Abstract
Purpose
Owing to the absence of the most recent evidence on the efficacy and safety of luseogliflozin, our study aimed to conduct a systematic review and meta-analysis of luseogliflozin in patients with type 2 diabetes mellitus.
Methods
A comprehensive search of electronic databases like PubMed, Cochrane CENTRAL, and Google Scholar was performed from the inception to the 31st of August 2023 to identify the randomized controlled trials (RCTs) that examined the glucose and body weight lowering efficacy and safety outcomes of luseogliflozin in comparison with control or other active treatments. The fixed or random-effect model was used based on the heterogeneity identified using the I2 statistic and Cochran’s Q test.
Results
Out of 50 non-duplicate articles identified through database searching, 8 RCTs (11 studies) with 1922 patients were included in this study. The efficacy outcomes like HbA1c (MD: −0.59%; 95% CI: −0.90, −0.29; P < 0.001), FPG (MD: −16.01 mg/dL; 95% CI: −19.46, −12.57; P < 0.001), PPG (MD: −36.63 mg/dL; 95% CI: −43.71, −29.55; P < 0.001) and body weight (MD: −1.66 kg; 95% CI: −2.23, −1.12; P < 0.001) were significantly reduced with luseogliflozin compared to the control group. Regarding the safety outcomes, there was no statistically significant difference between the two groups for hypoglycemia (OR: 1.14; 95% CI: 0.70, 1.84; P = 0.60). However, pollakiuria (OR: 4.08; 95% CI: 1.71, 9.69; P < 0.001) and any ADRs (OR: 2.04; 95% CI: 1.33, 3.14; P < 0.001) were significantly higher in the luseogliflozin group compared to the control.
Conclusion
The current study identified a significant improvement in efficacy outcomes of HbA1c, FPG, PPG, and body weight in the luseogliflozin group. Non-significant safety results may be due to a smaller population size and fewer studies. Hence, long-term multicentric RCTs are needed to identify the safety and efficacy in a diversified population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the International Diabetes Federation (IDF), over 536.6 million individuals will have diabetes in 2021 all over the world, and by 2045, this count is predicted to be 783.2 million [1]. Diabetes incidence increased from the 1990s to the mid-2000s and has been stable [2]. It is a chronic metabolic condition with diverse etiology, social risk factors, and genetic, behavioral, and environmental susceptibility variables. Although it is linked to significant problems, an early diagnosis and starting the treatment may stop or postpone the onset of long-term effects. The development of end-stage renal disease, retinopathy resulting in blindness, cardiovascular diseases, and limb amputations are some of the chronic complications of diabetes mellitus. All of these conditions raise the morbidity and mortality rates of type 2 diabetes mellitus (T2DM) patients [3].
The treatment for T2DM primarily focuses on lifestyle modification and pharmacological treatment. It is challenging to prescribe the best course of therapy for patients due to the wide range of recommendations in the area, which can result in inefficiencies and increase the financial burden on patients and healthcare systems [4, 5]. A novel anti-diabetic class, sodium-glucose cotransporter 2 (SGLT2) inhibitors, is getting prominence for managing T2DM in recent years. Inhibiting SGLT2 improves the excretion of urinary glucose by blocking the reabsorption of filtered glucose in the kidney’s proximal convoluted tubules, decreasing plasma glucose levels, and improving glycemic control [6,7,8]. Canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin are currently available SGLT2 inhibitors in the market. In addition to glycemic control, SGLT2 inhibitors improve cardiovascular and renal outcomes, including reduced hospitalization due to heart failure and decreased risk of renal disease progression [9, 10]. Even though SGLT2 inhibitors have beneficial effects, a recent overview of information from post-marketing studies indicates that they can also have adverse effects, including volume depletion, diabetic ketoacidosis, genital and urinary tract infections, bladder cancer, bone fractures, Fournier gangrene and foot & leg amputations [11,12,13,14].
Luseogliflozin, an orally active second-line SGLT2 inhibitor with an inhibitory concentration (IC50) of 2.26 nM, exhibits 1765-fold selectivity for inhibition of SGLT2 over SGLT1 [8]. It acts by inhibiting SGLT2-mediated renal reabsorption in the proximal convoluted tubule [7, 15, 16]. It was licensed by the regulatory authority of Japan in 2014. When used alone, luseogliflozin has produced favorable results for glycemic control and weight loss [17]. Luseogliflozin is an orally accessible, highly selective SGLT2 inhibitor and 1-thio-D-glucitol derivative that decreases blood sugar levels by encouraging glucose excretion through the urine [8, 18]. In db/db mice and streptozotocin-induced diabetic rats, it lowered hyperglycemia, improving glucose tolerance without increasing insulin secretion [8]. Luseogliflozin lowers the levels of glycated hemoglobin (HbA1c), body weight, serum lipid profile, serum uric acid (SUA), markers of renal and hepatic function, and adiponectin levels [17, 19]. It also decreases blood pressure compared to the dipeptidyl peptidase-4 inhibitor (DPP-4i) [20]. Haneda M et al. concluded that luseogliflozin was well tolerated and safer in patients with different renal functions regardless of baseline estimated glomerular filtration rate (eGFR), and no serious safety issues were shown in these patients [21]. Much research has been done on luseogliflozin and concluded that it has various beneficial activities on the human body, notably lowering HbA1c and cardioprotective, renoprotective, and SUA activity. However, there is no concrete evidence that the results are accurate.
Even though luseogliflozin shows favorable efficacy data, it has not been approved by countries beyond Japan. The current study aims to present a pooled estimate of all the published safety and efficacy parameters from the published randomized controlled trials (RCTs) of luseogliflozin for T2DM. The study thoroughly sought and evaluated the relevant research literature, found admissible studies, extracted data, and combined the findings utilizing appropriate statistical techniques.
Methods
A systematic review was performed as per the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [22]. An extensive search was performed in PubMed/MEDLINE, Cohrane CENTRAL, and Google Scholar from inception to 31st August 2023. The search was also performed in ClinicalTrials.gov and International Clinical Trials Registry Platform (ICTRP) to identify the grey literature. The keywords included in the search strategy were T2DM AND (“Luseogliflozin” OR “SGLT-2 inhibitor”) without any restrictions related to language or publication year. A specific search strategy is mentioned in Supplementary Table S1.
Phase II or III RCTs with subjects assigned to treatment with the 2.5 or 5 mg doses of luseogliflozin or control (placebo/active control), consisting of efficacy parameters like the mean change in HbA1c levels from the baseline, postprandial glucose (PPG), fasting plasma glucose (FPG), body weight, and the safety outcomes like hypoglycemia, any adverse events published were included. Case reports, case series, cohort studies, case-control studies, in-vitro studies, reviews, and non-randomized trials were excluded.
All the included studies were independently reviewed by the two reviewers (RRG and PNH) for further evaluation of the data, followed by the extraction of the following information from each study: first author’s last name, year of publication, study design, country of the population studied, sample size and defined efficacy and safety parameters, if applicable. A third reviewer (CT) re-evaluated the extracted data. The quality of the included RCTs was independently evaluated by two reviewers (RRG and PNH) using the JADAD scale [23], and the risk of bias was assessed using the Risk of Bias 2 (RoB2) tool [24]. Discrepancies were resolved by a joint revaluation of the original articles with a third reviewer (KU).
Meta-analysis was conducted using RevMan version 5.4.1. Mean Difference (MD) with 95% CI was used to estimate the pooled effect sizes for continuous variables and odds ratio (OR) for dichotomous variables. The Higgins inconsistency index (I2) and Cochran’s Q test were used to estimate the heterogeneity, and it was defined by an I2 > 50% and/or Cochran’s Q test P-value ≤ 0.1. The funnel plot was used to estimate publication bias by visual inspection. Subgroup analyses were conducted based on various doses of luseogliflozin as well as based on the comparator. We performed a sensitivity analysis by re-estimating the pooled estimate after excluding studies with the smallest sample size.
Results
Search results
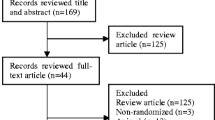
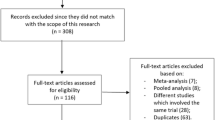
A systematic literature search found 50 records from databases like PubMed, Cochrane CENTRAL, and Google Scholar. No duplicates were found after the screening of the records. Further, 26 records featuring non-controlled trials that did not contain target drugs, post hoc sub-analysis, diseases other than T2DM, study protocols, systematic reviews, and meta-analysis were excluded. The remaining 24 full-text articles were assessed for eligibility. Furthermore, 13 articles were excluded because they didn’t fit into our prespecified criteria. Finally, 8 articles (including 11 studies) were finalized for the qualitative synthesis, and all were accounted for in the meta-analysis [17, 19, 25,26,27,28,29,30]. The PRISMA flow chart is demonstrated in Fig. 1.
Characteristics of included studies
The meta-analysis includes a total of 8 RCTs (11 studies) with 1922 participants. Among the included studies, eight were compared with the placebo [17, 19, 27, 29, 30], and three were with the active control [25, 26, 28] (voglibose, metformin, DPP-4is). The impact of luseogliflozin on HbA1c was assessed in 11 studies to determine its efficacy. Similarly, nine studies sought its effects on FPG, and eight focused on luseogliflozin’s effects on PPG. Ten studies investigated the impact of luseogliflozin on body weight, six evaluated hypoglycemia, and six investigated blood pressure. The JADAD scale assessed the quality of the RCTs, and nine studies scored more than three, considered as high quality [17, 19, 27,28,29,30], and two studies scored less than three as low quality [25, 26]. The RoB2 tool was used to assess the risk of bias; three studies were at high risk [17, 25, 28], two were at low risk [27, 30], and three raised some concerns [19, 26, 29] (Supplementary Fig. S26). The detailed characteristics of the included studies are demonstrated in Supplementary Table S2.
Primary outcomes
HbA1c
Findings from the 11 studies were combined with 1922 individuals (1011 in the luseogliflozin group and 911 in the control group). The primary analysis showed that the luseogliflozin group was more effective at lowering HbA1c than the control group (MD: −0.59%; 95% CI: −0.90, −0.29), with the significance of P < 0.001. Heterogeneity among the trials was considerably high (I2: 97%, P < 0.001). Hence, the random effects model was executed. A dose-based subgroup analysis found that treatment with luseogliflozin 2.5 and 5 mg showed statistically significant differences (2.5 mg: MD: −0.64%; 95% CI: −1.05, −0.24; P < 0.001; 5 mg: MD: −0.50%; 95% CI: −0.71 −0.29; P < 0.001) compared to the control (Fig. 2). However in the comparator-based subgroup analysis, luseogliflozin lowers HbA1c significantly in comparison with placebo (MD: −0.62%; 95% CI: −0.80, −0.44; P < 0.001), but not in comparison with the active control (MD: −0.55%; 95% CI: −1.57, 0.46; P < 0.28) (Table 1, Supplementary Fig. S22).
Fasting plasma glucose (FPG)
In nine studies, 652 patients received luseogliflozin treatment, while 557 received a control. The pooled estimate showed a significantly higher mean change from a baseline level of FPG in the luseogliflozin group than in the control group (MD: −16.01 mg/dL; 95% CI: −19.46, −12.57; P < 0.001) (Fig. 3). The studies were found to have significant heterogeneity (I2: 97%, P < 0.001), so a random effects model was applied. The subgroup analysis of luseogliflozin 2.5 mg and 5 mg was performed, and it was found to have a higher MD compared to control (2.5 mg: MD: −18.69 mg/dL; 95% CI: −34.79, −2.60; P = 0.02; 5 mg: MD: −18.61 mg/dL; 95% CI: −40.78, 3.55; P = 0.10).
Postprandial glucose (PPG)
The PPG was analyzed in eight studies with 1158 patients (626 luseogliflozin patients and 532 control groups). The statistical significance in the pooled results suggested that luseogliflozin was more beneficial in reducing PPG compared to the control group (MD: −36.63 mg/dL; 95% CI: −43.71, −29.55; P < 0.001) (Fig. 4). Because of considerable heterogeneity (I2: 98%, P < 0.001) random effects model was executed. Both the subgroups based on the dose (2.5 and 5 mg) revealed a lowering in PPG compared to the control (2.5 mg: MD: −45.08 mg/dL; 95% CI: −79.68, −10.48; P = 0.01; 5 mg: MD: −39.45 mg/dL; 95% CI: −86.53, 7.62; P = 0.10).
Body weight
Results from ten studies were combined with 1891 individuals (996 in the luseogliflozin group and 895 in the control group). The primary analysis showed the reduction in the body weight favors the luseogliflozin group (MD: −1.66 kg; 95% CI: −2.07, −1.24) with a significance, P < 0.001 (Fig. 5), and considerable heterogeneity was found among the studies (I2 = 80%, P < 0.001); hence random effects model was applied. The subgroup analysis was used to determine the statistical significance of luseogliflozin 2.5 mg and 5 mg compared to the control, and it was found that there was a statistical significance difference (2.5 mg: MD: −1.64 kg; 95% CI: −2.22, −1.06; P < 0.001; 5 mg: MD: −1.67 kg; 95% CI: −2.23, −1.12; P < 0.001) in lowering body weight. However, comparator-based subgroup analysis revealed that the reduction in body weight was significant when compared to placebo (MD: −1.54 kg; 95% CI: −1.80, −1.28; P < 0.001) but not in comparison with active control (MD: −1.93 kg; 95% CI: −4.78, 0.91; P = 0.18 (Table 1, Supplementary Fig. S23).
Secondary outcomes
The study evaluated additional outcomes, including intact proinsulin, fasting insulin, insulin (2 h), homa β, homa R, glycosylated albumin, and fasting insulin. The results showed that glycosylated albumin (2.5 mg: MD: −3.40; 95% CI: −4.31, −2.49; P < 0.001; 5 mg: MD: −3.38; 95% CI: −3.87, −2.88; P < 0.001), homa β (2.5 mg: MD: 4.26; 95% CI: 1.68, 6.85; P < 0.001; 5 mg: MD: 6.58; 95% CI: 3.25, 9.90; P < 0.001), homa R (2.5 mg: MD: −0.65; 95% CI: −1.18, −0.13; P < 0.001; 5 mg: MD: −0.82; 95% CI: −1.24, −0.40; P < 0.001), and urinary glucose (2.5 mg: MD: 7.75; 95% CI: 6.03, 9.47; P < 0.001; 5 mg: MD: 9.32; 95% CI: 7.57, 11.07; P < 0.001) were favors the luseogliflozin group compared to the control group. Luseogliflozin 5 mg was associated with significant reductions in insulin (2 h) (MD: −5.65; 95% CI: −9.25, −2.06; P < 0.001) and intact pro-insulin (MD: −1.88; 95% CI: −3.28, −0.48; P < 0.001) and in the meantime, the 2.5 mg subgroup showed no statistical significance. Luseogliflozin also led to significant reductions in systolic blood pressure (SBP) (2.5 mg: MD: −4.21; 95% CI: −5.78, −2.64; P < 0.001; 5 mg: MD: −5.26; 95% CI: −8.10, −2.41; P < 0.001), and diastolic blood pressure (DBP) (2.5 mg: MD: −1.84; 95% CI: −2.95, −0.73; P = 0.001; 5 mg: MD: −2.80; 95% CI: −4.79, −0.82; P < 0.001) (Table 1).
Safety outcomes
Hypoglycemia
This involved six studies with 1000 patients that noted the existence of hypoglycemia as a safety parameter (542 luseogliflozin-treated patients and 458 control group patients). The forest plot suggested that there was no statistical significance for the hypoglycemia, and it was not evident in either the luseogliflozin group (both 2.5 and 5 mg) or the control group (OR: 1.14; 95% CI: 0.70, 1.84; P = 0.60). There was no heterogeneity between the studies (I2: 0%; P = 0.63) (Fig. 6).
Notably, the safety outcome of pollakiuria (OR: 4.08; 95% CI: 1.71, 9.69; P = 0.001) and any ADRs (OR: 2.04; 95% CI: 1.33, 3.14; P = 0.001) were statistically significant in the luseogliflozin group compared to the control group. The most typical safety outcomes were mild and transitory. In subgroup analysis, all other safety outcomes, including nasopharyngitis, genital infections, and thirst, were not statistically significant (Table 2).
Assessment of publication bias
The publication bias of studies was visually examined by funnel plot. The plot reveals asymmetry in the pooled effect, indicating that publication bias exists (Fig. S20).
Sensitivity analysis
Sensitivity analysis was conducted by eliminating the study with the smallest sample size (Shibuya, 2018, sample size: 32, weight of the study: 1.4%), which specified that the outcome was stable, and there was no change in the outcome of HbA1c mean change (Fig. S21).
Discussion
Our meta-analysis compared luseogliflozin with placebo or other conventional oral antidiabetic medications (voglibose, metformin, DPP-4is), including 8 RCTs with 1922 T2DM patients. Our meta-analysis found that luseogliflozin significantly improved HbA1c and FPG in T2DM patients by systematically analyzing and combining the existing evidence. Furthermore, the luseogliflozin treatment resulted in clinically relevant lower body weight, PPG, and blood pressure than the control. Overall, it was identified that luseogliflozin effectively improves glycemic control, weight loss, and blood pressure in patients with T2DM. Compared to the previous meta-analysis on the safety and efficacy of luseogliflozin [31], this meta-analysis included comprehensive data from recently published studies [26, 27] with updated outcomes and a dose-based subgroup analysis of luseogliflozin 2.5 and 5 mg, as well as comparator based subgroup analysis.
Luseogliflozin was well tolerated, with no significant adverse events across treatment groups. Despite the decrease in FPG, hypoglycemia was infrequent in participants taking luseogliflozin due to its insulin-independent mechanism of action, and neither the drug nor a placebo showed a statistically significant variation in hypoglycemia. The results revealed a lower incidence of hypoglycemia in the luseogliflozin compared to the control.
Luseogliflozin was developed by Taisho Pharmaceutical Co., Ltd. got its first global approval in April 2013 in Japan for T2DM alone or in combination with other oral hypoglycemic medications [32]. The U.S. Food and Drug Administration (FDA) has yet to authorize using luseogliflozin, a new and highly selective SGLT2 inhibitor, in the United States. In our study, out of 11 studies, 10 were conducted on Japanese patients [17, 19, 25, 26, 28,29,30] and one in Russia [27], which could be the reason for the lack of approval by the U.S. FDA. With the assistance of our findings in this systematic review and meta-analysis, further long-term multicentre studies are needed to obtain drug approvals in other countries.
Canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin are FDA-approved for treating adult patients with T2DM to improve blood sugar control in addition to diet and exercise [33]. Current evidence suggests that SGLT2 inhibitors significantly improve the cardio-renal outcomes in T2DM patients [9, 10]. The most often observed adverse events with this class of medications are urinary tract infections, hypoglycemia, female genital mycotic infections, and increased urination [34]. Notably, luseogliflozin has a marginally similar reduction of HbA1c levels compared to canagliflozin, dapagliflozin, and empagliflozin [35,36,37]. However, there is a comparatively lower incidence of these adverse events in luseogliflozin than in previously established SGLT-2 inhibitors, and luseogliflozin has the lowest dose among the other SGLT2 inhibitors available on the market [8, 27].
In the Phase 2 clinical trial, all tested doses (0.5, 2.5, 5 mg) of luseogliflozin helped to achieve glycemic control, reduce body weight, and be well tolerated in T2DM patients over 12 weeks [29]. The 24-week Phase 3 clinical study showed that adding luseogliflozin to insulin therapy significantly improved weight loss and glycemic control in Japanese individuals with T2DM [30]. The dose-finding study of this drug in Japanese individuals with T2DM determined that doses greater than or equal to 2.5 mg improved glycemic control [38]. The MUSCAT-HF trial compared luseogliflozin with voglibose and found that luseogliflozin had no statistically significant effect on proBNP levels in heart failure with preserved ejection fraction in patients with T2DM [28]. Studies by Seino et al. and Ejiri et al. revealed that luseogliflozin significantly affects SBP and DBP. Like empagliflozin, luseogliflozin also prevents cardiovascular risk [17, 28,29,30].
The major limitation of our study was the smaller population size and the smaller number of outcomes evaluated in all included studies. Secondly, we evaluated the efficacy and safety of luseogliflozin in T2DM, which was not generalized to subjects with type 1 diabetes mellitus (T1DM).
Conclusion
This systematic review and meta-analysis identified a significant improvement in HbA1c, FPG, PPG, and body weight efficacy outcomes for luseogliflozin in comparison with placebo or active control. Nonsignificant safety results may be due to a smaller population size and a smaller number of outcomes. Further, there is a need for long-term multicentre studies to get marketing approvals for luseogliflozin in countries other than Japan.
References
IDF Diabetes Atlas. 10th ed. Available from: https://diabetesatlas.org/ (2023)
D.J. Magliano, R.M. Islam, E.L.M. Barr, E.W. Gregg, M.E. Pavkov, J.L. Harding et al. Trends in incidence of total or type 2 diabetes: systematic review. BMJ 366, l5003 (2019)
O.R. Temneanu, L.M. Trandafir, M.R. Purcarea, Type 2 diabetes mellitus in children and adolescents: a relatively new clinical problem within pediatric practice. J. Med Life 9(3), 235–239 (2016)
Y.A. Domínguez, M. Emiliano, L. Puigi, J.H. Rodríguezi, Contribución de la Epidemiología al estudio de la diabetes mellitus. Rev. Cubana Hig. Epidemiol. 55(2), 46–50 (2017)
A.E.I. Coria, A.A. Cortés, O.F.R. de la Roche, E.C. Hernández, La Diabetes Mellitus y sus implicaciones sociales y clínicas en México y Latinoamérica Diabetes Mellitus and its Social and Clinical Implications in Mexico and Latin America. Archivos en. Med. Fam. 19(4), 91–94 (2017)
S. Katayama, M. Hatano, M. Issiki, Clinical features and therapeutic perspectives on hypertension in diabetics. Hypertension Res. 41(4), 213 (2018)
G. Musso, R. Gambino, M. Cassader, G. Pagano, A novel approach to control hyperglycemia in type 2 diabetes: Sodium glucose co-transport (SGLT) inhibitors. Systematic review and meta-analysis of randomized trials. Ann. Med. 44(4), 375–393 (2012)
K. Yamamoto, S. Uchida, K. Kitano, N. Fukuhara, L. Okumura-Kitajima, E. Gunji et al. TS-071 is a novel, potent and selective renal sodium-glucose cotransporter 2 (SGLT2) inhibitor with anti-hyperglycaemic activity. Br. J. Pharm. 164(1), 181–191 (2011)
T.A. Zelniker, S.D. Wiviott, I. Raz, K. Im, E.L. Goodrich, M.P. Bonaca et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 393(10166), 31–39 (2019)
T. Yamada, M. Wakabayashi, A. Bhalla, N. Chopra, H. Miyashita, T. Mikami et al. Cardiovascular and renal outcomes with SGLT-2 inhibitors versus GLP-1 receptor agonists in patients with type 2 diabetes mellitus and chronic kidney disease: a systematic review and network meta-analysis. Cardiovasc. Diabetol. 20(1), 14 (2021)
M. Singh, A. Kumar, Risks Associated with SGLT2 Inhibitors: An Overview. Curr. Drug Saf. 13(2), 84–91 (2018)
J.B. McGill, S. Subramanian, Safety of Sodium-Glucose Co-Transporter 2 Inhibitors. Am. J. Cardiol. 124(Suppl 1), S45–S52 (2019)
A. Tentolouris, P. Vlachakis, E. Tzeravini, I. Eleftheriadou, N. Tentolouris. SGLT2 Inhibitors: A Review of Their Antidiabetic and Cardioprotective Effects. Int. J. Environ. Res. Public Health. 16, 2965 (2019)
R. Pelletier, K. Ng, W. Alkabbani, Y. Labib, N. Mourad, J.M. Gamble, Adverse events associated with sodium glucose co-transporter 2 inhibitors: an overview of quantitative systematic reviews. Ther. Adv. Drug Saf. 12, 2042098621989134 (2021)
W.L. Bennett, N.M. Maruthur, S. Singh, J.B. Segal, L.M. Wilson, R. Chatterjee et al. Comparative effectiveness and safety of medications for type 2 diabetes: An update including new drugs and 2-drug combinations. Ann. Intern Med 154(9), 602–618 (2011)
B. Peene, K. Benhalima, Sodium glucose transporter protein 2 inhibitors: Focusing on the kidney to treat type 2 diabetes. Ther. Adv. Endocrinol. Metab. 5(5), 124–136 (2014)
Y. Seino, T. Sasaki, A. Fukatsu, M. Ubukata, S. Sakai, Y. Samukawa, Efficacy and safety of luseogliflozin as monotherapy in Japanese patients with type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled, phase 3 study. Curr. Med Res Opin. 30(7), 1245–1255 (2014)
H. Kakinuma, T. Oi, Y. Hashimoto-Tsuchiya, M. Arai, Y. Kawakita, Y. Fukasawa et al. (1S)-1,5-anhydro-1-[5-(4-ethoxybenzyl)-2-methoxy-4-methylphenyl]-1-thio- d -glucitol (TS-071) is a potent, selective sodium-dependent glucose cotransporter 2 (SGLT2) inhibitor for type 2 diabetes treatment. J. Med. Chem. 53(8), 3247–3261 (2010)
Y. Seino, K. Kaku, N. Inagaki, M. Haneda, T. Sasaki, A. Fukatsu et al. Fifty-two-week long-term clinical study of luseogliflozin as monotherapy in Japanese patients with type 2 diabetes mellitus inadequately controlled with diet and exercise. Endocr. J. 62(7), 593–603 (2015)
R. Hashimoto-Kameda, K.Y. Cho, H. Nomoto, A. Nakamura, K. Omori, S. Nagai, et al. Lowering of blood pressure and pulse rate by switching from DPP-4 inhibitor to luseogliflozin in patients with type 2 diabetes complicated with hypertension: A multicenter, prospective, randomized, open-label, parallel-group comparison trial (LUNA study). Diabetes Res Clin Pract. 180, 109069 (2021)
M. Haneda, Y. Seino, N. Inagaki, K. Kaku, T. Sasaki, A. Fukatsu et al. Influence of Renal Function on the 52-Week Efficacy and Safety of the Sodium Glucose Cotransporter 2 Inhibitor Luseogliflozin in Japanese Patients with Type 2 Diabetes Mellitus. Clin. Ther. 38(1), 66–88.e20 (2016)
M.J. Page, J.E. McKenzie, P.M. Bossuyt, I. Boutron, T.C. Hoffmann, C.D. Mulrow et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71 (2021)
A.R. Jadad, R.A. Moore, D. Carroll, C. Jenkinson, D.J. Reynolds, D.J. Gavaghan et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin. Trials 17(1), 1–12 (1996)
J.A.C. Sterne, J. Savović, M.J. Page, R.G. Elbers, N.S. Blencowe, I. Boutron, et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 366, l4898 (2019)
T. Shibuya, N. Fushimi, M. Kawai, Y. Yoshida, H. Hachiya, S. Ito et al. Luseogliflozin improves liver fat deposition compared to metformin in type 2 diabetes patients with non-alcoholic fatty liver disease: A prospective randomized controlled pilot study. Diab. Obes. Metab. 20(2), 438–442 (2018)
M. Sugawara, M. Fukuda, I. Sakuma, Y. Wakasa, H. Funayama, A. Kondo, et al. Overall Efficacy and Safety of Sodium-Glucose Cotransporter 2 Inhibitor Luseogliflozin Versus Dipeptidyl-Peptidase 4 Inhibitors: Multicenter, Open-Label, Randomized-Controlled Trial (J-SELECT study). Diabetes Ther. 14(9), (2023)
M. Shestakova, B. Kvasnikov, E. Erina, E. Isachenko, A. Andreev, Efficacy and safety of luseogliflozin in Caucasian patients with type 2 diabetes: results from a phase III, randomized, placebo-controlled, clinical trial. BMJ Open Diab. Res Care 11(3), e003290 (2023)
K. Ejiri, T. Miyoshi, H. Kihara, Y. Hata, T. Nagano, A. Takaishi, et al. Effect of Luseogliflozin on Heart Failure With Preserved Ejection Fraction in Patients With Diabetes Mellitus. J Am Heart Assoc. 9(16), e015103 (2020)
Y. Seino, T. Sasaki, A. Fukatsu, S. Sakai, Y. Samukawa, Efficacy and safety of luseogliflozin monotherapy in Japanese patients with type 2 diabetes mellitus: a 12-week, randomized, placebo-controlled, phase II study. Curr. Med. Res. Opin. 30(7), 1219–1230 (2014)
Y. Seino, T. Sasaki, A. Fukatsu, H. Imazeki, H. Ochiai, S. Sakai, Efficacy and safety of luseogliflozin added to insulin therapy in Japanese patients with type 2 diabetes: a multicenter, 52-week, clinical study with a 16-week, double-blind period and a 36-week, open-label period. Curr. Med. Res. Opin. 34(6), 981–994 (2018)
D. Dutta, J. Kadian, K. Mahajan, A. Dhall, M. Sharma, Efficacy and safety of luseogliflozin in improving glycaemic and non-glycaemic outcomes in type-2 diabetes: A meta-analysis. Diabetes Metab Syndr. 17(3), 102742 (2023)
A. Markham, S. Elkinson, Luseogliflozin: first global approval. Drugs 74(8), 945–950 (2014)
Sodium-Glucose Transport Protein 2 (SGLT2) Inhibitors - PubMed. Available from: https://pubmed.ncbi.nlm.nih.gov/35015430/ (2023)
S. Halimi, B. Vergès, Adverse effects and safety of SGLT-2 inhibitors. Diab. Metab. 40(6 Suppl 1), S28–S34 (2014)
W. Xiong, M.Y. Xiao, M. Zhang, F. Chang, Efficacy and safety of canagliflozin in patients with type 2 diabetes. Medicine 95(48), e5473 (2016)
R. Devi, G. Mali, I. Chakraborty, M.K. Unnikrishnan, S. Abdulsalim, Efficacy and safety of empagliflozin in type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Postgrad. Med. 129(3), 382–392 (2017)
M. Feng, H. Lv, X. Xu, J. Wang, W. Lyu, S. Fu, Efficacy and safety of dapagliflozin as monotherapy in patients with type 2 diabetes mellitus. Medicine 98(30), e16575 (2019)
Y. Seino, T. Sasaki, A. Fukatsu, M. Ubukata, S. Sakai, Y. Samukawa, Dose-finding study of luseogliflozin in Japanese patients with type 2 diabetes mellitus: a 12-week, randomized, double-blind, placebo-controlled, phase II study. Curr. Med. Res. Opin. 30(7), 1231–1244 (2014)
Acknowledgements
The authors thank the authorities of the National Institute of Pharmaceutical Education and Research (NIPER) Guwahati for their constant support while conducting this study.
Author information
Authors and Affiliations
Contributions
Conceptualization, methodology, data curation: R.R.G., P.N.H., K.U.; formal analysis, investigation, writing (original draft preparation), visualization: R.R.G., P.N.H., C.T.; writing (review and editing): R.R.G., P.N.H., C.T.; review (systematic search): K.U., C.T.; review (reporting quality): K.U., C.T.; methodology, supervision: K.U.; All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Galigutta, R.R., Hasik, P.N., Thomas, C. et al. Efficacy and safety of luseogliflozin in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Endocrine (2024). https://doi.org/10.1007/s12020-024-03925-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12020-024-03925-x