysis results
All-cause mortality
12 studies reported all-cause mortality, involving 7066 individuals in the Met group and 17,404 in the non-Met group. Significant heterogeneity was observed in the studies (I² = 93%, P = 0.0004), warranting the utilization of a random effects model. The findings indicated that compared to the non-Met group, the Met group exhibited a significantly reduced risk of all-cause mortality, with a statistically significant difference between the two groups (RR = 0.72, 95%CI: 0.60–0.87). Additionally, a subgroup analysis was conducted based on different exposure drugs in the control group, specifically categorized into the sulfonylureas group and non-sulfonylureas group. The results indicated no statistically significant difference in the incidence of all-cause mortality between the Met group and the sulfonylureas subgroup (RR = 0.71, 95%CI: 0.45–1.13). In contrast, compared with the non-sulfonylureas subgroup, the Met group displayed a reduced risk of all-cause mortality, with the difference being statistically significant (RR = 0.72, 95%CI: 0.59–0.89) (Fig. 2).
Cardiovascular mortality rate
The cardiovascular mortality rate was reported in 2 studies, comprising 1013 cases in the Met group and 376 cases in the non-Met group. Significant heterogeneity was observed (I² = 91%, P = 0.03), necessitating the utilization of a random effects model. The findings indicated that, in comparison to the non-Met group, the Met group exhibited a reduced risk of cardiovascular death, and this distinction was statistically significant (RR = 0.52, 95% CI: 0.29–0.92) (Table 3).
Incidence of hospitalization for heart failure
Five studies reported the incidence of hospitalization for HF, involving 2585 patients in the Met group and 3214 in the non-Met group. Heterogeneity testing revealed substantial heterogeneity (I² = 88%, p = 0.12), necessitating the application of a random effects model. The findings indicated no statistically significant disparity in the risk of heart failure hospitalization between the two groups (RR = 0.85, 95% CI: 0.70–1.04) (Fig. 2).
Changes in cardiac function
Two studies assessed the impact of Met on LVEF and LVEDD, involving 99 patients in the Met group and 96 patients in the control group. Significant heterogeneity was observed across these studies. Thus, a random effects model was applied. The findings indicated that there were no statistically significant differences between the Met and control groups in terms of enhancing LVEF (WMD = 1.47, 95%CI: −1.79–4.72) and LVEDD (WMD = −2.79, 95%CI: −6.56 to 0.98). Additionally, two studies investigated the effects of Met on NT-proBNP in 139 Met group cases and 134 non-Met group cases. No significant heterogeneity was detected in these studies (I² = 0%). Thus, a fixed effects model was employed. The results revealed that the Met group exhibited superior improvement in NT-proBNP levels compared to the non-Met group, with a statistically significant difference (WMD = −132.91, 95%CI: −173.03, −92.79) (Table 3).
Publication bias and sensitivity analysis
In this study, Egger’s test was employed to identify potential publication bias within the scope of all-cause mortality and incidence of hospitalization for HF among patients afflicted with type 2 diabetes mellitus and chronic heart failure who underwent metformin treatment. The findings indicated that neither indicator exhibited substantial publication bias (Table 4). Furthermore, during Duval and Tweedie’s trim and fill sensitivity assessment, the effect sizes for all-cause mortality and incidence of hospitalization for HF remained unchanged, underscoring the stability of these effect sizes (Table 4).
Discussion
In recent years, guidelines have emphasized the comprehensive management of patients with T2DM. This emphasis extends beyond fundamental lifestyle enhancements and encompasses glycemic control, blood pressure regulation, lipid management, weight reduction, and other therapeutic modalities. Particular attention is directed toward the attenuation of cardiorenal complications in individuals with existing T2DM. This approach seeks to curtail the progression of diabetic complications, mitigate disability, reduce mortality, enhance patients’ quality of life, and extend their overall life expectancy. Met, established as the primary hypoglycemic agent for T2DM, has demonstrated its capacity to diminish the risk of cardiovascular events and mortality in obese patients afflicted with T2DM. Our meta-analysis encompassed 15 studies meeting the predefined criteria, involving 20,595 patients affected by T2DM and CHF. The meta-analysis outcomes signify that Met holds the potential to reduce the hazard of all-cause mortality and cardiovascular-related death among patients grappling with T2DM and CHF. Notably, its utilization does not escalate the susceptibility to HF hospitalization, and further evidence supports its ability to ameliorate NT-proBNP levels in these patients.
Since the inception of the UKPDS and its subsequent investigations in 1998, Met has garnered recognition as a hypoglycemic agent linked to a diminished risk of diabetes-related outcomes, encompassing both macrovascular and microvascular complications, particularly in overweight individuals afflicted by T2DM [30]. Observational inquiries into T2DM patients concomitantly experiencing HF have unveiled a favorable association between Met treatment and a reduced likelihood of all-cause mortality and hospitalization due to HF [23]. Notably, a study by Fácila L et al. disclosed that over an average follow-up period of 2.4 years, involving 835 subjects with acute heart failure, patients receiving Met exhibited markedly lower long-term all-cause mortality compared to their non-Met-receiving counterparts [31]. Thus, extant research underscores that Met treatment offers superior clinical outcomes for patients grappling with T2DM and HF compared to traditional antidiabetic agents like insulin. Furthermore, complementary animal experiments have substantiated Met’s protective effects against cardiovascular disease at the molecular level. These protective mechanisms include mitigating myocardial ischemia by promoting protein kinase B and adenylate-activated protein kinase activation and nitric oxide production. Additionally, Met exerts a modulatory role in post-vascular inflammation, myocardial preservation, and the retardation of atherosclerotic progression. Concurrently, Met demonstrates efficacy in diminishing cardiac fibrosis and improving lipid profiles by reducing triglyceride levels. A meta-analysis examining the impact of Met treatment on HF with preserved ejection fraction revealed its potential to reduce mortality [32]. In addition to its potential impact on heart failure outcomes, metformin, as an antidiabetic medication, significantly improves glycemic control in T2DM patients by reducing hepatic glucose production and increasing peripheral insulin sensitivity [33]. Good glycemic control is particularly important in patients with T2DM and CHF, as hyperglycemia can exacerbate the progression of heart failure. Studies suggest that improved glycemic control may positively influence heart failure outcomes by reducing hyperglycemia-related cardiovascular complications, such as inflammation and oxidative stress [34, 35]. Therefore, the use of metformin in patients with T2DM and CHF may improve overall clinical outcomes not only through direct cardiac protective mechanisms but also by optimizing blood glucose levels [36].
This study adopted a comprehensive approach by focusing on all patients with CHF, incorporating the latest high-quality cohort studies and RCTs for a comprehensive analysis. The findings further demonstrated that Metformin can diminish the risks associated with all-cause mortality and cardiovascular mortality in individuals afflicted with T2DM and CHF, all while not elevating the likelihood of hospitalization due to HF. Furthermore, this investigation revealed Met’s capacity to ameliorate NT-proBNP levels among T2DM patients with CHF. Nevertheless, the scarcity of RCTs to evaluate Met’s influence on clinical outcomes in patients with T2DM and HF remains a notable gap in the current literature. Notably, ongoing studies registered in the clinical trial registry are poised to provide a more precise answer to whether Met is indeed associated with enhanced outcomes for HF patients. These forthcoming studies hold the promise of shedding further light on this matter [37, 38].
Met, a first-line antidiabetic medication for T2DM, has garnered global recognition for its enduring utility due to its established safety, efficacy, and cost-effectiveness. With revisions made by the FDA concerning Met prescriptions for individuals with HF in 2006 and 2016 and the 2018 guidance from the European Society of Cardiology (ESC) suggesting its potential safety and efficacy in T2DM and HF patients, the utilization of Met is expected to continue its upward trajectory. Nonetheless, it remains unsuitable for those individuals with preexisting contraindications or who necessitate cautious administration [39]. This meta-analysis demonstrated that, compared to conventional antidiabetic agents, Met use within these patient cohorts substantially enhances long-term prognoses and retards the progression of comorbid conditions. Moreover, this study bolsters the assertion that Met can be judiciously employed within the CHF population, a demographic hitherto considered contraindicated for its use.
While the FDA currently permits the use of Met in patients with mild to moderate CHF, clinical practice often sees hesitancy, particularly among T2DM patients with cardiorenal comorbidities. This hesitation primarily arises from concerns regarding Met’s association with LA, which has an observed high mortality rate of approximately 50% [40]. Furthermore, it is noteworthy that most ongoing RCT studies involving Met treatment exclude individuals with comorbid conditions.
Most RCT studies registered on clinical trial registration websites previously categorized as contraindicated cases align with the recommended criteria. However, their progress has been hindered by the limited availability of high-quality RCT evidence that supports such clinical decisions. This study included only three RCTs, which impedes an in-depth examination of this issue. In conclusion, to ensure the safety of Met treatment for T2DM patients with comorbidities, there is an urgent need for more high-evidence research. Multi-center, randomized, placebo-controlled trials should be prioritized to comprehensively assess the long-term safety of Met’s clinical application, thereby instilling confidence in patients with comorbid conditions who may benefit from Met treatment.
The 2023 European Society of Cardiology (ESC) guidelines recommend the use of SGLT2 inhibitors, such as dapagliflozin or empagliflozin, in heart failure patients, regardless of ejection fraction. This recommendation reflects an evolving approach to heart failure management and could impact future research and treatment strategies [41]. This is particularly important for patients with T2DM and chronic heart failure, as the interaction between different antidiabetic medications and heart failure outcomes is becoming increasingly complex. Incorporating SGLT2 inhibitors into standard care may influence the future use and study of metformin in this patient population. Further research is needed to understand the long-term effects of combining metformin with SGLT2 inhibitors and their combined impact on cardiovascular outcomes.
Nonetheless, this study has certain limitations. Firstly, meta-analysis faced challenges in including all pertinent studies, as some were excluded due to unreported outcome data or the absence of valid outcome information. Secondly, handling the disparities between studies was complex, and the potential influence of heterogeneity had to be considered. The heterogeneity test in this study revealed high levels of heterogeneity among the included studies, necessitating a cautious interpretation of the results. Thirdly, the study was marked by variations in the types of interventions or exposure drugs within the control group, discrepancies in intervention periods, varying lengths of follow-up, and the presence of other confounding variables. As a result, certain outcome indicators could not undergo subgroup analysis, making it challenging to eliminate the impact of confounding factors from the comprehensive analysis results.
Conclusion
In individuals with T2DM and CHF, the use of Met is linked to a decreased likelihood of all-cause mortality and cardiovascular-related mortality. Furthermore, it can enhance cardiac function in CHF patients without elevating the risk of hospitalization due to heart failure, establishing its safety and potential benefits.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- Met:
-
Metformin
- T2DM:
-
Type 2 Diabetes Mellitus
- CHF:
-
Chronic Heart Failure
- LVEF:
-
Left Ventricular Ejection Fraction
- LVEDD:
-
Left Ventricular End-Diastolic Dimension
- RCTs:
-
Randomized Controlled Trials
- NOS:
-
Newcastle-Ottawa Scale
- SD:
-
Standard Deviation
- RR:
-
Relative Risks
- CI:
-
Confidence Intervals
- WMD:
-
Weighted Mean Difference
- SMD:
-
Standardized Mean Difference
References
D.J. Magliano, E.J. Boyko, committee IDAtes. International Diabetes Federation; 2021. 10th ed. Brussels.2021.
I.S. Thrainsdottir, T. Aspelund, T. Hardarson, K. Malmberg, G. Sigurdsson, G. Thorgeirsson et al. Glucose abnormalities and heart failure predict poor prognosis in the population-based Reykjavik Study. Eur. J. Cardiovasc Prev. Rehabil. 12, 465–471 (2005)
J.B. Buse, D.J. Wexler, A. Tsapas, P. Rossing, G. Mingrone, C. Mathieu et al. 2019 Update to: Management of hyperglycemia in type 2 diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diab. Care 43, 487–493 (2020)
R.I. Misbin, L. Green, B.V. Stadel, J.L. Gueriguian, A. Gubbi, G.A. Fleming, Lactic acidosis in patients with diabetes treated with metformin. N. Engl. J. Med. 338, 265–266 (1998)
S.E. Inzucchi, F.A. Masoudi, D.K. McGuire, Metformin in heart failure. Diab. Care 30, e129 (2007)
A.A. Tahrani, G.I. Varughese, J.H. Scarpello, F.W. Hanna, Metformin, heart failure, and lactic acidosis: is metformin absolutely contraindicated? BMJ 335, 508–512 (2007)
A. Holstein, E.H. Egberts, [Traditional contraindications to the use of metformin – more harmful than beneficial?]. Dtsch Med Wochenschr. 131, 105–110 (2006)
J.N. Cohn, R. Ferrari, N. Sharpe, Cardiac remodeling–concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J. Am. Coll. Cardiol. 35, 569–582 (2000)
M.R. MacDonald, M.C. Petrie, N.M. Hawkins, J.R. Petrie, M. Fisher, R. McKelvie et al. Diabetes, left ventricular systolic dysfunction, and chronic heart failure. Eur. Heart J. 29, 1224–1240 (2008)
G. Wells, B. Shea, D. O’Connell, J. Peterson, V. Welch, M. Losos et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
R. Harris, J. Deeks, D. Altman, M. Bradburn, R. Harbord, J. Sterne, Metan: fixed- and random-effects meta-analysis. Stata J. 8, 3–28 (2008)
V. Nyaga, M. Arbyn, M. Aerts, Metaprop: a Stata command to perform meta-analysis of binomial data. Arch. Public Health 72, 39 (2014)
M. Egger, Davey, G. Smith, M. Schneider, Bias in meta-analysis detected by a simple, graphical test. Br. Med. J. 315, 629–634 (1997)
S. Duval, R. Tweedie, Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56, 455–463 (2000)
R. Roussel, F. Travert, B. Pasquet, P.W. Wilson, S.C. Smith Jr., S. Goto et al. Metformin use and mortality among patients with diabetes and atherothrombosis. Arch. Intern Med 170, 1892–1899 (2010)
P. Liu, X.C. Zhou, T. Wang, Clinical study of Dapagliflozin in the treatment of type 2 diabetes complicated with heart failure. Chin. J. Clin. Pharmacol. 37, 227–230 (2021)
R.F. Hua, Q. Shu, Y. Liu, X.B. Zeng, L. Ji, B.Q. Xie et al. Efficacy of sitagliptin metformin combination with insulin in the treatment of obesity type 2 diabetes with heart failure. Shanghai Med. J. 37, 862–864 (2014)
Y. Su, D. Wang, J. Long, F. Yang, Q. Xu, L.Y. Si, Clinical study of Metformin improving cardiac function in patients with type 2 diabetes complicated with diastolic heart failure. J. Third Mil. Med. Univ. 35, 1862–1865 (2013)
C. Andersson, J.B. Olesen, P.R. Hansen, P. Weeke, M.L. Norgaard, C.H. Jorgensen et al. Metformin treatment is associated with a low risk of mortality in diabetic patients with heart failure: a retrospective nationwide cohort study. Diabetologia 53, 2546–2553 (2010)
J. Wang, Y. Lu, X. Min, T. Yuan, J. Wei, Z. Cai, The association between metformin treatment and outcomes in type 2 diabetes mellitus patients with heart failure with preserved ejection fraction: A retrospective study. Front Cardiovasc Med 8, 648212 (2021)
A. Retwinski, M. Kosmalski, M. Crespo-Leiro, A. Maggioni, G. Opolski, P. Ponikowski et al. The influence of metformin and the presence of type 2 diabetes mellitus on mortality and hospitalisation in patients with heart failure. Kardiol. Pol. 76, 1336–1343 (2018)
H.Y. Chang, Y.W. Su, A.N. Feng, M.C. Fong, K.C. Huang, E. Chong et al. Prescription patterns of diabetes medications influencing clinical outcomes of heart failure patients with reduced ejection fraction. ESC Heart Fail 7, 604–615 (2020)
F.A. Masoudi, S.E. Inzucchi, Y. Wang, E.P. Havranek, J.M. Foody, H.M. Krumholz, Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: an observational study. Circulation 111, 583–590 (2005)
D.T. Eurich, S.R. Majumdar, F.A. McAlister, R.T. Tsuyuki, J.A. Johnson, Improved clinical outcomes associated with metformin in patients with diabetes and heart failure. Diab. Care 28, 2345–2351 (2005)
J.M. Evans, A.S. Doney, M.A. AlZadjali, S.A. Ogston, J.R. Petrie, A.D. Morris et al. Effect of Metformin on mortality in patients with heart failure and type 2 diabetes mellitus. Am. J. Cardiol. 106, 1006–1010 (2010)
J. Benes, M. Kotrc, K. Kroupova, P. Wohlfahrt, J. Kovar, J. Franekova et al. Metformin treatment is associated with improved outcome in patients with diabetes and advanced heart failure (HFrEF). Sci. Rep. 12, 13038 (2022)
T.R. Godec, D.I. Bromage, M. Pujades-Rodriguez, A. Cannata, A. Gonzalez-Izquierdo, S. Denaxas et al. Cardiovascular outcomes associated with treatment of type 2 diabetes in patients with ischaemic heart failure. ESC Heart Fail 9, 1608–1615 (2022)
D. Aguilar, W. Chan, B. Bozkurt, K. Ramasubbu, A. Deswal, Metformin use and mortality in ambulatory patients with diabetes and heart failure. Circ. Heart Fail 4, 53–58 (2011)
D.D. Shah, G.C. Fonarow, T.B. Horwich, Metformin therapy and outcomes in patients with advanced systolic heart failure and diabetes. J. Card. Fail 16, 200–206 (2010)
Group UPDS, Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352, 854–865 (1998)
L. Facila, O. Fabregat-Andres, V. Bertomeu, J.P. Navarro, G. Minana, S. Garcia-Blas et al. Metformin and risk of long-term mortality following an admission for acute heart failure. J. Cardiovasc Med (Hagerstown) 18, 69–73 (2017)
A. Halabi, J. Sen, Q. Huynh, T.H. Marwick, Metformin treatment in heart failure with preserved ejection fraction: a systematic review and meta-regression analysis. Cardiovasc Diabetol. 19, 124 (2020)
G. Rena, E.R. Pearson, K. Sakamoto, Molecular mechanism of action of metformin: old or new insights? Diabetologia 56, 1898–1906 (2013)
X.F. Wang, J.Y. Zhang, L. Li, X.Y. Zhao, H.L. Tao, L. Zhang, Metformin improves cardiac function in rats via activation of AMP-activated protein kinase. Clin. Exp. Pharm. Physiol. 38, 94–101 (2011)
M.J. Crowley, C.J. Diamantidis, J.R. McDuffie, C.B. Cameron, J.W. Stanifer, C.K. Mock et al. Clinical outcomes of metformin use in populations with chronic kidney disease, congestive heart failure, or chronic liver disease: A systematic review. Ann. Intern Med 166, 191–200 (2017)
N. Papanas, E. Maltezos, D.P. Mikhailidis, Metformin and heart failure: never say never again. Expert Opin. Pharmacother. 13, 1–8 (2012)
CZ EUCTR, Cardioprotective and metabolic effects of metformin in patients with heart failure and diabetes. Available at: https://clinicaltrials.gov/show/NCT01690091,2012. Accessed 26 October 2023.
EUCTR DK. DanHeart. Available at: https://trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2015-002150-12-DK. Accessed 26 October 2023.
P.M. Seferovic, M.C. Petrie, G.S. Filippatos, S.D. Anker, G. Rosano, J. Bauersachs et al. Type 2 diabetes mellitus and heart failure: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail 20, 853–872 (2018)
A.M. Stades, J.T. Heikens, D.W. Erkelens, F. Holleman, J.B. Hoekstra, Metformin and lactic acidosis: cause or coincidence? A review of case reports. J. Intern Med 255, 179–187 (2004)
Authors/Task Force M, T.A. McDonagh, M. Metra, M. Adamo, R.S. Gardner, A. Baumbach et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail 26, 5–17 (2024)
Author contributions
H.W.X., and Z.R.C. conceived of the study, participated in its design, conducted the systematic literature review, performed data analyses, prepared the draft, and critically revised the manuscript. All authors have approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, W., Zhao, R. Clinical outcomes in type 2 diabetes patients with chronic heart failure treated with metformin: a meta-analysis. Endocrine (2024). https://doi.org/10.1007/s12020-024-04025-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12020-024-04025-6