Abstract
Purpose
No study has comprehensively assessed the relationship of metabolic factors including insulin resistance, hypertension, hyperuricemia, and hypercholesterolemia with the development of carotid plaque. Therefore, we constructed metabolic scores based on the above metabolic factors and examined its association with carotid plaque in young and older Chinese adults.
Methods
This study included 17,396 participants who underwent carotid ultrasound examinations, including 14,173 young adults (<65 years) and 3,223 older adults (≥65 years). Individual metabolic score was calculated using triglyceride-glucose (TyG) index, mean arterial pressure (MAP), uric acid, and total cholesterol (TC). Logistic regression models were conducted to examine the role of metabolic score and its components in the prevalence of carotid plaque. The nonlinear relationship was examined using restricted cubic spline regression. Meanwhile, subgroup, interaction, and sensitivity analyses were conducted.
Results
The multivariate logistic regression analysis showed that TyG (OR: 1.088; 95%CI: 1.046-1.132), MAP (OR: 1.121; 95%CI: 1.077–1.168), TC (OR: 1.137; 95%CI: 1.094–1.182) and metabolic score (OR: 1.064; 95%CI: 1.046–1.082) were associated with carotid plaque prevalence in young adults rather than older adults. The nonlinear association was not observed for metabolic scores and carotid plaque. Subgroup analyses showed significant associations between metabolic scores and carotid plaque prevalence in men, women, normal-weight, and overweight young adults. No interaction of metabolic score with sex and BMI were observed.
Conclusions
The results support that control of TyG, MAP, TC, and metabolic scores is a key point in preventing the prevalence of carotid plaque in the young adults.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular disease has become the leading cause of disability and premature death worldwide. By 2030, about 23.6 million people are expected to die of cardiovascular disease each year globally [1]. Cardiovascular disease is no longer confined to high-income countries. In recent decades, the prevalence of cardiovascular disease has surged significantly in low-income and middle-income countries, resulting in over 80 percent of cardiovascular disease deaths transpiring in these regions [2]. Atherosclerosis is the main pathologic process underlying most cardiovascular disease, which can begin early in life and remain latent and asymptomatic for long periods of time before entering advanced stages of life [3]. Plaque rupture and thrombosis are major causes of stroke and myocardial infarction, resulting in atherosclerosis is an independent risk factor for cardiovascular disease [4]. Carotid plaque is present in 20.2% of Chinese adults aged 30–80 years, according to a systematic review [5]. Therefore, early identification and intervention of risk factors associated with carotid plaque can help to reduce the burden of carotid atherosclerosis and cardiovascular disease on the public [6].
Many studies have shown that metabolic syndrome, defined as a group of interrelated metabolic risk factors (including abdominal obesity, elevated fasting glucose, hypertriglyceridemia, hypertension, and low high-density lipoprotein (HDL) cholesterol levels), can increase the risk of atherosclerosis and cardiovascular disease [7, 8]. Various metabolic components have also been associated with increased carotid artery stiffness and plaque volume [9]. Recent scientific interest has focused on the association of insulin resistance with involvement in carotid plaque, and the triglyceride-glucose (TyG) index has emerged as a valuable biomarker of insulin resistance [10, 11]. Some studies have shown that an elevated TyG index increases the likelihood of plaque formation in public carotid arteries [12]. However, Zhao et al. reported no association between these two variables, and there was no general consensus on whether TyG index has a substantial relationship with the incidence of carotid plaque [13]. In addition, a 7-year prospective cohort study found that carotid plaque was associated with higher blood pressure, pulse pressure, and mean arterial pressure (MAP) [14]. Observational studies suggest that high blood uric acid (UA) levels may also be a risk factor for carotid plaque [15, 16]. Previous clinical studies have consistently demonstrated a significant association between metabolic syndrome and the incidence of ischemic stroke [7, 17]. However, no study has comprehensively examined the relationship between various metabolic factors and carotid plaque.
In this study, we utilized four metabolic factors (TyG index, MAP, UA, and total cholesterol (TC)) to establish a metabolic score to reflect the overall metabolic status of the body. Similar to previous studies, four metabolic factors were chosen a priori without any data exploration [18]. Then, we estimated the effect of each of the major factors involved in metabolic score on the prevalence of carotid plaque in young and older adults. The associations between metabolic score and carotid plaque in young and older adults were also examined, which will contribute to the development and implementation of preventive measures against carotid plaque.
Methods
Setting and participants
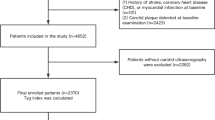
In this cross-sectional study, we extracted 42,900 records from individuals who participated in the annual physical examination and underwent carotid symmetry ultrasound examinations at the Health Management Center of a tertiary hospital in Henan Province from 2017 to 2020. We further excluded data with missing data on the blood pressure, blood lipid, blood glucose, UA, or BMI (n = 4339). Last, one recent examination record for each participant was retained for this study and 17,396 available participants were finally analyzed. The flowchart for the screening of participants was shown in Supplement Fig. 1. This study was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University (2018-KY-56) and all subjects had informed consent.
Physical examination
All participants were asked to complete a physical assessment, laboratory investigations, and carotid symmetry ultrasound. Physical assessment, including height, weight, and blood pressure, were performed by trained medical workers in line with standard instruments and protocols. All participants were asked to rest for at least 5 min in a quiet environment before blood pressure was measured. Trained staff members measured blood pressure two times using a standard mercury sphygmomanometer to average the values, and recorded systolic blood pressures (SBP) and diastolic blood pressures (DBP). After 12 h of fasting, 5 mL of blood was drawn by venipuncture in the morning for measurement of biochemical indices including TC, triglyceride (TG), HDL, fasting plasma glucose (FPG), and UA. Senior clinicians performed and reported on physical examination, laboratory tests, and bilateral carotid ultrasound. The participants were sequentially examined on both sides of the main carotid artery, common carotid artery bifurcation, internal carotid artery, external carotid artery, and subclavian artery using high-resolution B-mode ultrasound. Carotid plaque are focal structures encroaching into the arterial lumen of at least 0.5 mm or 50% of the surrounding intima-media thickness value or demonstrates a thickness >1.5 mm as measured from the intima-lumen interface to the media-adventitia interface. According to expert consensus, the overall diagnostic accuracy could reach 90% [19].
Definitions
BMI was calculated as weight divided by height squared (kg/m2). The mean arterial pressure (MAP) was calculated as DBP + 1/3(SBP-DBP). The TyG index was considered a surrogate for identifying insulin resistance and determining metabolic health status. The calculation formula of TyG was ln[TG(mg/dL)*FPG(mg/dL)/2] [20, 21]. Then, we constructed metabolic score as the sum of the z-transformed values of TyG index, MAP, UA, and TC. It is important to note that the UA Z-scores for men and women are calculated separately because of the differences in the reference ranges of UA for men and women. The continuous variables of TyG index, MAP, UA, TC, and metabolic score were transformed into categorical variables using quartile methods.
Statistical analysis
Since the data distribution was skewed, the continuous variables were shown using median and interquartile range (P50 (P25–P75)), while categorical variables were expressed using frequency (%). The distribution difference of baseline demographics and other covariables among different age group was estimated by chi-square test for a categorical variable and by Wilcoxon rank-sum test for a continuous variable. Logistic regression models were performed to estimate the association of carotid plaque prevalence with TyG index, MAP, TC, UA, and metabolic score in young adults and older adults. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated with and without adjustment for covariates. The trend test is performed by assigning medians to each quartile of the index and entering them as continuous variables in the model. In model 1, no confounders were adjusted; in model 2, age, sex, and BMI were adjusted based on model 1. The potential nonlinear relationship was examined using restricted cubic spline (RCS) regression (knots on the 25th, 50th, and 75th percentiles). RCS analyses were performed to avoid the potential effects of inappropriate linearity and to examine the precise dose-response association relationships between predictor and response variable. Stratified analysis was conducted by sex (male or female), and BMI (<18.5, 18.5–23.9, 24–27.9, or ≥28 kg/m2). The multiplicative interaction of metabolic score with sex and BMI was estimated respectively using the likelihood ratio test, with a comparison of the likelihood scores of the two models with and without the interaction terms. For sensitivity analyses, we reconstructed metabolic scores by deleting variables that were not meaningful for carotid plaque prevalence. All statistical analyses were performed using R 4.1.2. Tests were two-tailed and a P value less than 0.05 was considered statistically significant.
Results
Characteristics of the study population
A total of 17,396 participants were included in this study, which was consisted of 10,742 (61.75%) participants with carotid plaque, 11,486 (66.03%) males, and 14,173 (81.47%) participants less than 65 years of age (Table 1). Of all participants, the P50 (P25–P75) of age, BMI, FPG, TG, TyG index, SBP, DBP, MAP, TC, HDL, UA, and metabolic score was 55 (48–62) year, 24.89 (22.82–26.92) kg/m2, 5.30 (4.93–5.82) mmol/L, 1.34 (0.95–1.90) mmol/L, 8.67 (8.29–9.07), 127 (116–141) mmHg, 77 (69–86) mmHg, 94.33 (85.67–103.67) mmHg, 4.65 (4.04–5.28) mmol/L, 1.28 (1.09–1.53) mmol/L, 325 (270–383) mmol/L and −0.09 (−1.72 to 1.61). More participants in the ≥65 years group than in the <65 years group were carotid plaque and male, were high MAP, low TC, UA, and metabolic score.
Relationship of the metabolic score and its components with carotid plaque
The results of univariate and multivariate logistic regression analysis of TyG, MAP, UA, TC, and metabolic score with the prevalence of carotid plaque were consistent, as shown in Table 2. When adjusting for age, sex, and BMI, our study found that TyG (OR: 1.088; 95%CI: 1.046–1.132), MAP (OR: 1.121; 95%CI: 1.077–1.168), TC (OR: 1.137; 95%CI: 1.094–1.182) and metabolic score (OR: 1.064; 95%CI: 1.046–1.082) were associated with the prevalence of carotid plaque rather than UA (OR: 1.015; 95%CI: 0.970–1.062) in young adults. When continuous variables were transformed into categorical variables using quartile methods, compared with the lowest metabolic score group (<−1.72), participants with metabolic score at Q3 (−0.09–1.61) and Q4 (≥1.61) was significantly associated with a higher prevalence of carotid plaque with OR (95% CI) of 1.240 (1.109–1.386) and 1.434 (1.275–1.612), respectively. Whether or not confounders were adjusted, our study did not find TYG, MAP, UA, TC, and metabolic score were associated with the prevalence of carotid plaque in older adults. Figure 1 displayed the non-linear associations of carotid plaque prevalence with metabolic score through the RCS curve, and no significant non-linear associations were found. For the age <65 years, Pnonlinear was = 0.561, and for the age ≥65 years, Pnonlinear was = 0.641.
Restricted cubic spline of the relationship of carotid plaque risk with metabolic score, ((a) Age < 65 years, (b) Age ≥ 65 years). Potential nonlinear relationships were examined using restricted cubic splines (knots on the 25th, 50th, and 75th percentiles). The dash line represents the OR equals to 1. The ORs were adjusted for age, sex, and BMI
Subgroup analyses and interaction analyses
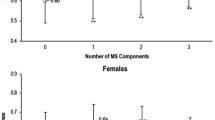
Figure 2 showed the results of subgroup analyses for the association between the continuous TyG index, MAP, UA, TC, metabolic score, and the carotid plaque prevalence. Relationships between TyG index, MAP, TC, metabolic score, and carotid plaque prevalence were observed in men, women, and normal-weight (18.5–23.9 kg/m2) and overweight (24–27.9 kg/m2) participants when participants were younger than 65 years. When the age of the participants was ≥65 years, the relationship between TyG index, MAP, TC, metabolic score, and carotid plaque prevalence was not observed in subgroup analyses. Figure 3 showed the results of the interaction between metabolic score and sex or BMI on the prevalence of carotid plaque. The results showed no interaction of metabolic score with sex and BMI in either the age <65 years, or ≥65 years population (Pinteraction > 0.05).
The interaction between metabolic score, sex, and BMI on the prevalence of carotid plaque ((a) Interaction of metabolic score and sex in the population <65 years; (b) Interaction of metabolic score and sex in the population ≥65 years; (c) Interaction of metabolic score and BMI in the population <65 years; (d) Interaction of metabolic score and BMI in the population ≥65 years). The population of individuals with underweight status (BMI < 18.5 Kg/m2) is relatively small, and therefore, this particular group is not taken into consideration in the analysis of interactions
Sensitivity analyses
Because the associations between UA and carotid plaque prevalence was not statistically significant in the previous analysis, we constructed metabolic scores using three index, TyG index, MAP, and TC, in a sensitivity analysis (Supplement Table 1). Our study found that elevated metabolic scores were a risk factor for the prevalence of carotid plaque in people younger than 65 years with OR (95%CI) = 1.084 (1.063–1.106), regardless of whether or not adjusted for confounding factors. Similarly, compared with the lowest metabolic score group (< − 1.40), participants with metabolic score at Q2 (−1.40 to −0.07), Q3 (−0.07–1.30), and Q4 (≥1.30) was significantly associated with a higher prevalence of carotid plaque. However, in those population aged >65 years, without adjusting for confounders, elevated metabolic score reduced the risk of developing carotid plaque, and this association disappeared after adjusting for age, sex, and BMI.
Discussion
The present study demonstrated a strong correlation between TyG index, MAP, TC, metabolic score, and the prevalence of carotid plaque among the Chinese young adults. Subgroup analyses showed significant associations between metabolic scores and carotid plaque prevalence in men, women, normal-weight, and overweight young adults.
According to a large-scale study conducted in China, the TyG index was easier to use and better for identifying metabolically unhealthy individuals and those with a high likelihood of cardiometabolic disease [22]. However, the TyG index is only a valid proxy for insulin resistance and does not provide a comprehensive picture of other metabolic indicators in the body. In addition, it has been suggested that indicators of hypertension may induce carotid plaque through a variety of potential mechanisms, including genetic processes, endothelial dysfunction, or neurohumoral, humoral, and metabolic pathways [23]. Several studies have shown elevated UA leads to impaired vascular function and atherosclerosis, and hyperuricemia has also been suggested as a candidate risk factor for metabolic syndrome [24, 25]. Therefore, we established a new index, metabolic score, to synthesize the metabolic status of the body using TyG index, MAP, UA, and TC, and found a correlation between these indices and the prevalence of carotid plaque in young adults. But, in older adults, we did not find this association. A possible explanation is that aging is a significant risk factor for the prevalence of carotid plaque, which in our study was also found to be 92% in those ≥65 years of age [26]. A previous study found that 87% of Norwegian cohort participants aged 63 to 65 years had carotid plaque, consistent with our study [27]. In addition, using the RCS, we found that after adjusting for confounders, metabolic score had a linear rather than a nonlinear association with carotid plaque prevalence, the risk of carotid plaque prevalence to increase with an increasing score.
Subgroup analyses found that the TyG index, MAP, UA metabolic score was also significantly associated with the progression of carotid plaque in men, women, and normal-weight and overweight populations aged <65 years but not among individuals aged ≥65 years. Our study was consistent with a previous study, which showed a higher prevalence in male than in female patients with the prevalence of carotid plaque [28]. Possible reasons for this are the different levels of sex hormones in men and women, which affect vascular reactivity, inflammation, and lipoprotein metabolism, all of which contribute to the development of carotid plaque [29]. Besides, it is interesting that the association of metabolic score, TYG index, MAP, and TC with carotid plaque prevalence disappeared in the obese population (BMI ≥ 28 kg/m2). One possible explanation is that the association between the metabolic score, TyG index, MAP, and the prevalence of carotid plaque is more pronounced in the normal population. When the body is in a relatively unhealthy state, such as obesity or aging, the prevalence of carotid plaque increases significantly, regardless of normal metabolic status. In the future, it may be necessary to develop tailored interventions for different metabolic populations in order to reduce the occurrence of carotid plaque. The associations between UA and carotid plaque prevalence were not observed in young and older people. A previous study also supported our conclusion that UA level can be considered as an important risk factor for arterial stiffness in the Korean population, however, none of the carotid intima-media thicknesses were associated with UA [30]. Therefore, we removed UA to reconstruct the metabolic score and also found a strong association between the metabolic score and the incidence of carotid plaque in young adults in the sensitivity analyses.
There were some limitations of this study. First, the study design was cross-sectional, and all the collected data were based on carotid plaque prevalence. The causal relationship between metabolic score and prevalence of carotid plaque could not be clarified. Second, the lack of information on certain covariates, such as socioeconomic status and lifestyle, limited the reliability of the conclusions. Third, we only assessed the presence or absence of plaque, whereas assessing the stability of carotid plaque may provide more detailed information. Nonetheless, the presence of plaque in the carotid artery provided important information about the risk of cardiovascular events. Fourth, the population included in this study was those who had annual medical checkups at a tertiary care hospital, and the extent to which this finding applies to all populations deserves further exploration.
Conclusions
TyG index, MAP, TC, and metabolic score were modifiable risk factors for the prevalence of carotid plaque in young adults. Accordingly, the potential significance of metabolic factors controls in combating carotid plaque need to be emphasized.
Abbreviations
- TyG:
-
Triglyceride-glucose
- MAP:
-
Mean arterial pressure
- TC:
-
Total cholesterol
- HDL:
-
High-density lipoprotein
- UA:
-
Uric acid
- SBP:
-
Systolic blood pressures
- DBP:
-
Diastolic blood pressures
- TG:
-
Triglyceride
- FPG:
-
Fasting plasma glucose
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- RCS:
-
Restricted cubic spline
References
G.A. Roth, C. Johnson, A. Abajobir et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J. Am. Coll. Cardiol. 70, 1–25 (2017). https://doi.org/10.1016/j.jacc.2017.04.052
S. Mendis, S. Davis, B. Norrving, Organizational update: the world health organization global status report on noncommunicable diseases 2014; one more landmark step in the combat against stroke and vascular disease. Stroke 46, e121–e122 (2015). https://doi.org/10.1161/STROKEAHA.115.008097
P. Song, Z. Fang, H. Wang et al. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: a systematic review, meta-analysis, and modelling study. Lancet Glob. Health 8(5), e721–e729 (2020). https://doi.org/10.1016/S2214-109X(20)30117-0
Y. Jiao, Y. Qin, Z. Zhang, H. Zhang, H. Liu, C. Li, Early identification of carotid vulnerable plaque in asymptomatic patients. BMC Cardiovasc. Disord. 20(1), 429 (2020). https://doi.org/10.1186/s12872-020-01709-5
P. Song, W. Xia, Y. Zhu et al. Prevalence of carotid atherosclerosis and carotid plaque in Chinese adults: A systematic review and meta-regression analysis. Atherosclerosis 276, 67–73 (2018). https://doi.org/10.1016/j.atherosclerosis.2018.07.020
Y. Zhang, Z. Wu, X. Li, J. Wei, Q. Zhang, J. Wang, Association between the triglyceride-glucose index and carotid plaque incidence: a longitudinal study. Cardiovasc. Diabetol. 21(1), 244 (2022). https://doi.org/10.1186/s12933-022-01683-6
E.J. Benjamin, P. Muntner, A. Alonso et al. Heart disease and stroke statistics-2019 update: A report from the American Heart Association. Circulation 139(10), e56–e528 (2019). https://doi.org/10.1161/CIR.0000000000000659
L. Roever, E.S. Resende, A.L.D. Diniz et al. Metabolic syndrome and risk of stroke: Protocol for an update systematic review and meta-analysis. Medicine 97(15), e9862 (2018). https://doi.org/10.1097/MD.0000000000009862
F. Servadei, L. Anemona, M. Cardellini et al. The risk of carotid plaque instability in patients with metabolic syndrome is higher in women with hypertriglyceridemia. Cardiovasc. Diabetol. 20(1), 98 (2021). https://doi.org/10.1186/s12933-021-01277-8
J. Alizargar, C.H. Bai, N.C. Hsieh, S.V. Wu, Use of the triglyceride-glucose index (TyG) in cardiovascular disease patients. Cardiovasc. Diabetol. 19(1), 8 (2020). https://doi.org/10.1186/s12933-019-0982-2
W. Li, D. Chen, Y. Tao, Z. Lu, D. Wang, Association between triglyceride-glucose index and carotid atherosclerosis detected by ultrasonography. Cardiovasc. Diabetol. 21(1), 137 (2022). https://doi.org/10.1186/s12933-022-01570-0
Z. Wu, J. Wang, Z. Li et al. Triglyceride glucose index and carotid atherosclerosis incidence in the Chinese population: A prospective cohort study. Nutr. Metab. Cardiovasc. Dis. 31(7), 2042–2050 (2021). https://doi.org/10.1016/j.numecd.2021.03.027
S. Zhao, S. Yu, C. Chi et al. Association between macro- and microvascular damage and the triglyceride glucose index in community-dwelling elderly individuals: the Northern Shanghai Study. Cardiovasc. Diabetol. 18(1), 95 (2019). https://doi.org/10.1186/s12933-019-0898-x
J. Liu, X. Ma, X.L. Ren et al. The role of blood pressure in carotid plaque incidence: interactions with body mass index, age, and sex-based on a 7-year cohort study. Front. Physiol. 12, 690094 (2021). https://doi.org/10.3389/fphys.2021.690094
S. Takayama, R. Kawamoto, T. Kusunoki, M. Abe, M. Onji, Uric acid is an independent risk factor for carotid atherosclerosis in a Japanese elderly population without metabolic syndrome. Cardiovasc. Diabetol. 11, 2 (2012). https://doi.org/10.1186/1475-2840-11-2
L. Feng, C. Hua, H. Sun et al. Association between serum uric acid level and carotid atherosclerosis in chinese individuals aged 75 years or older: a hospital-based case-control study. J. Nutr. Health Aging 22(4), 508–512 (2018). https://doi.org/10.1007/s12603-017-0984-2
X. Li, X. Li, F. Fang, X. Fu, H. Lin, Q. Gao, Is metabolic syndrome associated with the risk of recurrent stroke: a meta-analysis of cohort studies. J. Stroke Cerebrovasc. Dis. 26(12), 2700–2705 (2017). https://doi.org/10.1016/j.jstrokecerebrovasdis.2017.03.014
J. Fritz, W. Brozek, H. Concin et al. The association of excess body weight with risk of ESKD is mediated through insulin resistance, hypertension, and hyperuricemia. J. Am. Soc. Nephrol. 33(7), 1377–1389 (2022). https://doi.org/10.1681/ASN.2021091263
P.J. Touboul, M.G. Hennerici, S. Meairs et al. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). Cerebrovasc. Dis. 34(4), 290–296 (2012). https://doi.org/10.1159/000343145
F. Guerrero-Romero, L.E. Simental-Mendia, M. Gonzalez-Ortiz et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J. Clin. Endocrinol. Metab. 95(7), 3347–3351 (2010). https://doi.org/10.1210/jc.2010-0288
X. Ma, L. Dong, Q. Shao et al. Triglyceride glucose index for predicting cardiovascular outcomes after percutaneous coronary intervention in patients with type 2 diabetes mellitus and acute coronary syndrome. Cardiovasc. Diabetol. 19(1), 31 (2020). https://doi.org/10.1186/s12933-020-01006-7
X. Yu, L. Wang, W. Zhang et al. Fasting triglycerides and glucose index is more suitable for the identification of metabolically unhealthy individuals in the Chinese adult population: A nationwide study. J. Diabetes Investig. 10(4), 1050–1058 (2019). https://doi.org/10.1111/jdi.12975
Y. Chen, H. Xiong, D. Wu et al. Relationship of short-term blood pressure variability with carotid intima-media thickness in hypertensive patients. Biomed. Eng. Online 14, 71 (2015). https://doi.org/10.1186/s12938-015-0059-8
H. Liu, J. Liu, H. Zhao et al. Relationship between serum uric acid and vascular function and structure markers and gender difference in a real-world population of China-from Beijing Vascular Disease Patients Evaluation Study (BEST) study. J. Atheroscler. Thromb. 25(3), 254–261 (2018). https://doi.org/10.5551/jat.39685
N. Ikeda, E. Saito, N. Kondo et al. What has made the population of Japan healthy? Lancet 378(9796), 1094–1105 (2011). https://doi.org/10.1016/S0140-6736(11)61055-6
S. Laurent, P. Boutouyrie, Arterial stiffness and hypertension in the elderly. Front. Cardiovasc. Med. 7, 544302 (2020). https://doi.org/10.3389/fcvm.2020.544302
H. Ihle-Hansen, T. Vigen, H. Ihle-Hansen, et al. Prevalence of carotid plaque in a 63- to 65-year-old norwegian cohort from the general population: The ACE (Akershus Cardiac Examination) 1950 Study. J. Am. Heart Assoc. 7(10) (2018) https://doi.org/10.1161/JAHA.118.008562
K. Gasbarrino, D. Di Iorio, S.S. Daskalopoulou,, Importance of sex and gender in ischaemic stroke and carotid atherosclerotic disease. Eur. Heart J. 43(6), 460–473 (2022). https://doi.org/10.1093/eurheartj/ehab756
A.P. Arnold, L.A. Cassis, M. Eghbali, K. Reue, K. Sandberg, Sex hormones and sex chromosomes cause sex differences in the development of cardiovascular diseases. Arterioscler. Thromb. Vasc. Biol. 37(5), 746–756 (2017). https://doi.org/10.1161/ATVBAHA.116.307301
J.S. Bae, D.H. Shin, P.S. Park et al. The impact of serum uric acid level on arterial stiffness and carotid atherosclerosis: the Korean Multi-Rural Communities Cohort study. Atherosclerosis 231(1), 145–151 (2013). https://doi.org/10.1016/j.atherosclerosis.2013.08.017
Funding
This research was supported by the National Natural Science Foundation of China (No.82073670).
Author information
Authors and Affiliations
Contributions
J.W.F. performed data analysis and wrote the manuscript. Y.L.Y. and X.C.J. contributed suggestions for manuscript revision and revised the manuscript. Y.P.W., C.Y.Z., and N.N.W. revised the manuscript. S.Y.D. and X.Z.S. conceived and initiated this project provided advice on experimental design, oversaw the implementation of the statistical method, and revised/finalized the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University (2018-KY-56) and all subjects had informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fan, J., Yang, Y., Jia, X. et al. Metabolic score and its components are associated with carotid plaque prevalence in young adults. Endocrine (2024). https://doi.org/10.1007/s12020-024-03903-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12020-024-03903-3