Abstract
Purpose
In this study, we evaluated the biological role of miRNA-31-5p in papillary thyroid cancer (PTC).
Methods
By using the real-time PCR, we measured miRNA-31-5p expression levels in 25 PTC tissues and in two human PTC cell lines (K1 and TPC-1). Then, K1 cells were transiently transfected with mirVana inhibitor or mirVana mimic to miRNA-31-5-p. Cell proliferation was determined by MTT and colony formation assays. The in vitro metastatic ability of thyroid cancer cells was evaluated by adhesion, migration and invasion assays. Epithelial mesenchymal transition (EMT) and Hippo pathway related gene and protein levels were evaluated by using the TaqMan™ Gene Expression Assays and western blot analysis, respectively.
Results
We found a significant increase of miR-31-5-p expression in tumor tissue and in K1 cells harboring the BRAF p.V600E mutation. Knockdown of miR-31-5p determined a reduction of cell proliferation, associated with a significant decrease in cell adhesion, migration and invasion properties. A downregulation of EMT markers and YAP/β-catenin axis was also observed.
Conclusions
Our findings suggest that miRNA-31-5p acts as oncogenic miRNA in human thyrocytes and its overexpression may be involved in the BRAF-related tumorigenesis in PTCs, providing new understanding into its pathological role in PTC progression and invasiveness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
PTC is the most common histological type of TC and generally has an excellent prognosis. However, the management of invasive tumors and/or distant metastases unresponsive to radioactive iodine (RAI) treatment is still a major clinical challenge [1, 2]. Therefore, there is the need to understand the mechanisms underlying the progression of PTC for providing new diagnostic and therapeutic options [3]. miRNAs are single-stranded RNAs containing between 19 and 24 nucleotides involved in the regulation of a large number of proteins responsible for many cell functions [4, 5]. Several studies demonstrated that the dysregulation of miRNAs occurs in various cancers, playing a key role in biological processes such as cell migration, invasion, and EMT [6]. In the recent years, literature data highlighted that changes in miRNAs expression are correlated with the clinicopathological features of PTCs, including large tumor size, multifocality, extrathyroidal extension, lymph node and distant metastasis [7, 8]. Furthermore, expression of several miRNAs has been positively/negatively associated with TC recurrence risk, therefore the miRNA profiling could improve PTC screenings, clinical management and treatment evaluations [6, 9]. In a recent investigation, Maggisano et al. [10] observed a deregulation of several exosomal miRNAs in PTC cells and three of them (miR-31-5p, miR-222-3p, and let-7i-3p) were significantly overexpressed. Data on miR-31 in TC are few and discordant. Wang et al. [11] reported that miR-31 overexpression represses PTC cell proliferation, invasion and migration abilities by regulating the extracellular reg-ulated protein kinases (ERK), protein kinase B (Akt) signaling pathways and EMT. In addition, miR-31 restoration significantly suppressed the tumor growth of xenograft PTC models [11]. More recently, it has been reported that the overexpression of the long non-coding RNA LIFR-AS1 inhibited proliferation, migration, invasion, and vascular endothelial growth factor (VEGF) secretion of PTC cells by targeting miR-31-5p [12]. Moreover, the clinical significance and the biological role of miR-31-5p in PTC are unknown.
In the present study, we investigated the clinical role of miR-31-5p in human PTC tissues and compared the expression levels of miR-31-5p between K1 cells, an in vitro model of aggressive PTC harboring the BRAF p.V600E mutation, and TPC-1 cells, less aggressive cells with wild type (wt) BRAF. Also, we investigated the influence of miR-31-5p on the tumor pathologic properties in K1 cells, by its inhibition.
Materials and methods
Patients
Fresh frozen tumor tissues of 25 PTC patients were collected at Policlinico Umberto I, University of Roma ‘Sapienza’ with the informed consent by each patient and the approval of the local ethical committee according to guidelines of the Declaration of Helsinki (protocol code 1184/17 and date of approval 21/12/2017). The enrolled PTCs were well characterized for their clinical-pathological and genetic features. Tumors were staged according to the AJCC/UICC TNM classification [13] and the risk of recurrence was classified as LR or IR in accordance with the 2015 ATA Guidelines for the Management of Adult Patients with Thyroid Nodules and Differentiated TC [14]. PTC histotypes were determined by histopathological examination. All snap-frozen tumor specimens were reviewed by an experienced pathologist who confirmed the adequacy of the cancer cell population in each sample analyzed (at least 70% of cancer cells). The clinical and pathological characteristics are summarized in Table S1. BRAF mutational status was previously determined by Sanger sequencing, as previously described [15].
Cell culture
The human PTC K1, that harbors the BRAF p.V600E mutation as well as the p.E542K and C228T mutations in PI3K and TERT promoter respectively, and TPC-1 cells, characterized by the presence of RET/PTC1 rearrangement and CDKN2A p.Ala68fs, STAG2 p.Gln1089Ter and TERT C228T mutations [16, 17], were grown in DMEM medium (Thermo Fisher Scientific Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS, Thermo Fisher Scientific Inc.), penicillin (100 IU/ml), streptomycin (100 mg/ml), and amphotericin B (2.5 mg/ml) (Sigma Aldrich, Milan, Italy) at 37 °C in a humidified 5% CO2 atmosphere. To confirm the identity of the cell lines, short tandem repeat analysis was performed by using the AmpFLSTR NGM SElect PCR Amplification Kit (Thermo Fisher Scientific Inc.).
Extraction of RNA and real-time PCR array analysis of miR-31-5p and genes expression
Total RNA was isolated from tissues and cells using TRIzol reagent (Thermo Fisher Scientific Inc.) according to the manufacturer’s protocol [18]. The RNA concentration and purity were determined using a NanoDrop Spectrophotometer (Thermo Fisher Scientific Inc.). 10 ng of total RNA from tumor tissues and cells were reverse-transcribed with the TaqMan Advanced miRNA cDNA Synthesis Kit (Thermo Fisher Scientific Inc.) and the expression levels of miR-31-5p were analyzed using the TaqMan Advanced MicroRNA Assay (Thermo Fisher Scientific Inc.). For EMT and Hippo pathway related gene expression, 1 µg of RNA from cells was reverse-transcribed using the High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific Inc.), and the gene expression levels were quantified using the TaqMan Assays (Thermo Fisher Scientific Inc.). All qPCR reactions were run on the 7900HT Fast Real-Time PCR System (Thermo Fisher Scientific Inc.), and results were expressed using the 2−ΔΔCt method [19]. Mir-16-5p and beta-2-microglobulin were used as endogenous control for miR-31-5p and gene expression analysis respectively. Negative Control (NC) for the miR-31-5p inhibitor or mimic was used as calibrator sample.
Cell transfection
K1 cells were transiently transfected with 30 nM of mirVana inhibitor or 30 nM of mirVana mimic to miR-31-5p using lipofectamine RNAiMAX (Thermo Fisher Scientific Inc.), following the manufacturer’s instructions. For each set of cells, a corresponding mirVana negative control (NC) for the inhibitor or mimic was used. Cells were seeded in six well plates (100 × 103/well) with medium containing FBS 10%. The day after, transfection was performed on 60–80% confluence starved cells. Transfection efficiency was evaluated after 24 h by RT-PCR assay.
Proliferation assay
The cell proliferation rate was determined via the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay [20]. Briefly, after transfection with miR-31-5p inhibitor, miR-31-5p mimic or NC, cells were seeded in 96 well plates at a density of 3.0 × 103 followed by 24, 48, and 72 h incubation. Thereafter, 10 µL of MTT (0.5 mg/mL) were added to the cells and the solubilized product was quantified with a microplate spectrophotometer (xMark, Biorad, Milan, Italy) at a wavelength of 540 nm and a reference wavelength of 690 nm. Results are expressed as percentages over cells transfected with negative controls.
Soft agar assay
Cell ability to grow in three-dimensional soft agar was evaluated as follows [21]. First, 24-well plates were loaded with agar noble-DMEM medium mixture at a concentration of 0.5%. Besides, we also established and mixed the sterile 0.4% top low melting agarose with a transfected cell number (see Cell transfection paragraph) of about 1.25 × 103 per well. When the top agar is solidified, the soft agar plate was maintained under the condition of 37 °C for 20 days. We added the fresh medium into the wells twice every week. Finally, colonies with diameter of more than 50 µm were counted under an inverted microscope, after being photographed with a DFC 3000 G camera mounted on a Leica microscope (DM IL LED, Leica Microsystems Srl, Milan, Italy).
Adhesion, migration and invasion assays
Adhesion assays were performed as previously described [22]. Briefly, after transient transfection, 3 × 104 cells were seeded into 24-well plates coated with collagen I (BD, Milan, Italy) and incubated for 30 min. Adherent cells were fixed in 4% paraformaldehyde, stained with 0.1% Crystal Violet solution, washed with distilled water and then solubilized in 10% acetic acid. Cell adhesion was quantified by measurement of absorbance at 560 nm.
Transwell inserts with a diameter pore of 8 µm (Costar, Euroclone, Milan, Italy) not coated or coated with Geltrex (Thermo Fisher Scientific Inc.) were used for cell migration and invasion assays, respectively. 60 × 103 transfected cells were suspended in FBS-free medium containing 1% bovine serum albumin (BSA), for migration assay, or 1% FBS, for invasion assay, and added in the upper chamber of the inserts; the bottom chamber contained growth media supplemented with 10% FBS. After 6 h or 24 h of incubation for migration and invasion assays, respectively, cells in the upper well were removed with a cotton swab while cells of the underside of the membrane were fixed and stained with Diff-Quick Stain (Bio Map, Monza, Italy). Cells were counted using a microscope provided with an eyepiece and equipped with a counting grid. Results from three independents experiments are expressed as percentages over NC for miRNA inhibitor or miRNA mimic.
Protein extraction and western blot analysis
Total proteins were extracted as previously described [23]. Thirty µg of proteins were run on a 7.5, 9, or 12% SDS-PAGE gel, transferred to PVDF membranes (VWR, Milan, Italy), blocked with Tris buffered saline, 1% Tween 20, 5% non-fat dry milk (TTBS/milk) and incubated overnight with the following antibodies: anti-β-catenin diluted 1:1000, anti-CDH2 diluted 1:1000 and anti-YAP-1 diluted 1:1000 (Cell Signaling, Danvers, MA, USA); anti-FN1 diluted 1:250 and anti-GAPDH diluted 1:1000 (Sigma Aldrich); anti-TAZ diluted 1:500 (Thermo Fisher Scientific Inc.). Then, the membranes were washed in TTBS and incubated with horseradish peroxidase-conjugated antibody (Transduction Laboratories, Lexington, KY, USA) in TTBS/milk. Western blot detection system ECL Plus (Perkin Elmer, Monza, Italy) was used to visualize proteins.
Immunofluorescence assay
Cells grown on coverslips, after mir-31-5p transfection, were fixed with 4% paraformaldehyde, permeabilized with Triton X-100 and processed for immunofluorescence as previously described [24]. Briefly, cells were immunostained with anti-YAP-1 antibody (Abcam) diluted 1:1000, and anti-TAZ antibody (Thermo Fisher Scientific Inc.) diluted 1:200, and then incubated with Alexa Fluor 555 anti-mouse antibody from Molecular Probes (Thermo Fisher Scientific Inc.), at 1:500 dilution. Finally, nuclei were stained with Hoechst 33258 (Thermo Fisher Scientific Inc). Cells were visualized under a Leica microscope (DM IL LED, Leica Microsystems Srl, Milan, Italy) equipped with a DFC 3000 G camera.
Statistical analysis
All statistical analyses were performed with the GraphPad Prism version 9.3.0 software (GraphPad Software Inc., San Diego, CA, USA) by using T-Test with Welch’s correction. P values lower than 0.05 were considered statistically significant.
Results
MiR-31-5p expression in PTC tissues and cell lines
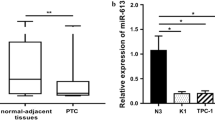
As reported in Table S1, the 25 PTC tissues analyzed, included 11 that were classified as intermediate risk (IR) and 14 as low risk (LR) for recurrence or relapse of TC according to ATA criteria [14]. These tumors were also characterized by the presence (N = 12) or absence (N = 13) of the BRAF p.V600E mutation. Real-time PCR analysis revealed that the expression levels of miR-31-5p were significantly increased in tumors harboring the BRAF p.V600E mutation compared to the levels in BRAF wt PTCs (p < 0.05) (Fig. 1A). The expression levels of the miR-31-5p between PTCs grouped considering other clinical features (i.e., ATA risk, extrathyroidal invasion, tumor size and multifocality) didn’t show any difference (data not shown). In PTC cells the expression of miR-31-5p was significantly higher in K1 compared to TPC-1 cells (p < 0.01) (Fig. 1B). Subsequent experiments were thus focused on the BRAF-mutated K1 cells.
Effects of miR-31-5p knockdown on viability, adhesion, migration and invasiveness of PTC cells
The specific miR-31-5p inhibitor and miR-31-5p mimic significantly reduced and increased respectively the expression of miR-31-5p in K1 cells (p < 0.001), (Fig. 2).
Transfection efficiency. K1 cells were transfected for 24 h with: (A) miR-31-5p inhibitor, (B) miR-31-5p mimic or their corresponding controls (NC miR inhibitor and NC miR mimic). The expression of miR-31-5p was detected by qRT-PCR as described in the section Materials and Methods. Cells transfected with NC miR inhibitor or mimic were arbitrarily set at 1 and miRNA levels are indicated as relative expression values. All samples were run in duplicate. Results are shown as mean ± SD. *** p < 0.001 vs NC miR inhibitor or NC miR mimic
In K1 transfected cells, analysis of cell proliferation after 24, 48, and 72 h showed a significant reduction of viability (~20%, p < 0.05 vs NC miR inhibitor) (Fig. 3A) and after 20 days, the growth ability in three-dimensional soft agar resulted reduced (p < 0.05 vs NC miR inhibitor) (Fig. 3B). Next, we assessed the effect of miR-31-5p inhibitor on cell adhesion, migration and invasion, key component of malignant tumor progression. As shown in Fig. 3C, treatment with miR-31-5p inhibitor significantly reduced cell adhesion (~30%, p < 0.001 vs NC miR inhibitor), migration (~50%, p < 0.001 vs NC miR inhibitor) and invasion (~40%, p < 0.001 vs NC miR inhibitor).
miR-31-5p knockdown effects on cell growth, adhesion, migration and invasion properties of K1 cells. Cells were transfected with miR-31-5p inhibitor or with NC miR inhibitor as reported in Materials and Methods. (A): Cell viability was analyzed by MTT assay after 24, 48, and 72 h of incubation. Results are expressed as a percentage over NC miR inhibitor. B A three-dimensional colony formation test was conducted in soft agar (Scale bar, 100 mM). Quantification of assays were showed on the right statistical panel. C For adhesion assays, after 30 min of incubation, cells were fixed and stained with crystal violet. For migration and invasion assays after 6 or 24 h, respectively, filters were stained, and the cells counted. Values are expressed as means ± SD from three (A and C) or two (B) independents experiments. Statistical analysis was performed using the T-Test with Welch’s correction. * p < 0.05, ***p < 0.001 vs NC miR inhibitor
Conversely, miR-31-5p overexpression by synthetic miR-31-5p mimic transfection into K1 cells increased cell motility and invasiveness without affecting tumor cell proliferation (Fig. 4).
Effects of miR-31-5p mimic on cell growth, adhesion migration and invasion properties of K1 cells. Cells were transfected with miR-31-5p mimic or with NC miR mimic as reported in Materials and Methods. A Cell viability was analyzed by MTT assay after 24, 48, and 72 h of incubation. Results are expressed as a percentage over NC miR mimic and were obtained from three independent experiments. B A three-dimensional colony formation test was conducted in soft agar (Scale bar, 250 mM). Quantification of assays was showed on the right statistical panel. C For adhesion assays, after 30 min of incubation, cells were fixed and stained with crystal violet. For migration and invasion assays after 6 or 24 h, respectively, filters were stained, and the cells counted. Values are expressed as means ± SD from three independents experiments. Statistical analysis was performed using the T-Test with Welch’s correction. * p < 0.05, vs NC miR mimic
MiR-31-5p knockdown influences the EMT acting on the Hippo and Wnt/β-catenin pathways
In order to investigate if miR-31-5p can influence the metastatic invasiveness of K1 cells, we evaluated the expression of some of the most representative markers of EMT. The analysis of the mRNA and protein levels of fibronectin 1 (FN1) and N-cadherin (CDH2) showed a significant reduction in the expression of these two markers in cells transfected with miR-31-5p inhibitor (Fig. 5A, B). Moreover, we assessed the expression of Yes-associated protein 1 (YAP-1) and PDZ-binding motif (TAZ), two players of the Hippo pathway with a driving role in cellular migration and metastasis, by qRT-PCR, western blot and immunofluorescence staining. As shown in Fig. 5, the miR-31-5p inhibitor determined a considerable downregulation of these markers in K1 cells. Furthermore, we also observed a modulation of Wnt/β-catenin signaling pathway consisting in a decreased protein expression of β-catenin (Fig. 5B).
miR-31-5p knockdown regulates the expression of EMT and Hippo pathway markers. K1 cells were transfected for 24 h with miR-31-5p inhibitor or its corresponding control (NC miR inhibitor). A The gene expression was detected by qRT-PCR. Cells transfected with NC miR inhibitor were arbitrarily set at 1 and miRNA levels are indicated as relative expression values. All samples were run in duplicate. Results are shown as mean ± SD. * p < 0.05, **p < 0.01, *** p < 0.001 vs NC miR inhibitor. B Western blotting analysis showed different FN1, CDH2, YAP-1, TAZ and β-catenin expression levels in cells transfected with miR-31-5p inhibitor compared with NC miR inhibitor. GAPDH was used as a loading control. C Representative immunofluorescence images of cells transfected with miR-31-5p inhibitor or its corresponding control (NC miR inhibitor). YAP-1 and TAZ proteins were detected with Alexa Fluor 555 conjugated secondary antibody; nuclei were stained with Hoechst 33258 (Scale bar, 100 µM)
On the contrary, K1 cells transfected with miR-31-5p mimic showed a recover of the expression levels of both Hippo-related proteins and β-catenin (Fig. 6).
miR-31-5p mimic restores the expression of Hippo pathway markers and β-catenin. K1 cells were transfected for 24 h with miR-31-5p mimic or its corresponding control (NC miR mimic). A The gene expression was detected by qRT-PCR. Cells transfected with NC miR mimic were arbitrarily set at 1 and miRNA levels are indicated as relative expression values. All samples were run in duplicate. Results are shown as mean ± SD. B Western blotting analysis shows different YAP-1, TAZ and β-catenin expression levels in cells transfected with miR-31-5p mimic compared with NC miR mimic. GAPDH was used as a loading control. C Representative immunofluorescence images of cells transfected with miR-31-5p mimic or its corresponding control (NC miR mimic). YAP-1 and TAZ proteins were detected with Alexa Fluor 555 conjugated secondary antibody; nuclei were stained with Hoechst 33258 (Scale bar, 100 µM)
Discussion
The current approaches based on surgery plus radioiodine therapy are effective for the majority of PTC but the management of the most aggressive subtypes that are unresponsive to radioiodine therapy, needs novel diagnostic and prognostic tools. In this context, it is essential to elucidate the molecular mechanisms underlying the enhanced aggressiveness of certain PTCs. Recent studies suggest that some miRNAs can play an important role in tumor progression and metastasis acting as oncogenes or tumor suppressors in PTC [25]. It has been demonstrated that mir-31-5p has an oncogenic role in several human cancers [26,27,28,29]. In particular, it resulted overexpressed in bone tissues cancer and its downregulation suppressed proliferation and invasion of osteosarcoma cells as well as tumor growth in vivo [26]. In colorectal cancer, it was demonstrated that knockdown of miR-31-5p inhibited proliferation, colony formation, cell migration and invasion by re-pressing YAP/β-catenin axis as well as the chemoresistance to 5-fluorouracil [28].
In the present study we observed a significant increase of miR-31-5p expression levels in tumors harboring the BRAF p.V600E mutation, currently investigated as molecular target for personalized treatment [30]. This finding is supported by the TCGA data obtained on a huge cohort of PTC patients from cBioPortal (available at https://www.cbioportal.org/). Moreover, comparing the miR-31-5p expression levels in two PTC cell lines, we observed higher levels in the BRAF mutated cells (i.e., K1 cells) with an aggressive behavior in terms of growth rate and invasiveness. These results suggested an involvement of the miR-31-5p in the BRAF associated cancer progression, prompting us to analyze the role of this miRNA on the tumor pathologic properties in K1 cells by its inhibition, although we cannot exclude that other genetic alterations besides or in addition to the BRAF V600E mutation (i.e., PI3K and TERT promoter mutations), may be involved in the miRNA overexpression. A real proof of the functional relationship between miRNA-31-5p and the BRAF mutation would be produced only using a cell line in which the BRAF mutation is removed, for example by using the CRISPR/CAS9 technique. We found that miR-31-5p knockdown resulted in a reduction of proliferation rate and cell growth in soft agar as well as a significant decrease in cell adhesion, migration, and invasion properties. Several studies have established that the invasiveness of TC is associated with multiphase processes like EMT, a phenomenon in which epithelial cells acquire the ability to invade the extracellular matrix and migrate into the bloodstream [31, 32].
Thus, we hypothesized that miR-31-5p could influence the metastatic invasiveness of K1 cells by modulating EMT-related proteins such as FN1 and CDH2. We found that FN1 and CDH2 gene and protein expression was significantly downregulated in K1 cells after miR-31-5p inhibition. We also demonstrated that its overexpression using specific miR-31-5p mimic determined an increase of cell invasiveness. Interestingly, some evidence suggested that a high expression of FN1 promotes the EMT process and tumor aggressiveness resulting overexpressed in PTC patients with lymph node metastasis and with the BRAF p.V600E mutation [33, 34].
The molecular mechanism by which miR-31-5p can induce EMT process is still unknown. It has been postulated that miR-31-5p may regulate the Wnt/β-catenin and Hippo signaling pathways, two of the pathways with a crucial role in activation of EMT-related transcription factors [28, 35,36,37]. Recently, several studies have investigated the function of YAP-1 and TAZ in driving proliferation of cancer cells and tumor tissue, as well as cellular migration, metastasis and resistance to therapeutics [38,39,40]. High levels of YAP-1 were also detected in TC tissues and correlated with advanced tumor progression and poor prognosis [41, 42]. The activation of YAP-1 accelerates the malignant progression and metastasis conferring chemoresistance in TC cells [43]. Furthermore, deregulation of miRNAs is also one of the most crucial mechanisms for Wnt/β-catenin signaling activation [44] and recent data support the role of this pathway in PTC progression, upregulating EMT-related genes and increasing the thyroid tumor cell invasion [8, 45]. Herein, knockdown of miR-31-5p resulted in a downregulation of YAP-1, TAZ and β-catenin. A recover of their expression levels was observed when miR-31-5p was overexpressed confirming, for the first time, the influence of miR-31-5p on Hippo and Wnt/β-catenin pathways in TC cells.
Conclusions
The findings illustrated in the present paper suggest that the miRNA-31-5p acts as oncogenic miRNA in human thyrocytes and its overexpression may be involved in the BRAF-related tumorigenesis in PTCs. It may play an important role in thyroid tumor progression by altering migration and invasion properties of neoplastic thyroid cells by influencing the EMT process at least in part through the modulation and of YAP-1/β-catenin signaling pathways. These results will advance our understanding the process of PTC progression and may provide evidence of miR-31-5p as novel therapeutic target for PTC treatment.
References
S. Bulotta, M. Celano, G. Costante, D. Russo, Novel therapeutic options for radioiodine-refractory thyroid cancer: redifferentiation and beyond. Curr. Opin. Oncol. 32(1), 13–19 (2020). https://doi.org/10.1097/CCO.0000000000000593
R. Niciporuka, J. Nazarovs, A. Ozolins, Z. Narbuts, E. Miklasevics, J. Gardovskis. Can we predict differentiated thyroid cancer behavior? role of genetic and molecular markers. Medicina (Kaunas, Lithuania) 57(10), 1131 (2021). https://doi.org/10.3390/medicina57101131.
M. Rogucki, A. Buczynska, A.J. Kretowski, A. Poplawska-Kita, The Importance of miRNA in the Diagnosis and Prognosis of Papillary Thyroid Cancer. J. Clin. Med. 10(20), 4738 (2021). https://doi.org/10.3390/jcm10204738
M. Hussain, Micro-RNAs (miRNAs): Genomic Organisation, Biogenesis and Mode of Action. Cell. Tissue Res. 349, 405–413 (2012). https://doi.org/10.1007/s00441-012-1438-0
C.R. Lima, C.C. Gomes, M.F. Santos, Role of microRNAs in endocrine cancer metastasis. Mol. Cell. Endocrinol. 456, 62–75 (2017). https://doi.org/10.1016/j.mce.2017.03.015
H. Shakib, S. Rajabi, M.H. Dehghan, F.J. Mashayekhi, N. Safari-Alighiarloo, M. Hedayati, Epithelial-to-mesenchymal transition in thyroid cancer: a comprehensive review. Endocrine 66, 435–455 (2019). https://doi.org/10.1007/s12020-019-02030-8
Y. Sun, S. Yu, Y. Liu, F. Wang, Y. Liu, H. Xiao, Expression of miRNAs in Papillary Thyroid Carcinomas Is Associated with BRAF Mutation and Clinicopathological Features in Chinese Patients. Int. J. Endocrinol. 2013, 128735 (2013). https://doi.org/10.1155/2013/128735
M. Papaioannou, A.G. Chorti, A. Chatzikyriakidou, K. Giannoulis, S. Bakkar, T.S. Papavramidis, MicroRNAs in Papillary Thyroid Cancer: What Is New in Diagnosis and Treatment. Front. Oncol. 11, 755097 (2022). https://doi.org/10.3389/fonc.2021.755097
M. Celano, F. Rosignolo, V. Maggisano, V. Pecce, M. Iannone, D. Russo, S. Bulotta, MicroRNAs as Biomarkers in Thyroid Carcinoma. Int. J. Genomics. 2017, 6496570 (2017). https://doi.org/10.1155/2017/6496570
V. Maggisano, F. Capriglione, A. Verrienti, M. Celano, A. Gagliardi, S. Bulotta, M. Sponziello, C. Mio, V. Pecce, C. Durante, G. Damante, D. Russo, Identification of Exosomal microRNAs and Their Targets in Papillary Thyroid Cancer Cells. Biomedicines 10(5), 961 (2022). https://doi.org/10.3390/biomedicines10050961
Y. Wang, B.G. Liu, C.X. Zhou, MicroRNA-31 inhibits papillary thyroid carcinoma cell biological progression by directly targeting SOX11 and regulating epithelial-to-mesenchymal transition, ERK and Akt signaling pathways. Eur. Rev. Med. Pharmacol. Sci. 23, 5863–5873 (2019). https://doi.org/10.26355/eurrev_201907_18329
D. Yi, D. Zhang, J. He, Long non-coding RNA LIFR-AS1 suppressed the proliferation, angiogenesis, migration and invasion of papillary thyroid cancer cells via the miR-31-5p/SIDT2 axis. Cell. Cycle 20, 2619–2637 (2021). https://doi.org/10.1080/15384101.2021.1995129
R.M. Tuttle, B. Haugen, N.D. Perrier, Updated American Joint Committee on Cancer/Tumor-Node-Metastasis Staging System for Differentiated and Anaplastic Thyroid Cancer (Eighth Edition): What Changed and Why? Thyroid 27(6), 751–756 (2017). https://doi.org/10.1089/thy.2017.0102
B.R. Haugen, E.K. Alexander, K.C. Bible, G.M. Doherty, S.J. Mandel, Y.E. Nikiforov, F. Pacini, G.W. Randolph, A.M. Sawka, M. Schlumberger, K.G. Schuff, S.I. Sherman, J.A. Sosa, D.L. Steward, R.M. Tuttle, L. Wartofsky, 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 26, 1–133 (2016). https://doi.org/10.1089/thy.2015.0020
N. Passon, E. Bregant, M. Sponziello, M. Dima, F. Rosignolo, C. Durante, M. Celano, D. Russo, S. Filetti, G. Damante, Somatic amplifications and deletions in genome of papillary thyroid carcinomas. Endocrine 50, 453–464 (2015). https://doi.org/10.1007/s12020-015-0592-z
R.E. Schweppe, J.P. Klopper, C. Korch, U. Pugazhenthi, M. Benezra, J.A. Knauf, J.A. Fagin, L.A. Marlow, J.A. Copland, R.C. Smallridge, B.R. Haugen, Deoxyribonucleic acid profiling analysis of 40 human thyroid cancer cell lines reveals cross-contamination resulting in cell line redundancy and misidentification. J. Clin. Endocrinol. Metab. 93, 4331–4341 (2008). https://doi.org/10.1210/jc.2008-1102
A. Bairoch, The Cellosaurus, a Cell-Line Knowledge Resource. J. Biomol. Tech. 29(2), 25–38 (2018). https://doi.org/10.7171/jbt.18-2902-002
V. Maggisano, M. Celano, S.M. Lepore, M. Sponziello, F. Rosignolo, V. Pecce, A. Verrienti, F. Baldan, C. Mio, L. Allegri, M. Maranghi, R. Falcone, G. Damante, D. Russo, S. Bulotta, Human telomerase reverse transcriptase in papillary thyroid cancer: gene expression, effects of silencing and regulation by BET inhibitors in thyroid cancer cells. Endocrine 63, 545–553 (2019). https://doi.org/10.1007/s12020-018-01836-2
K.J. Livak, S.J. Flood, J. Marmaro, W. Giusti, K. Deetz, Oligonucleotides with fluorescent dyes at opposite ends provide a quenched probe system useful for detecting PCR product and nucleic acid hybridization. PCR Methods Appl. 4, 357–362 (1995). https://doi.org/10.1101/gr.4.6.357
M. Celano, V. Maggisano, S. Bulotta, L. Allegri, V. Pecce, L. Abballe, G. Damante, D. Russo, Quercetin improves the effects of sorafenib on growth and migration of thyroid cancer cells. Endocrine 67, 496–498 (2020). https://doi.org/10.1007/s12020-019-02140-3
S. Borowicz, M. Van Scoyk, S. Avasarala, M.K.K. Rathinam, J. Tauler, R.K. Bikkavilli, R.A. Winn, The soft agar colony formation assay. J. Vis. Exp. 92, e51998 (2014). https://doi.org/10.3791/51998
S. Bulotta, M.V. Ierardi, J. Maiuolo, M.G. Cattaneo, A. Cerullo, L.M. Vicentini, N. Borgese, Basal nitric oxide release attenuates cell migration of HeLa and endothelial cells. Biochem. Biophys. Res. Commun. 386, 744e749 (2009). https://doi.org/10.1016/j.bbrc.2009.06.118
M. D’Agostino, P. Voce, M. Celano, M. Sponziello, S. Moretti, V. Maggisano, A. Verrienti, C. Durante, S. Filetti, E. Puxeddu, D. Russo, Sunitinib exerts only limited effects on the proliferation and differentiation of anaplastic thyroid cancer cells. Thyroid 22, 138–144 (2012). https://doi.org/10.1089/thy.2011.0060
S. Bulotta, A. Cerullo, R. Barsacchi, C. De Palma, D. Rotiroti, E. Clementi, N. Borgese, Endothelial nitric oxide synthase is segregated from caveolin-1 and localizes to the leading edge of migrating cells. Exp. Cell Res. 312(6), 877–889 (2006). https://doi.org/10.1016/j.yexcr.2005.12.014
N. Mastronikolis, E. Tsiambas, D. Roukas, P. Fotiades, A. Chrysovergis, V. Papanikolaou, E. Kyrodimos, S. Mastronikoli, A. Niotis, V. Ragos, Micro-RNAs signatures in papillary thyroid carcinoma. J. Buon. 25(5), 2144–2146 (2020)
X. Chen, L. Zhong, X. Li, W. Liu, Y. Zhao, J. Li, Down-regulation of microRNA-31-5p inhibits proliferation and invasion of osteosarcoma cells through Wnt/β-catenin signaling pathway by enhancing AXIN1. Exp. Mol. Pathol. 108, 32–41 (2019). https://doi.org/10.1016/j.yexmp.2019.03.001
Z. Lu, Q. He, J. Liang, W. Li, Q. Su, Z. Chen, Q. Wan, X. Zhou, L. Cao, J. Sun, Y. Wu, L. Liu, X. Wu, J. Hou, K. Lian, A. Wang, miR-31-5p Is a Potential Circulating Biomarker and Therapeutic Target for Oral Cancer. Mol. Ther. Nucleic Acids 16, 471–480 (2019). https://doi.org/10.1016/j.omtn.2019.03.012
Y.L. Du, Y. Liang, G.Q. Shi, Y. Cao, J. Qiu, L. Yuan, Z. Yong, L. Liu, J. Li, LINC00689 participates in proliferation, chemoresistance and metastasis via miR-31-5p/YAP/beta-catenin axis in colorectal cancer. Exp. Cell. Res. 395, 112176 (2020). https://doi.org/10.1016/j.yexcr.2020.112176
F. Song, Z. Xuan, X. Yang, X. Ye, Z. Pan, Q. Fang, Identification of key microRNAs and hub genes in non-small-cell lung cancer using integrative bioinformatics and functional analyses. J. Cell. Biochem. 121, 2690–2703 (2020). https://doi.org/10.1002/jcb.29489
H.G. Vuong, A.M.A. Altibi, U.N.P. Duong, L. Hassell, Prognostic implication of BRAF and TERT promoter mutation combination in papillary thyroid carcinoma-A meta-analysis. Clin. Endocrinol. (Oxf.). 87, 411–417 (2017). https://doi.org/10.1111/cen.13413
V. Vasko, A.V. Espinosa, W. Scouten, H. He, H. Auer, S. Liyanarachchi, A. Larin, V. Savchenko, G.L. Francis, A. de la Chapelle, M. Saji, M.D. Ringel, Gene expression and functional evidence of epithelial-to-mesenchymal transition in papillary thyroid carcinoma invasion. Proc. Natl Acad. Sci. USA. 104, 2803–2808 (2007). https://doi.org/10.1073/pnas.0610733104
P. Baquero, E. Jimenez-Mora, A. Santos, M. Lasa, A. Chiloeches, TGF beta induces epithelial-mesenchymal transition of thyroid cancer cells by both the BRAF/MEK/ERK and Src/FAK pathways. Mol. Carcinog. 55, 1639–1654 (2016). https://doi.org/10.1002/mc.22415
M. Sponziello, F. Rosignolo, M. Celano, V. Maggisano, V. Pecce, R.F. De Rose, G.E. Lombardo, C. Durante, S. Filetti, G. Damante, D. Russo, S. Bulotta, Fibronectin-1 expression is increased in aggressive thyroid cancer and favors the migration and invasion of cancer cells. Mol. Cell. Endocrinol. 431, 123–132 (2016). https://doi.org/10.1016/j.mce.2016.05.007
S. Xia, C. Wang, E.L. Postma, Y. Yang, X. Ni, W. Zhan, Fibronectin 1 promotes migration and invasion of papillary thyroid cancer and predicts papillary thyroid cancer lymph node metastasis. Onco Targets Ther. 10, 1743–1755 (2017). https://doi.org/10.2147/OTT.S122009
Y. Lei, L. Chen, G. Zhang, A. Shan, C. Ye, B. Liang, J. Sun, X. Liao, C. Zhu, Y. Chen, J. Wang, E. Zhang, L. Deng, MicroRNAs target the Wnt/betacatenin signaling pathway to regulate epithelial mesenchymal transition in cancer (Review). Oncol. Rep. 44, 1299–1313 (2020). https://doi.org/10.3892/or.2020.7703
B. Mi, Q. Li, T. Li, G. Liu, J. Sai, High miR-31-5p expression promotes colon adenocarcinoma progression by targeting TNS1. Aging (Albany NY) 12, 7480–7490 (2020). https://doi.org/10.18632/aging.103096
I. Akrida, V. Bravou, H. Papadaki, The deadly cross-talk between Hippo pathway and epithelial-mesenchymal transition (EMT) in cancer. Mol. Biol. Rep. 49(10), 10065–10076 (2022). https://doi.org/10.1007/s11033-022-07590-z
T. Pei, Y. Li, J. Wang, H. Wang, Y. Liang, H. Shi, B. Sun, D. Yin, J. Sun, R. Song, S. Pan, Y. Sun, H. Jiang, T. Zheng, L. Liu, YAP is a critical oncogene in human cholangiocarcinoma. Oncotarget 6, 17206–17220 (2015). https://doi.org/10.18632/oncotarget.4043
S.E. Hiemer, L. Zhang, V.K. Kartha, T.S. Packer, M. Almershed, V. Noonan, M. Kukuruzinska, M.V. Bais, S. Monti, X. Varelas, A YAP/TAZ-Regulated Molecular Signature Is Associated with Oral Squamous Cell Carcinoma. Mol. Cancer Res. 13, 957–968 (2015). https://doi.org/10.1158/1541-7786.MCR-14-0580
W. Zhang, Y. Gao, F. Li, X. Tong, Y. Ren, X. Han, S. Yao, F. Long, Z. Yang, H. Fan, L. Zhang, H. Ji, YAP promotes malignant progression of Lkb1-deficient lung adenocarcinoma through downstream regulation of survivin. Cancer Res. 75, 4450–4457 (2015). https://doi.org/10.1158/0008-5472.CAN-14-3396
M. Celano, C. Mignogna, F. Rosignolo, M. Sponziello, M. Iannone, S.M. Lepore, G.E. Lombardo, V. Maggisano, A. Verrienti, S. Bulotta, C. Durante, C. Di Loreto, G. Damante, D. Russo, Expression of YAP1 in aggressive thyroid cancer. Endocrine 59(1), 209–212 (2018). https://doi.org/10.1007/s12020-017-1240-6
Z. Liu, W. Zeng, Y. Maimaiti, J. Ming, Y. Guo, Y. Liu, C. Liu, T. Huang, High Expression of Yes-activated Protein-1 in Papillary Thyroid Carcinoma Correlates With Poor Prognosis. Appl. Immunohistochem. Mol. Morphol. 27(1), 59–64 (2019). https://doi.org/10.1097/PAI.0000000000000544.
M. Wang, M. Dai, D. Wang, W. Xiong, Z. Zeng, C. Guo, The regulatory networks of the Hippo signaling pathway in cancer development. J. Cancer 12, 6216–6230 (2021). https://doi.org/10.7150/jca.62402
P. Ma, J. Han, Overexpression of miR-100-5p inhibits papillary thyroid cancer progression via targeting FZD8. Open Med. (Wars.) 17(1), 1172–1182 (2022). https://doi.org/10.1515/med-2022-0490
B. Basu, M.K. Ghosh, Ubiquitination and deubiquitination in the regulation of epithelial-mesenchymal transition in cancer: Shifting gears at the molecular level. Biochim. Biophys. Acta Mol. Cell. Res. 1869, 119261 (2022). https://doi.org/10.1016/j.bbamcr.2022.119261
Acknowledgements
This paper was financially supported by the Department of Health Sciences, University of Catanzaro “Magna Græcia”.
Funding
This study was funded by grants from the Italian Ministry of Universities and Research, PRIN2017EKMFTN_003.
Author information
Authors and Affiliations
Contributions
Conceptualization, V.M., F.C., and D.R.; Investigation, V.M., F.C., A.V., and M.C.; Data curation, A.V., M.S., V.P., M.C.; Writing-original draft preparation, V.M., S.B., and D.R.; Writing-review and editing, C.D., S.B., and D.R.; Supervision, C.D., D.R., and S.B. All authors have read and agreed to the published version of the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
This study was conducted according to guidelines of the Declaration of Helsinki and approved by the ethics committee of Sapienza University of Rome (protocol code 1184/17 and date of approval 21/12/2017).
Consent for publication
Informed consent was obtained from all subjects involved in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Maggisano, V., Capriglione, F., Verrienti, A. et al. Expression of miR-31-5p affects growth, migration and invasiveness of papillary thyroid cancer cells. Endocrine 79, 517–526 (2023). https://doi.org/10.1007/s12020-022-03267-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-022-03267-6