Abstract
Background
There have been few studies on the role of autophagy in pancreatic neuroendocrine tumours (PNETs). SQSTM1/p62 (also called Sequestosome 1) is a potential autophagy regulator, and its biological roles and clinical significance in PNETs remain poorly understood.
Purpose
The purpose of this study was to evaluate the clinical significance of SQSTM1/p62 in human PNET specimens and to evaluate its potential value as a therapeutic target by studying its biological function in PNET cell lines.
Methods
SQSTM1/p62 protein expression was assessed in 106 PNET patient specimens by immunohistochemistry, and the relationship between SQSTM1/p62 protein expression and the clinicopathological features of PNETs in patients was analysed. The proliferation, invasion and apoptosis of SQSTM1/p62-knockdown QGP-1 and INS-1 cells were assessed by the MTT assay, a Transwell assay and flow cytometry. Cell autophagy was assessed by western blotting and mCherry-GFP-LC3B.
Results
The protein expression of SQSTM1/p62 in PNET patient specimens was significantly correlated with tumour recurrence (p = 0.005) and worse prognosis (log rank p = 0.020). Downregulation of the SQSTM1/p62 gene inhibited tumour cell proliferation and migration and induced PNET cell death. Downregulation of SQSTM1/p62 activated autophagy in PNET cell lines but blocked autophagic flow. Knockdown of the SQSTM1/p62 gene inhibited mTOR phosphorylation.
Conclusion
The SQSTM1/P62 protein could be an independent prognostic marker for PNET patients. Downregulating SQSTM1/P62 can inhibit PNET progression, inhibit mTOR phosphorylation and block autophagic flow.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic neuroendocrine tumours (PNETs) make up approximately 2–3% of all pancreatic tumours, and the incidence and prevalence of PNETs have steadily increased worldwide over the past 30 years [1,2,3]. The most common primary site of gastroenteropancreatic neuroendocrine tumours (GEP-NETs) is the pancreas (32%) in China [3]. However, the prognosis of patients with PNETs has not improved obviously in the past decades. Studying prognostic factors for PNET patients and finding new treatment methods are the keys to improving the prognosis of patients [4].
Recent studies have shown that blocking autophagic flow can inhibit the growth of PNET cells and enhance the antitumour effect of everolimus and sunitinib [5,6,7]. These findings indicated that autophagy blockade could inhibit PNET progression and treatment resistance. However, the regulatory mechanism needs to be further studied. Dysregulation of autophagosomes induces autophagy-dependent cell death. Sami et al. found that treatment with the pandeacetylase inhibitor panobinostat causes autophagic cell death in PNET cells, suggesting that promoting autophagic cell death could be a promising strategy for PNETs [8].
SQSTM1/p62 (also called sequestosome 1) is a multifunctional protein and signalling hub involved in cell autophagy, apoptosis and tumorigenesis. When autophagic flow is blocked or activated, SQSTM1/p62 expression is increased or decreased, respectively. Kaito and Nihira et al. showed that inhibition of SQSTM1/p62 results in autophagic cell death in several human carcinoma cell lines [9]. Some studies have shown that SQSTM1/p62 accumulation in the cytoplasm is associated with worse clinical outcomes in patients with colorectal cancer [10], non-small cell lung cancer [10], prostate cancer [11] and breast cancer [12]. The role of SQSTM1/p62 in PNET cells is largely unknown. Thus, in the present study, we examined the expression of SQSTM1/p62 in PNET patient specimens and explored its biological functions in PNET cell lines.
Materials and methods
Patients and tumor
A total of 106 PNETs were collected by tumour resection at Peking Union Medical College Hospital between 1998 and 2017. Clinical follow-up data of 90 patients were obtained from 2000 to 2020. The diagnostic criteria for PNETs were reported previously [13, 14]. The pathological diagnosis of all PNETs was made by 2 experienced pathologists. Tumour grade and stage were defined in 95 and 103 patients, respectively, according to the ENETS guidelines.
Immunohistochemical staining
Immunohistochemistry was performed on formalin-fixed, paraffin-embedded (FFPE) sections of 106 PNETs and 46 para-tumoral tissue specimens. The primary antibody used in this study was an anti-SQSTM1/p62 antibody (PM045; rabbit polyclonal, MBL; sc-48402; mouse monoclonal) at a 1:1000 dilution. All tissue sections were antigen-retrieved in a microwave oven 2 times for 10 min in 10 mM citrate buffer and counterstained with haematoxylin.
PNET cell lines and cell culture
The INS-1 rat insulinoma cell line (RRID: CVCL_0352) and QGP1 human PNET cell line 9 (RRID: CVCL_3143) were used in the present study and were kindly provided by Dr. Zai Wang from China-Japan Friendship Hospital. The INS-1 cell line was identified by testing its ability to secrete insulin, and the QGP1 cell line was identified by STR matching analysis (see Supplementary Fig. 1).
INS-1 cells were cultured in RPMI-1640 medium containing 11.2 mM glucose (11875-093, Gibco) and supplemented with 10% foetal bovine serum (10091-148, Gibco) and 1% penicillin–streptomycin solution (15070-063, Gibco). The release of insulin was measured to confirm INS-1 cells as an insulinoma cell line. QGP1 cells were cultured in DMEM (11965-092, Gibco) supplemented with 10% foetal bovine serum (10091-148, Gibco) and 1% penicillin–streptomycin solution (15070-063, Gibco). The two cell lines were maintained in a humidified incubator at 37 °C in 5% CO2. All experiments were performed with mycoplasma-free cells.
Small interfering RNA transfection
Small interfering RNAs (siRNAs) targeting SQSTM1/p62 along with a negative control siRNA (siRNA-NC) were purchased from GenePharma. The sequences of the siRNAs targeting SQSTM1/p62 are shown in Supplementary Table 1. Cells were transfected with siRNA (final concentration, 10 nmol/L) using LipofectamineTM 2000 (11668-019, Invitrogen).
Reverse transcription (RT)–PCR analysis
Total cellular RNA was extracted using TRIzol reagent (Ambion) according to the manufacturer’s protocol, and cDNA was prepared from RNA using HiScript Reverse Transcriptase (RNase H) (VAZYME). The primer sequences used for amplification are shown in Supplementary Table 2. Quantitative RT–PCR was conducted with a 10 min denaturation step at 95 °C in a final volume of 20 µL using Taq Plus DNA Polymerase (TIANGEN) according to the manufacturer’s instructions. The delta (Ct) method was used for quantification. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control.
Protein extraction
Total protein was extracted by adding 200 µl of RIPA buffer (Beyotime) supplemented with PMSF (Beyotime) and phosphatase inhibitors (Beyotime). Cell fragments and lysates were centrifuged at 12,000 rpm for 5 min at 4 °C, and the supernatant was stored at −20 °C. The protein concentration was determined by a BCA protein concentration test kit (Beyotime).
Western blot analysis
Total protein lysates were denatured by boiling for 10 min, separated by electrophoresis on SDS–PAGE gels, and then transferred to PVDF membranes. The membrane was subsequently incubated with the primary antibody in Tris-buffered saline/Tween 20 (TBST) with 5% skim milk and 1% BSA overnight at 4 °C. After 3 washes in TBST, the membranes were incubated with the secondary antibody in TBST with 5% skim milk for 2 h at 37 °C. Immunoblotting was performed using the ECL Detection System (P1050, Applygen), and the film grey value was analysed by BandScan.
The primary antibodies used in western blotting were as follows: anti-SQSTM1/p62 antibody (PM045; rabbit polyclonal, MBL, at a 1:1000 dilution), anti-LC3B antibody (NB100-2220, rabbit polyclonal, NOVUS, at a 1:50 dilution), anti-p-mTOR antibody (#5536, rabbit monoclonal, Cell Signaling, at a 1:200 dilution), anti-mTOR antibody (20657-1-AP, rabbit polyclonal, Proteintech Group, Inc., at a 1:500 dilution) and anti-GAPDH antibody (ab9485, rabbit polyclonal, Santa Cruz, at a 1:2500 dilution).
Transwell migration/invasion assays
Migrating/invasion assays were performed using transwells (Corning). The lower chambers were filled with 0.8 ml culture media, and cells (2 × 105 in 0.2 mL serum-free media) were seeded onto the upper chambers and incubated at 37 °C for 24 h. After removing the cells on the upper surface of the filters, the remaining cells were fixed with 70% glacial ethanol and stained with crystal violet dye solution, and the number of cells that migrated into or infiltrated the lower surface was counted in five randomly selected high-magnification fields (200×) for each sample.
MTT proliferation assay
Cell proliferation was examined using the MTT cell proliferation and cytotoxicity test kit (Sigma). siRNA-transfected cells (5 × 103 cells/well) were seeded onto 96-well plates. After 0 h, 24 h, 48 h, 72 h, and 96 h of culture, 10 µl MTT was added to each well and cultured for another 4 h. The medium was removed, and the cells were lysed in DMSO. Then, the absorbance was measured at 568 nm by an enzyme labelling instrument. It was repeated 3 times at each time point, and we took the average value to draw a cell growth curve.
Flow cytometry
Apoptosis was detected by an Annexin V-APC/7-AAD detection kit (KeyGen). Cells (5 × 105 cells/well) were seeded onto 6-well plates, collected after 48 h of culture, washed with PBS three times and resuspended in 500 μl binding buffer. Then, 5 μl Annexin V-APC and 5 μl 7-AAD were added, mixed, and incubated in the dark for 10 mins, and cell apoptosis was detected by flow cytometry. Each group of experiments was repeated 3 times.
Autophagy fluorescent double-label study
Cell autophagy was detected by adenovirus expressing mCherry-GFP-LC3B fusion protein (Beyotime). A virus solution with an MOI value of 30 was added to cells (5 × 105 cells/well) seeded on 6-well plates. After 24 h of culture, the medium containing virus was replaced with fresh medium, and the cells were cultured for another 24 h. The change in LC3B fluorescence was observed under a fluorescence microscope. Autophagosomes were counted under three 1:600 high magnification lenses.
Statistical analysis
All statistical analyses were performed using SPSS software, version 23.0 (IBM). Student’s t test was used to analyse differences between continuous values of two independent groups. The χ2 test or Fisher’s exact test was applied to analyse the categorical variables. The survival of patients was analysed by Kaplan–Meier analysis and log-rank test. Cox’s proportional hazard model was used for multivariate analysis. A two-tailed test was used in all statistical analyses. P < 0.05 was considered significant.
Results
Clinicopathological characteristics
A total of 106 PNETs were enrolled in our study, including 65 insulinomas, 9 gastrinomas, 1 VIPoma, 4 glucagon omas, and 27 nonfunctional PNETs. Ninety patients (85%) were followed up, and the median follow-up interval was 70 (range 2–183) months. Twelve patients died of the tumors, 70 patients survived without disease, 7 patients survived with progressive disease, and one patient was alive with unknown status. All tumors were well differentiated, and most of these tumors were classified as grade 1 and grade 2 except for 1 tumour with grade 3. The clinicopathological features of each patient are listed in Supplemental Table 3 and summarized in Table 1.
Expression of SQSTM1/p62 protein in PNET tissues
We detected the expression of SQSTM1/p62 protein in 106 PNET and 46 para-tumour specimens. A total of 49 (46%) PNET samples were SQSTM1/p62 positive, which was characterized by brown or yellow cytoplasm. Among the para-tumoral tissues, SQSTM1/p62 protein was only expressed in most islets, but its immunostaining was negative in acini and duct cells (Fig. 1A).
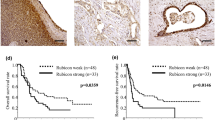
SQSTM1/p62 protein expression in PNETs and its correlation with prognosis. A Immunostaining of SQSTM1/p62 in PNETs. Left panel: HE staining of tumoral and peritumoral tissues; right panel: immunostaining of SQSTM1/p62 in PNETs. The SQSTM1/p62 protein was not expressed in the cytoplasm of one tumour (T1) or acini cells but was expressed in islet cells (Bar = 500 µm). The SQSTM1/p62 protein was expressed in the cytoplasm of tumour cells (T2) as well as in islet cells but was not expressed in acini or duct cells (Bar = 100 µm). B Kaplan‒Meier plot showing that the protein expression of SQSTM1/p62 in PNETs was correlated with worse disease-free survival (right) but not with worse overall survival (left)
Correlation between SQSTM1/p62 expression and clinicopathological features and prognosis of PNET patients
The expression of SQSTM1/p62 protein was significantly associated with larger tumour size (n = 103, p = 0.003), metastasis (n = 102, p = 0.018) and recurrence (n = 85, p = 0.002) but was not associated with age or sex (Table 1). SQSTM1/p62 was more commonly expressed in tumors in the pancreatic body or tail than in those in the pancreatic head or neck (n = 69, p = 0.017) (Table 1). The expression of SQSTM1/p62 protein was significantly associated with death (n = 90, p = 0.043) and poor disease free survival (n = 89, p = 0.009).
Kaplan–Meier survival analysis showed that patients with SQSTM1/p62-positive tumour tissue had shorter disease-free survival times (n = 86, p = 0.020) but not overall survival times (n = 88, p = 0.077) (Fig. 1B). Multivariate analysis (Cox’s proportional hazard model) demonstrated that SQSTM1/p62 expression correlated with worse disease-free survival, but it did not reach statistical significance [HR = 0.367, 95% CI: 0.114–1.181, p = 0.093] (Table 2).
Effects of SQSTM1/P62 expression on PNET cell proliferation, invasion and apoptosis
We further explored the biological functions of SQSTM1/p62 in PNET cell lines (QGP-1 and INS-1). First, we found that SQSTM1/p62 protein levels were highly and moderately expressed in QGP-1 and INS-1 cells, respectively (Fig. 2). Subsequently, we knocked down SQSTM1/p62 expression in QGP-1 and INS-1 cells by transfecting SQSTM1/p62-targeting siRNA (siRNA-Homo SQSTM1/p62 and siRNA-Rat SQSTM1/p62, respectively). SQSTM1/p62 mRNA expression was significantly decreased in PNET cells transfected with SQSTM1/p62-targeting siRNA 48 h after transfection compared to those transfected with siRNA-NC (n = 3, p < 0.001, Fig. 2). The protein expression level of SQSTM1/p62 in these cells was confirmed by immunoblotting (Fig. 4A).
We assessed cell proliferation by MTT assay and found that the number of cells was significantly lower in QGP-1 cells transfected with siRNA-Homo SQSTM1/p62 than in QGP-1 cells transfected with siRNA-NC after 24, 48, 72 and 96 h (n = 3, p = 1.0E-4, 2.0E-4, 2.0E-4, 6.0E-4, respectively; Fig. 3A). A significant association was also detected in INS-1 cells transfected with siRNA-rat SQSTM1/p62 (n = 3, p = 1.0E-5, 0.034, 0.016, 1.0E-5, respectively; Fig. 3A).
Effects of SQSTM1/P62 expression on cell proliferation, invasion and apoptosis. A The MTT assay showed that the proliferation of INS-1 and QGP-1 cells decreased significantly after knockdown of SQSTM1/p62 (INS1: n = 3, 24 h: p = 1.0E-4, 48 h: p = 2.0E-4, 72 h: p = 2.0E-4, 96 h: p = 6.0E-4; QGP1: n = 3, 24 h: p = 1.0E-5, 48 h: p = 0.034, 72 h: p = 0.016, 96 h: p = 1.0E-5). B The Transwell cell migration assay showed that the invasion ability of INS-1 and QGP-1 cells decreased significantly after knockdown of SQSTM1/p62 (n = 3, p = 2.0E-4 and n = 3, p = 1.0E-5, respectively). C Annexin V-FITC/PI flow cytometry showed that the number of dead INS-1 and QGP-1 cells (UR + LR) increased significantly after knockdown of SQSTM1/p62 (n = 3, p = 1.0E-5 and n = 3, p = 5.0E-4, respectively)
We used a classical transwell system to investigate the effect of SQSTM1/p62 on PNET cell invasion. The results showed that the number of QGP-1 cells and INS-1 cells that migrated through the membrane significantly decreased 48 h after transfection with SQSTM1/p62-targeting siRNA (n = 3, p = 2.0E-4 and n = 3, p = 1.0E-5, respectively; Fig. 3B), indicating that SQSTM1/p62 increased the invasive ability of PNET cells.
It has been reported that downregulating SQSTM1/p62 expression leads to autophagic cell death in multiple tumour cells [9]. PNET cells also undergo autophagic cell death [8]. Therefore, we performed annexin V-FITC/PI flow cytometry to detect the cell death induced by decreasing SQSTM1/p62 expression in PNET cells and found that the number of dead cells increased significantly 48 h after transfection with SQSTM1/p62-targeting siRNA in QGP-1 and INS-1 cells (n = 3, p = 1.0E-5 and n = 3, p = 5.0E-4, respectively; Fig. 3C).
Downregulating SQSTM1/P62 expression in PNET cells inhibited mTOR phosphorylation and activated cell autophagy
We detected the protein expression of LC3, SQSTM1/p62, mTOR and phosphorylated mTOR by WB. We also detected autophagic flux with mCherry-GFP-LC3B, in which yellow fluorescence represents early autophagosomes, while red fluorescence represents late autophagosomes. The results showed that after knockdown of SQSTM1/P62 in INS-1 cells and QGP-1 cells, the proportion of LC3-II/LC3-I was significantly increased (n = 3, p = 0.004 and p = 0.015, respectively; Fig. 4A), and the number of early autophagic bodies was also increased (n = 3, p = 0.012 and p = 0.018, respectively; Fig. 4B). A small number of late autophagic bodies were observed both before and after SQSTM1/P62 knockdown in INS-1 cells and QGP-1 cells, indicating that downregulation of SQSTM1/p62 activated autophagy in PNET cells while blocking autophagic flow. We also found that downregulation of SQSTM1/p62 resulted in a significant decrease in phosphorylated mTOR protein (n = 3, p = 0.001 and p = 1.1E−4, respectively; Fig. 4A), while there was no change in total mTOR protein.
Downregulation of SQSTM1/P62 expression in PNET cells inhibits the phosphorylation of mTOR and activates cell autophagy. A WB showed that the ratio of LC3-II to LC3-I increased significantly (n = 3; p = 0.004 and p = 0.015, respectively). The level of total mTOR protein did not change significantly, but the phosphorylated mTOR protein level decreased significantly (n = 3; p = 0.001 and p = 1.1E−4, respectively). B Analysis of autophagic flow by mCherry-GFP-LC3B double labelling. Yellow fluorescence represents early autophagosomes, and red fluorescence represents late autophagosomes. The number of early autophagosomes increased significantly after knockdown of SQSTM1/p62 in INS-1 and QGP-1 cells (n = 3; p = 0.012 and p = 0.018, respectively)
Discussion
Autophagy plays important roles in the pathogenesis and development of tumours. The role of autophagy in the survival of tumour cells has been controversial during tumour pathogenesis and development. Some in vitro and in vivo studies have concluded that autophagy is a tumour suppressor in the initial stage of cancer and a tumour promoter in the late stage of cancer [15]. Autophagy is a dynamic and complex regulatory process that has different functions in different tumours. A few studies have explored the role of autophagy in PNETs, but the significance of autophagy and the potential of its modulation have not yet been fully investigated in PNETs.
Kosmas Daskalaki analysed the number of LC3B-positive puncta to evaluate autophagy levels and showed that the autophagy level in PNETs was increased compared to that in normal pancreatic tissue and that a higher autophagy level was significantly associated with higher tumour grade [16]. Matrood Sami et al. assessed the expression of autophagy-related genes and showed that they were expressed at lower levels in G2 PNETs than in G1 PNETs and at lower levels in metastasized PNETs than in nonmetastasized PNETs. SQSTM1/p62 is considered an autophagy-related gene [17]. In the present study, we investigated the clinical prognostic value of SQSTM1/p62 in a large number of patients with a rare disease. Similar to what was observed in Matrood Sami et al.’s study [18], patients in which SQSTM1/p62 was expressed in PNETs had a larger tumour size, higher metastasis rate and higher recurrence rate. Our survival analysis also showed that patients with tumor expressed SQSTM1/p62 had a shorter disease-free survival time. Although multivariate survival analysis showed that difference did not reach significance (p = 0.093, Table 2), this might be due to the small number of rare tumors.
We further explored the biological function of SQSTM1/p62 in PNET cell lines and found that down-regulation of SQSTM1/p62 reduced tumour cell proliferation and invasion but promoted PNET cell death, revealing that SQSTM1/p62 can promote PNET progression in vitro. A few studies have demonstrated that SQSTM1/p62 could be a potential tumour therapeutic target [16, 18, 19]; our findings seem to be in line with those results that interference with p62 could inhibit the growth of tumour cells [20].
mTOR is the central checkpoint for the negative regulation of autophagy [21]. The mTOR inhibitor everolimus is the main drug for advanced PNETs [22]. Both SQSTM1/p62 and mTOR are important autophagy regulators; however, there have been few reports on their relationship. Sugiyama et al. found that SQSTM1/p62 can recruit mTORC1 to the lysosomal membrane and catalyse mTOR polyubiquitination under amino acid-rich conditions, resulting in mTORC1 activation [23]. Our results showed that the level of phosphorylated mTOR protein decreased significantly after SQSTM1/p62 was downregulated in PNET cell lines, while the total mTOR protein level did not change significantly, suggesting that SQSTM1/p62 might promote PNET progression by activating mTOR.
Chloroquine (CQ)/hydroxychloroquine (HCQ) inhibits late autophagy by inhibiting autophagosome-lysosome fusion and the enzymatic activity of lysosomal hydrolases, which has anticancer action and enhances the antiproliferative action of chemotherapeutics [23]. According to Shani Avniel-Pola’s study, knockdown of the early autophagy genes Atg5 and Atg7 in PNET cells had little effect on tumour growth and showed much smaller effects than CQ/HCQ [24]. Tabea, Wiedmer’s study reported a similar conclusion: PNET cells that interfere with early autophagy can be partially recovered and survive, but blocking late autophagy promotes the death of PNET cells [25]. The above studies suggest that the key to autophagy-mediated tumour cell death lies in the abnormal accumulation of autophagosomes. We observed the activation of early autophagy in a PNET cell line after downregulation of SQSTM1/p62, but there was no significant change in the number of late autophagic bodies, indicating that autophagic flow was blocked while autophagy was activated, leading to greater autophagic substrate accumulation; this indicated that downregulating SQSTM1/p62 decreased PNET cell proliferation and invasion and increased cell death, possibly by disturbing autophagosomes and causing autophagic death.
mTOR inhibitors exert a synergistic effect with CQ, reducing cell viability and enhancing cytotoxicity and apoptosis in lung neuroendocrine neoplasma cells [26]. CQ combined with an mTOR inhibitor also showed a stronger antitumour effect than CQ alone in PNET, as it more strongly suppressed PNET cell viability and proliferation and more markedly decreased the PNET size and weight [24]. Our research shows that inhibiting SQSTM1/p62 can inhibit mTOR activation and lead to autophagy substrate accumulation; this indicates that SQSTM1/p62 has the effects of both an mTOR inhibitor and CQ/HCQ, indicating that is/p62 is a potential ideal therapeutic target for PNETs.
We have revealed that SQSTM1/p62 expression could predict a worse prognosis in patients with PNETs. We also observed that inhibiting SQSTM1/p62 could decline the progression of PNETs by inhibiting mTOR activation and promoting autophagic death. Our studies provide an evidence that SQSTM1/p62 might be a therapeutic target for PNETs. The limitation of our study is that the most of patients were retrospectively followed up and fresh frozen PNET tumour specimens were not studied, it might lead to the potential bias. The strength of present study is that we study SQSTM1/p62 not only in vitro experiments but also in a cohort of patients with rare tumors.
Conclusions
Expression of SQSTM1/P62 protein correlates with worse prognosis in PNETs patients. Knockdown of SQSTM1/P62 gene in vitro is able to inhibit PNET progression by suppressing mTOR activation and inducing autophagic death.
Abbreviations
- PNET:
-
pancreatic neuroendocrine tumour
- FFPE:
-
formalin-fixed paraffin-embedded
- siRNAs:
-
small interfering RNAs
- CQ:
-
chloroquine
- HCQ:
-
hydroxychloroquine
References
A. Dasari, C. Shen, D. Halperin, B. Zhao, S. Zhou, Y. Xu et al. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol 3, 1335–1342 (2017)
M. Cives, J.R. Strosberg, Gastroenteropancreatic Neuroendocrine Tumors. CA: a cancer journal for clinicians 68, 471–487 (2018)
J.-H. Fan, Y.-Q. Zhang, S.-S. Shi, Y.-J. Chen, X.-H. Yuan, L.-M. Jiang et al. A nation-wide retrospective epidemiological study of gastroenteropancreatic neuroendocrine neoplasms in china. Oncotarget 8, 71699–71708 (2017)
R.T. Jensen, L. Bodei, J. Capdevila, A. Couvelard, M. Falconi, S. Glasberg et al. Unmet Needs in Functional and Nonfunctional Pancreatic Neuroendocrine Neoplasms. Neuroendocrinology 108, 26–36 (2019)
S. Avniel-Polak, G. Leibowitz, Y. Riahi, B. Glaser, D.J. Gross, S. Grozinsky-Glasberg, Abrogation of Autophagy by Chloroquine Alone or in Combination with mTOR Inhibitors Induces Apoptosis in Neuroendocrine Tumor Cells. Neuroendocrinology 103, 724–737 (2016)
T. Wiedmer, A. Blank, S. Pantasis, L. Normand, R. Bill, P. Krebs et al. Autophagy Inhibition Improves Sunitinib Efficacy in Pancreatic Neuroendocrine Tumors via a Lysosome-dependent Mechanism. Mol Cancer Therap 16, 2502–2515 (2017)
S. Avniel-Polak, G. Leibowitz, V. Doviner, D.J. Gross, S. Grozinsky-Glasberg, Combining chloroquine with RAD001 inhibits tumor growth in a NEN mouse model. Endocr Relat Cancer 25, 677–686 (2018)
S. Matrood, N. de Prisco, T.T. Wissniowski, D. Wiese, S. Jabari, H. Griesmann et al. Modulation of Pancreatic Neuroendocrine Neoplastic Cell Fate by Autophagy-Mediated Death. Neuroendocrinology 111, 965–985 (2021)
K. Nihira, Y. Miki, K. Ono, T. Suzuki, H. Sasano, An inhibition of p62/SQSTM1 caused autophagic cell death of several human carcinoma cells. Cancer Sci 105, 568–575 (2014)
S. Nakayama, H. Karasawa, T.Suzuki, p62/sequestosome 1 in human colorectal carcinoma as a potent prognostic predictor associated with cell proliferation. Cancer Med 6, 1264–1274 (2017). https://doi.org/10.1002/cam4.1093
C. Burdelski, V. Reiswich, C. Hube-Magg, M. Kluth, S. Minner, C. Koop et al. Cytoplasmic accumulation of sequestosome 1 (p62) is a predictor of biochemical recurrence, rapid tumor cell proliferation, and genomic instability in prostate cancer. Clin Cancer Res 21, 3471–3479 (2015)
S.-S. Li, L.-Z. Xu, W. Zhou, S. Yao, C.-L. Wang, J.-L. Xia et al. p62/SQSTM1 interacts with vimentin to enhance breast cancer metastasis. Carcinogenesis 38, 1092–1103 (2017)
B. Liu, L.H. Tang, Z. Liu, M. Mei, R. Yu, D. Dhall et al. α-Internexin: a novel biomarker for pancreatic neuroendocrine tumor aggressiveness. J Clin Endocrinol Metab 99, E786–795 (2014)
M. Pavel, K. Oberg, M. Falconi, E.P. Krenning, A. Sundin, A. Perren et al. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 31, 844–860 (2020)
R. Rakesh, L.C. PriyaDharshini, K.M. Sakthivel, R.R. Rasmi, Role and regulation of autophagy in cancer. Biochim Biophys Acta 1868, 166400 (2022)
K. Daskalakis, K.I. Alexandraki, I. Kloukina, E. Kassi, E. Felekouras, E. Xingi et al. Increased autophagy/mitophagy levels in primary tumours of patients with pancreatic neuroendocrine neoplasms. Endocrine 68, 438–447 (2020)
M. Tao, T. Liu, Q. You, Z. Jiang, p62 as a therapeutic target for tumor. Eur J Med Chem 193, 112231 (2020)
S. Matrood, L.E. Melms, D.K. Bartsch, P. Di Fazio, The Expression of Autophagy-Associated Genes Represents a Valid Footprint for Aggressive Pancreatic Neuroendocrine Neoplasms. Int J Mol Sci 24, 3636 (2023)
Y. Chen, Q. Li, Q. Li, S. Xing, Y. Liu, Y. Liu et al. p62/SQSTM1, a Central but Unexploited Target: Advances in Its Physiological/Pathogenic Functions and Small Molecular Modulators. J Med Chem 63, 10135–10157 (2020)
H. Denk, C. Stumptner, P.M. Abuja, K. Zatloukal, Sequestosome 1/p62-related pathways as therapeutic targets in hepatocellular carcinoma. Expert Opin Ther Targets 23, 393–406 (2019)
Y.C. Kim, K.L. Guan, mTOR: a pharmacologic target for autophagy regulation. J Clin Investig 125, 25–32 (2015)
C.K. Maharjan, P.H. Ear, C.G. Tran, J.R. Howe, C. Chandrasekharan, D.E. Quelle, Pancreatic Neuroendocrine Tumors: Molecular Mechanisms and Therapeutic Targets. Cancers 13, 5117 (2021)
P.M.P. Ferreira, R.W.R.D. Sousa, J.R.D.O. Ferreira, G.C.G. Militão, D.P. Bezerra, Chloroquine and hydroxychloroquine in antitumor therapies based on autophagy-related mechanisms. Pharmacol Res 168, 105582 (2021)
A.-P. Shani, L. Gil, R. Yael, G. Benjamin, J.G. David, G.-G. Simona, Abrogation of Autophagy by Chloroquine Alone or in Combination with mTOR Inhibitors Induces Apoptosis in Neuroendocrine Tumor Cells. Neuroendocrinology 103, (2016) https://doi.org/10.1159/000442589-000442537.
W. Tabea, B. Annika, P. Sophia, N. Lea, B. Ruben, K. Philippe, et al. Autophagy Inhibition Improves Sunitinib Efficacy in Pancreatic Neuroendocrine Tumors via a Lysosome-dependent Mechanism. Mol Cancer Ther 16, (2017) https://doi.org/10.1158/1535-7163.MCT-1117-0136-2515.
A. Knigin, S. Avniel-Polak, G. Leibowitz, K. Oleinikov, D.J. Gross, S. Grozinsky-Glasberg, The Autophagy Inhibitor Chloroquine, Alone or in Combination with mTOR Inhibitors, Displays Anti-Tumor Effects in In Vitro and In Vivo Lung Carcinoid Models. Cancers 13, 6327 (2021)
Funding
This work was supported by the Beijing Tongzhou District Science and Technology Plan Project in 2018 and 2019 (Grant numbers KJ2018CXś008-23 and KJ2019CX014-20).
Author information
Authors and Affiliations
Contributions
Y-LS designed the project, performed the experiment and followed up with patients. J-HW performed the experiment. D-CZ pathologically diagnosed tumours. Y-JC and BX supervised and supported the project. Y-LS and J-LZ prepared the tumoral samples. Y-LS and Y-JC wrote and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Consent to participate
Written informed consent was obtained from the participants or their legally authorized representatives.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Scientific Ethics Committee of Peking Union Medical College Hospital (S-K431).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, YL., Weng, JH., Zhao, DC. et al. SQSTM1/p62 is a prognostic molecular marker and potential therapeutic target for pancreatic neuroendocrine tumours. Endocrine 85, 407–416 (2024). https://doi.org/10.1007/s12020-023-03546-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-023-03546-w