Abstract
Purpose
The aims of the current study were to describe clinical and biochemical features of patients with Paget disease of bone (PDB) followed at our medical center, and to examine the long-term effectiveness of zoledronate.
Methods
Retrospective cohort study included consecutive patients≥18 years with a diagnosis of PDB, followed in the Rabin Medical Center (RMC) Institute of Endocrinology from 1973 to 2023. The cohort comprised two groups: patients treated/not treated with zoledronic acid (ZOL/NZOL). The primary outcome was the percentage of patients who achieved a biochemical therapeutic response.
Results
Overall, 101 patients with PDB were included, 68 in the ZOL group and 33 in the NZOL group. The mean age was 65.2 ± 10.0 years, and 47% were female. Notably, 77% exhibited monostotic involvement, and only 3% had experienced fractures attributed to PDB. Mean ALP level at diagnosis was 160 ± 70.6 U/L. The median follow-up duration was 17 years since PDB diagnosis, comparable between the groups. Primary outcome was more prevalent in the ZOL compared to the NZOL group [42 patients (88%) VS 11 patients (52%) respectively, P = 0.004]. At the end of follow-up, mean ALP levels in the NZOL group were significantly higher than the levels in the ZOL group irrespective of the number of infusions received.
Conclusion
The majority of patients with PDB experience a mild disease course, marked by monostotic involvement and a low prevalence of fractures. Zoledronic acid effectively manages PDB, providing sustained biochemical response. The necessity for multiple zoledronic acid injections remains questionable, often implemented due to osteoporosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Paget disease of bone (PDB) is a focal disorder of bone metabolism characterized by an abnormal, accelerated rate of bone remodeling at one (monostotic PDB) or more (polyostotic PDB) skeletal sites [1]. The prevalence of PDB in the elderly population is estimated to be 1–7% worldwide [2, 3]. In recent decades, a discernible trend of decreasing severity and incidence of PDB has emerged [4,5,6,7]. This trend is characterized by the diagnosis of the disease occurring at an older age, a lower number of affected bones, and lower alkaline phosphatase (ALP) levels. Additionally, there has been a decrease in the rate of pathological fractures associated with PDB.
Diagnosis of PDB relies on clinical manifestations, characteristic imaging findings, and laboratory tests, particularly serum ALP levels. Due to its low cost and widespread availability, ALP is the bone turnover marker usually recommended to monitor disease activity [8].
The goal of the treatment in PDB is to alleviate symptoms and prevent complications by inhibiting osteoclastic activity [8, 9]. Calcitonin, a weak antiresorptive medication with osteoclast-inhibiting properties, was used in the past [10]. Currently, bisphosphonates, and particularly nitrogen-containing bisphosphonates, are considered to be the treatment of choice for PDB [8, 9]. Bisphosphonates often normalize biochemical markers, may heal lytic bone lesions [11], and may alleviate disease-induced bone pain [12]. In the past, intravenous pamidronate was frequently used to treat active PDB [13]. Subsequently, zoledronic acid, a highly potent intravenous bisphosphonate, was introduced and demonstrated a higher response rate and longer duration of remission, a decrease in bone markers, and improvement in quality of life compared to other oral or intravenous bisphosphonates [14,15,16,17]. However, there is still a debate regarding the optimal treatment strategy in PDB. While Endocrine Society guidelines recommend treating most patients with active disease [9], more recent guidelines advise only treatment for pain relief [8]. A single infusion of zoledronic acid was found to be effective for up to 6.5 years [15]; however, limited data is available on the impact of this treatment in maintaining disease control and preventing pagetic complications. The aims of the current study were to characterize a large group of patients with PDB, assess the long-term efficacy of zoledronic acid, and determine the indication for repeated administrations of this treatment.
Methods
Study design
A retrospective cohort study conducted at the Institute of Endocrinology of RMC, a tertiary hospital in the center of Israel. The study received approval from the RMC institutional review board (RMC-23-0140).
Study population
All consecutive patients aged ≥ 18 years old with a diagnosis of PDB, followed at the Institute of Endocrinology at RMC from January 1973 to January 2023, were included. A single patient received a diagnosis in the seventies, and another in the eighties. The vast majority of patients were diagnosed with PDB from the nineties onwards. Excluded from the study were patients with a bone disease other than PDB and those with PDB who were lost of follow-up.
The diagnosis of PDB was determined by the treating endocrinologist based on clinical findings, ALP levels, and characteristic findings on imaging studies during the diagnostic process.
The final cohort comprised two groups: patients treated with 5 mg zoledronic acid (ZOL) and a group not treated with zoledronic acid (NZOL). The decision to commence zoledronic acid treatment relied on the clinical judgment of the attending endocrinologist, primarily relying on clinical manifestations such as bone pain and imaging findings indicative of active disease. Treatment with zoledronic acid was not given in the presence of contraindications such as renal failure, drug sensitivity or hypocalcemia.
Data collection, surveillance and follow-up
Data were retrospectively collected from the RMC computerized system, including demographic details, co-morbidities, specifically osteoporosis (OP) diagnosis and treatment, hypertension, diabetes mellitus (DM), dyslipidemia and ischemic heart disease (IHD), date of PDB diagnosis, clinical manifestations such as localized pain, relevant imaging studies including x-rays and bone scans and bone biopsy to confirm and evaluate the extent of the disease. Complications of the disease were also recorded, including bone fractures, osteosarcoma, hearing loss and heart failure. Laboratory tests, especially ALP levels, and details of medical therapy for PDB were recorded, with follow-up duration from diagnosis to last ALP test and follow-up after zoledronic acid treatment initiation.
Outcome assessments
The primary outcome was to evaluate the percentage of patients achieving a therapeutic response, defined as a composite of ALP levels decreasing to the normal range and at least a 75% decrease relative to a deviation from the mid-point of the reference range (Beckman Colter, USA, normal range 30–120 U/L), as defined in previous studies [14]. Each component of the composite outcome was also analyzed separately. Outcomes were compared between the ZOL and NZOL groups as based on the difference between ALP levels at diagnosis and at the end of follow-up. Univariate analysis was performed to identify predictive factors of response to therapy in the ZOL group before treatment initiation and six months after therapy.
A comparison based on the number of zoledronic acid infusions was made to identify features of patients receiving more than one infusion. Mortality among the study groups was also documented.
Statistical analysis
IBM SPSS version 27.0 (IBM Corp., Armonk, NY) was used for statistical analysis. Continuous variables were presented using mean ± SD or median (IQR), as appropriate. Categorical variables were presented by (N, %). Differences between study groups were assessed using the t-test, Mann–Whitney test, and Chi-square test for normally distributed continuous, non-normally distributed continuous, and categorical variables, respectively. ANOVA was used to compare the values of normally distributed continuous variables between three study groups. Two-sided P-values less than 0.05 were considered statistically significant.
Results
Study population
In total, 101 patients were included in the final analysis, with 68 in the ZOL group, and 33 in the NZOL group. The median overall follow-up period for the study cohort was 17 years since PDB diagnosis, and this duration was similar between the two groups (16 VS 18 years in the ZOL and NZOL groups, respectively, p = 0.41). The baseline characteristics of the study cohort are detailed in Table 1. The mean age at diagnosis was 65.2 ± 10.0 years (range, 30–88), and there were 47 females (47%). The most prevalent chronic comorbidities were hypertension and dyslipidemia (60% and 62%, respectively). Chronic renal failure was documented in 13 patients (13%) and was more prevalent in the NZOL compared to the ZOL group (27% VS 6%, respectively, P = 0.008). Additionally, the NZOL group exhibited a lower mean calculated glomerular filtration rate (GFR) compared to the ZOL group (36.0 ± 5.9 VS 51.9 ± 5.8 mL/min/1.73 m², respectively).
Paget’s disease characteristics
The most common clinical manifestation at diagnosis was localized pain in 81 patients (80%), significantly more common in the ZOL group compared to the NZOL group (87% VS 67% respectively, P = 0.03). Monoostotic presentation was more common than polyostotic [78 patients (77%) VS 23 patients (23%) respectively]. Bone involvement included the pelvis and sacrum in 70 patients (69%), femur in 19 patients (19%), and less commonly vertebral, cranial and humerus involvement (Table 1). The mean ALP at diagnosis was 160.0 ± 70.6 U/L. Treatment prior to zoledronic acid included calcitonin in four patients and bisphosphonates other than zoledronic acid, in 62 patients, 39/68 in the ZOL group received other bisphosphonates before zoledronate, and 23/33 received any bisphosphonate other than zoledronate in the NZOL group, without significant difference between the two groups (Table 1). Pamidronate emerged as the non-zoledronic acid predominant bisphosphonate utilized, with approximately 50% of patients in both groups receiving this treatment.
Treatment with zoledronic acid
In total, 68 patients received zoledronic acid, with 42 patients receiving only one infusion and 26 patients receiving two or more infusions. The median follow-up duration following zoledronic acid initiation was seven years. In the ZOL group, the mean ALP before zoledronic acid initiation was 144.7 U/L, after six months of treatment 72.8 U/L, and at the end of follow-up 86.2 U/L. The last ALP during the follow-up was higher in the NZOL group compared to the ZOL group (109.7 U/L VS 86.2 U/L, respectively, P = 0.03) (Table 1). Hypocalcemia following zoledronic acid treatment was not observed in any of the patients.
Paget’s disease complications
Out of 101 patients, a total of eight (8%) experienced bone fractures. Among these, only three fractures were directly linked to Paget’s disease of bone (one femoral, one lumbar spine, and one radius fracture), while the remaining five were classified as osteoporotic fractures. All three pagetic fractures occurred in the ZOL group around the time of diagnosis, prior to the initiation of treatment. From the five osteoporotic fractures, three occurred in the ZOL group, and two in the NZOL group. None of the patients in our cohort had osteosarcoma, three patients had hearing loss and six had heart failure. According to our retrospective impression, disease relapse after treatment initiation was very rare.
Study’s outcomes
The study’s outcomes are presented in Table 2. ALP normalization was not significantly different in the ZOL and NZOL groups [32 patients (67%) VS nine patients (43%) respectively, P = 0.109]. A decrease of at least 75% in the ALP levels relative to a deviation of ALP from the mid-point of the reference range was more prevalent in the ZOL group compared to the NZOL group [34 patients (71%) VS seven patients (33%) respectively, P = 0.007]. The composite outcome of ALP normalization and a decrease of at least 75% in the ALP levels relative to a deviation of ALP from the mid-point of the reference range was also more prevalent in the ZOL group compared to the NZOL group [42 patients (88%) VS 11 patients (52%) respectively, P = 0.004].
Table 3 represents a univariate analysis for the occurrence of the primary outcome of, a composite of ALP normalization and a decrease of at least 75% in the ALP levels relative to a deviation of ALP from the mid-point of the reference range, in the zoledronic acid-treated patients, before treatment initiation and after six months of therapy. Cranial involvement was associated with a significantly lower rate of response to therapy (P = 0.03). ALP levels before zoledronic acid initiation were significantly higher in patients who responded to treatment compared to non-responders (154.4 ± 51.2 U/L VS 95.5 ± 34.5 U/L, P < 0.001). Since there were no clear parameters influencing the response to therapy, a multivariate analysis was not completed.
Comparison between single and multiple infusions of zoledronic acid
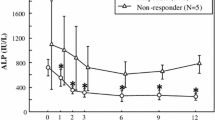
When comparing ALP levels at diagnosis and at the end of follow-up according to the number of zoledronic acid infusions (Fig. 1), mean ALP levels at diagnosis were found to be similar between patients treated with zoledronic acid with a single or more than one infusion and the NZOL group. However, at the end of follow-up, mean ALP levels in the NZOL group were significantly higher than the levels in the treated groups, regardless of the number of infusions (119 U/L VS 83 U/L and 83 U/L in the NZOL group, the ZOL group with a single infusion and the ZOL group with multiple infusions, respectively, p = 0.007). There was no difference in mean ALP levels at the end of follow-up between the group that was treated with one infusion and the group that received two or more infusions. The reported indications for more than one zoledronic acid infusions were osteoporosis in 16 patients and Paget’s disease in 10 patients and were based on clinical judgment of the treating physician.
When comparing characteristics of patients treated with one infusion and those treated with two or more infusions, female gender, cranial involvement, and a prior diagnosis of osteoporosis were the only parameters that were different between the two groups. Osteoporosis and female gender were more common in the group that was treated with two or more infusions. Cranial involvement was also more common in the multiple infusions group (Table 4).
Mortality
During the follow-up, thirty-one patients passed away, with 16 patients (24%) in the ZOL group and 15 patients (46%) in the NZOL group, yielding a significant difference P = 0.038. The mean age at the time of death was 85.9 ± 8.4 for the ZOL group and 88.8 ± 7.4 years for the NZOL group, with no significant difference observed, P = 0.311. The median follow-up of for the 11 patients who died after a single zoledronic acid infusion was 5 years (IQR 2–8 years), while the median follow-up for the five patients who received two or more infusions of zoledronic acid was 8 years (IQR 6–10 years). We did not have access to death certificates to ascertain the cause of death.
Discussion
In our retrospective study of patients with PDB, the majority had mild disease with predominantly monostotic involvement and a low prevalence of complications. The biochemical response to zoledronic acid persisted for up to 13 years following the initial infusion.
Our findings align with prior research on the effectiveness of zoledronic acid in managing PDB, further substantiateing the prolonged duration of efficacy of this medication. A study by Reid and colleagues in 2005 [14] found that a solitary administration of zoledronic acid results in quicker, more comprehensive, and longer-lasting improvements in individuals with Paget’s disease compared to using risedronate for treatment. In a study of Merlotti et al. [16], a single zoledronate infusion effectively achieved biochemical remission in as many as 90% of patients who did not respond to pamidronate, and therapeutic response was maintained in most patients for up to 15 months. In a study assessing long-term follow-up following a single infusion of zoledronic acid in Paget’s disease patients, remission duration of 6.5 years was demonstrated [15]. However, these studies focused on a single infusion of zoledronic acid with a shorter follow-up period than the follow-up in our study (six months plus 190 days of extension [14], 15 months [16] and 6.5 years [15], respectively). Our study extends prior research by emphasizing the enduring effectiveness of zoledronic acid. The overall follow-up period in our study was a median of 17 years, with a median follow-up of seven years after zoledronic acid treatment initiation and maximum follow-up of 13 years under treatment, highlighting the durability of zoledronic acid’s benefits.
The majority of patients in our study had mild disease predominantly involving a single bone in more than 75%. Localized pain was the most common clinical manifestation, significantly more common in the ZOL group compared to the NZOL group, likely serving as an incentives for treatment. Our study noted a low rate of skeletal complications, with only a small fraction of patients experiencing pathological fractures before starting treatment. This observation aligns with a global trend of decreasing PDB severity and incidence [4,5,6,7]. Pathological fracture rate was also low in a previous British cohort [18]. A systematic review reported a decline in the prevalance of fractures as a presenting feature of PDB over recent decades, but regional differences in referral patterns, missing data and reporting bias were noted [18]. The reduction in the clinical severity of PDB may be associated with environmental factors, though their specific nature remains unidentified [4]. Over the past three decades, bisphosphonates have become widely utilized, primarily for the treatment of osteoporosis. Their application for alternative purposes may contributes to alleviate the clinical manifestations of PDB.
In our cohort, approximately 70% of patients in the NZOL group recieved bisphosphonate treatment other than zoledronate, predominantly pamidronate. This may have mitigated the biochemical and clinical characteristics of PDB. Similarly, in the ZOL group, 57% of patients had previously received other bisphosphonates before transitioning to zoledronate, which may have affected the effectiveness of zoledronic acid.
Chronic renal failure was the only chronic condition that exhibited a significant difference between the two groups, being more prevalent in the NZOL group. Additionally, the NZOL group exhibited a lower mean calculated GFR compared to the ZOL group. This finding might cause prescription bias related to renal failure, such that this variance likely influenced the decision to avoid administering zoledronic acid treatment to these patients.
The Endocrine Society guidelines from 2014 recommend treatment of all patients with active PDB at risk of complications, termed intensive treatment strategy [9]. However, more recent guidelines from 2019 suggest treating symptomatic patients primarily for pain relief [8]. In a recent retrospective study, metabolically active patients with PDB treated with intensive zoledronic acid therapy had a negligible risk of complications and long-term disease control [19]. In a large prospective trial comparing intensive versus symptom control strategy, complications and bone pain didn’t differ after 3 years of follow-up between study groups [20]. In a 3-years extension study [21], zoledronic acid was implemented with similar strategies, and patients in the intensive treatment group had a non-significant increase in fractures and orthopedic procedures.
Interestingly, ALP levels before zoledronic acid initiation were significantly higher in patients who responded to treatment compared to non-responders. This finding is not in line with literature and clinical practice, as patients with higher ALP pre-treatment values are generally more at risk for disease persistence after Zol [15].
When comparing ALP levels at diagnosis and at the end of follow-up according to number of zoledronic acid infusions, we found a similar level of ALP at diagnosis in all groups. However, at the end of follow-up, mean ALP levels in the group that was not treated with zoledronic acid were significantly higher than the levels in the treated groups with single or multiple infusions, emphasizing the effectiveness of zoledronic acid treatment on the biochemical response. Nevertheless, the statistically-significant difference in ALP levels between the NZOL and the ZOL groups at the end of follow-up may not necessarily translate into clinical significance, especially as the values in the NZOL group remains within the reference range and data regarding clinical symptoms at the end of follow-up are lacking. The comparable levels of ALP at the end of follow-up between single or multiple injections is in line with previous studies assessing a single infusion of zoledronic acid [14,15,16] and does not support an added value of repeated treatments. Similarly, a study examining the durability of response to zoledronate treatment showed that the majority of elderly patients with Paget’s disease responded to a single intravenous infusion of zoledronate with indefinite disease suppression [22]. At the same time, Reid et al. reported a beneficial effect of re-treatment for the rare situation of relapsed Paget’s disease [23]. The results of our study align with the widely accepted approach of a single administration of zoledronic acid for disease control. It is important to note that most of our patients (16/26) who received more than one infusion of zoledronic acid, received it for the indication of osteoporosis and not for PDB. An alternative explanation for the need for multiple infusions to achieve a therapeutic response, rather than treating osteoporosis, could be more severe phenotype characteristics of PDB. However, upon comparing patients who underwent a single infusion with those who received two or more infusions, there were no discernible differences in factors such as age, extent of disease, local pain, type of treatment before zoledronic acid, comorbidities (except osteoporosis) and ALP levels at diagnosis and before treatment initiation between the two groups. Therefore, it seems less likely to assume that patients who received more than one infusion had a more severe form of the disease. Unfortunately, information related to genetic tests, such as the presence of SQSTM1 mutation, which often necessitate additional infusions, was not available.
The administration of zoledronate proved to be safe in our patients diagnosed with PDB, as no instances of hypocalcemia were observed.
Our study documented a considerable mortality rate, with approximately one-third of patients succumbing during follow-up, primarily at an advanced age. It is worth noting that mortality was more prevalent in the NZOL group, possibly attributed to a higher incidence of chronic kidney disease. However, this difference may not be clinically significant, given the exceptionally advanced age at which these deaths occurred.
The study has several limitations. Firstly, there are missing data of ALP levels at diagnosis, resulting in the inclusion of only 69 patients in the outcome analysis. Although we considered using ALP levels obtained at a different time point from the initial diagnosis, we opted against it because of the potential impact of other treatments these patients might have received, which could have influenced their ALP levels. Secondly, subjective clinical improvement was not specified as an outcome measure. Instead, we focused on using biochemical response as the primary outcome because it is more objective and can be easily quantified. Lastly, the retrospective nature of this study is another limitation; the patients were not randomly assigned to receive zoledronic acid or placebo, and the treatment and follow-up were therefore not standardized. Several expert endocrinologists participated in the medical care of the patients and since there is a lack of standardized criteria for initiation timing and number of injections of zoledronate, decisions were largely made based on individual practice or on the recommendation of our multidisciplinary team.
Conclusion
The majority of patients with PDB in this cohort exhibited a mild disease course, characterized by monostotic involvement and a low prevalence of complications. Our study underscores the enduring efficacy of zoledronic acid, revealing sustained biochemical response for up to 13 years following the initial infusion in PDB patients. Our findings suggest that a single infusion may suffice for most patients, with additional infusions primarily warranted for concurrent osteoporosis.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
M. Davie, M. Davies, R. Francis, W. Fraser, D. Hosking, R. Tansley, Paget’s disease of bone: a review of 889 patients. Bone 24(5), 11S–12S (1999). https://doi.org/10.1016/S8756-3282(99)00027-7
R.D. Altman, D.A. Bloch, M.C. Hochberg, W.A. Murphy, Prevalence of pelvic Paget’s disease of bone in the United States. J. Bone Min. Res 15(3), 461–465 (2010). https://doi.org/10.1359/jbmr.2000.15.3.461
C. Cooper, K. Schafheutle, E. Dennison, S. Kellingray, P. Guyer, D. Barker, The epidemiology of Paget’s disease in Britain: is the prevalence decreasing? J. Bone Min. Res 14(2), 192–197 (1999). https://doi.org/10.1359/jbmr.1999.14.2.192
E. Gendron, F. Bouchard, N. Singbo, J.P. Brown, L. Michou, Decline in clinical severity of Paget’s disease of bone: comparison between a contemporary cohort and a historical cohort. Bone 170, 116721 (2023). https://doi.org/10.1016/j.bone.2023.116721
A.A. Morales-Piga, F.J. Bachiller-Corral, V. Abraira, J. Beltrán, A. Rapado, Is clinical expressiveness of Paget’s disease of bone decreasing? Bone 30(2), 399–403 (2002). https://doi.org/10.1016/S8756-3282(01)00674-3
C. Britton, S. Brown, L. Ward, S.L. Rea, T. Ratajczak, J.P. Walsh, The changing presentation of Paget’s disease of bone in Australia, a high prevalence region. Calcif. Tissue Int 101(6), 564–569 (2017). https://doi.org/10.1007/s00223-017-0312-1
R.D. Tiegs, C.M. Lohse, P.C. Wollan, L.J. Melton, Long-term trends in the incidence of Paget’s disease of bone. Bone 27(3), 423–427 (2000). https://doi.org/10.1016/S8756-3282(00)00333-1
S.H. Ralston, L. Corral-Gudino, C. Cooper, R.M. Francis, W.D. Fraser, L. Gennari, N. Guañabens, M.K. Javaid, R. Layfield, T.W. O’Neill, R.G.G. Russell, M.D. Stone, K. Simpson, D. Wilkinson, R. Wills, M.C. Zillikens, S.P. Tuck, Diagnosis and management of Paget’s disease of bone in adults: a clinical guideline: diagnosis and management of Paget’s disease of bone. J. Bone Min. Res 34(4), e3657 (2019). https://doi.org/10.1002/jbmr.3657
F.R. Singer, H.G. Bone, D.J. Hosking, K.W. Lyles, M.H. Murad, I.R. Reid, E.S. Siris; Endocrine Society, Paget’s disease of bone: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 99(12), 4408–4422 (2014). https://doi.org/10.1210/jc.2014-2910
J.P. Lavender, I.M.A. Evans, R. Arnot, S. Bowring, F.H. Doyle, G.F. Joplin, I. MacIntyre, A comparison of radiography and radioisotope scanning in the detection of Paget’s disease and in the assessment of response to human calcitonin. BJR 50(592), 243–250 (1977). https://doi.org/10.1259/0007-1285-50-592-243
I.R. Reid, G.C. Nicholson, R.S. Weinstein, D.J. Hosking, T. Cundy, M.A. Kotowicz, W.A. Murphy, S. Yeap, S. Dufresne, A. Lombardi, T.A. Musliner, D.E. Thompson, A. John Yates, Biochemical and radiologic improvement in Paget’s disease of bone treated with alendronate: a randomized, placebo-controlled trial. Am. J. Med. 101(4), 341–348 (1996). https://doi.org/10.1016/S0002-9343(96)00227-6
P.D. Miller, J.P. Brown, E.S. Siris, M.S. Hoseyni, D.W. Axelrod, P.J. Bekker, A randomized, double-blind comparison of risedronate and etidronate in the treatment of Paget’s disease of bone. Am. J. Med. 106(5), 513–520 (1999). https://doi.org/10.1016/S0002-9343(99)00062-5
J.P. Walsh, L.C. Ward, G.O. Stewart, R.K. Will, R.A. Criddle, R.L. Prince, B.G.A. Stuckey, S.S. Dhaliwal, C.I. Bhagat, R.W. Retallack, G.N. Kent, P.J. Drury, S. Vasikaran, D.H. Gutteridge, A randomized clinical trial comparing oral alendronate and intravenous pamidronate for the treatment of Paget’s disease of bone. Bone 34(4), 747–754 (2004). https://doi.org/10.1016/j.bone.2003.12.011
I.R. Reid, P. Miller, K. Lyles, W. Fraser, J.P. Brown, Y. Saidi, P. Mesenbrink, G. Su, J. Pak, K. Zelenakas, M. Luchi, P. Richardson, D. Hosking, Comparison of a single infusion of zoledronic acid with risedronate for Paget’s disease. N. Engl. J. Med 353(9), 898–908 (2005). https://doi.org/10.1056/NEJMoa044241
I.R. Reid, K. Lyles, G. Su, J.P. Brown, J.P. Walsh, J. del Pino-Montes, P.D. Miller, W.D. Fraser, S. Cafoncelli, C. Bucci-Rechtweg, D.J. Hosking, A single infusion of zoledronic acid produces sustained remissions in Paget disease: data to 6.5 years. J. Bone Min. Res 26(9), 2261–2270 (2011). https://doi.org/10.1002/jbmr.438
D. Merlotti, L. Gennari, G. Martini, F. Valleggi, V. De Paola, A. Avanzati, R. Nuti, Comparison of different intravenous bisphosphonate regimens for Paget’s disease of bone. J. Bone Min. Res 22(10), 1510–1517 (2007). https://doi.org/10.1359/jbmr.070704
D. Hosking, K. Lyles, J.P. Brown, W.D. Fraser, P. Miller, M.D. Curiel, J.-P. Devogelaer, M. Hooper, G. Su, K. Zelenakas, J. Pak, T. Fashola, Y. Saidi, E.F. Eriksen, I.R. Reid, Long-term control of bone turnover in Paget’s disease with zoledronic acid and risedronate. J. Bone Min. Res 22(1), 142–148 (2007). https://doi.org/10.1359/jbmr.061001
A. Tan, S.H. Ralston, Clinical presentation of Paget’s disease: evaluation of a contemporary cohort and systematic review. Calcif. Tissue Int 95(5), 385–392 (2014). https://doi.org/10.1007/s00223-014-9904-1
M. Barale, S. Sigrist, F. Bioletto, F. Maiorino, E. Ghigo, R. Mazzetti, M. Procopio, Long-term efficacy of intensive zoledronate therapy and predictors of retreatment in Paget’s disease of bone. Calcif. Tissue Int 109(4), 383–392 (2021). https://doi.org/10.1007/s00223-021-00848-x
A.L. Langston, M.K. Campbell, W.D. Fraser, G.S. MacLennan, P.L. Selby, S.H. Ralston, Randomized trial of intensive bisphosphonate treatment versus symptomatic management in Paget’s disease of bone. J. Bone Min. Res 25(1), 20–31 (2010). https://doi.org/10.1359/jbmr.090709
A. Tan, K. Goodman, A. Walker, J. Hudson, G.S. MacLennan, P.L. Selby, W.D. Fraser, S.H. Ralston; for the PRISM-EZ Trial Group, Long-term randomized trial of intensive versus symptomatic management in Paget’s disease of bone: the PRISM-EZ study: intensive bisphosphonate therapy in Paget’s disease of bone. J. Bone Min. Res 32(6), 1165–1173 (2017). https://doi.org/10.1002/jbmr.3066
T. Cundy, K. Maslowski, A. Grey, I.R. Reid, Durability of response to zoledronate treatment and competing mortality in Paget’s disease of bone. J. Bone Min. Res 32(4), 753–756 (2017). https://doi.org/10.1002/jbmr.3029
I.R. Reid, J.P. Brown, N. Levitt, J.A. Román Ivorra, J. Bachiller-Corral, I.L. Ross, G. Su, O. Antunez-Flores, R.P. Aftring, Re-treatment of relapsed Paget’s disease of bone with zoledronic acid: results from an open-label study. Bonekey Rep. 2, 442 (2013). https://doi.org/10.1038/bonekey.2013.176
Funding
No funds, grants, or other support was received.
Author information
Authors and Affiliations
Contributions
Study Conception and design: Gorshtein A, Ayalon-Dangur I.Acquisition of data: All authors.Analysis and interpretation of data: Gorshtein A, Ayalon-Dangur I, Rudman Y.Drafting of the work: Gorshtein A, Ayalon-Dangur I.Critical Revision of the manuscript: All authors.Study supervision: Gorshtein A.Final approval of the manuscript: All authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ayalon-Dangur, I., Rudman, Y., Tsvetov, G. et al. Long-term effectiveness of zoledronic acid in patients with Paget’s disease of bone – a retrospective cohort study. Endocrine 85, 873–882 (2024). https://doi.org/10.1007/s12020-024-03791-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-024-03791-7