Abstarct
Aims
We aimed to assess the dose–response relationship between triglyceride–glucose (TyG) index and the incidence of type 2 diabetes mellitus (T2DM).
Methods
We performed a comprehensive systematic literature search using PubMed, Scopus, and Embase for records published from inception until 9 February 2021. The effect estimates were reported as relative risks (RRs).
Results
270,229 subjects from 14 studies were included in this systematic review and meta-analysis. The pooled incidence of T2DM was 9%. Meta-regression analysis indicates that baseline age (coefficient: 0.67, p = 0.026), drinking (coefficient: 0.03, p = 0.035), and HDL (coefficient: −0.89, p = 0.035) affected the incidence of T2DM in future. High TyG index was associated with increased incidence of T2DM in pooled unadjusted (RR 4.68 [3.01, 7.29], p < 0.001; I2: 96.6%) and adjusted model (adjusted RR 3.54 [2.75, 4.54], p < 0.001; I2: 83.7%). Dose–response meta-analysis for the adjusted RR showed that the linear association analysis was not significant per 0.1 increase in TyG index (RR 1.01 [0.99, 1.03], p = 0.223). There is a non-linear trend (p < 0.001) for the association between TyG index and incidence of T2DM. The dose–response curve became increasingly steeper at TyG index above 8.6.
Conclusions
TyG index was associated with the incidence of T2DM in a non-linear fashion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes mellitus (T2DM) is a major cause of cardiovascular disease and mortality, present in ~9.3% of the population [1]. Early identification and intervention of individuals at high risk for T2DM are important to reduce mortality and morbidity posed by diabetes-related complications. Patients with T2DM tend to have a higher weight, body mass index (BMI), waist circumference, systolic and diastolic blood pressure, fasting glucose, triglycerides, low-density lipoprotein (LDL), and high-density lipoprotein (HDL) cholesterol [2, 3]. However, which of these parameters are valuable for predicting future T2DM is uncertain.
Insulin resistance (IR) plays a pivotal role in the development of T2DM. Homeostasis model assessment estimated insulin resistance (HOMA-IR) has been shown to be an independent predictor for incident T2DM [4]. Euglycemic-hyperinsulinemic clamp (clamp-IR) remained the gold standard approach in identifying IR [5], however, its use is far too impractical. triglyceride–glucose (TyG) index, an accurate surrogate marker of IR [6], is a product of fasting glyceride and glucose, usually checked in apparently healthy individuals [7]. Thus, routine TyG index measurement is practical, feasible, and cost-effective. Recent studies have shown the potential use of TyG index to predict the development of T2DM [8]. However, the dose–response relationship between TyG index and T2DM remains unclear. In this systematic review and dose–response meta-analysis, we aimed to assess the dose–response relationship between TyG index and incidence of T2DM. The population is subjects from cohorts without T2DM who have TyG index measured at baseline and followed-up for the incidence of T2DM.
Materials and methods
This systematic review and meta-analysis follow the Meta-analysis of Observational Studies in Epidemiology and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines.
Eligibility criteria
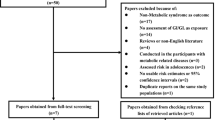
Studies that fulfilled all of these criteria were included: (1) prospective or retrospective cohorts reporting healthy subjects and (2) assessing the association between TyG index and the incidence of T2DM with at least three quantitative classifications. There were no language restrictions applied.
Studies that fulfilled one of the following criteria were excluded: (1) pre-prints, (2) abstract-only publication, (3) review articles, and (4) editorial/letters/commentaries.
Search strategy and study selection
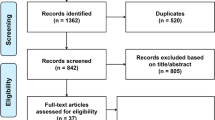
We performed a comprehensive systematic literature search using PubMed, Scopus, and Embase with keywords ((triglyceride–glucose index OR TyG) AND (diabetes)) for records published from the inception up until 9 February 2021. Hand searching from reference lists was also performed. Duplicates were removed from the initial record, and then two independent authors (qualification: medical doctors with extensive experience performing systematic reviews) screened the title/abstracts. The full-text of potential articles was then assessed using the inclusion and exclusion criteria. Authors were contacted for additional data whenever possible.
Data extraction
Two independent authors performed data extraction from the included studies for the first author, study design, year of publication, age, gender, hypertension, dyslipidemia, family history of T2DM, BMI, lipid profile, smoking, drinking, and the outcome of interest.
The exposure was TyG index was determined by the formula: ln (fasting TG [mg/dL] × fasting glucose [mg/dL]) [7].
The outcome of interest was the incidence of T2DM. T2DM was defined as fasting glucose >126 mg/dL or >HbA1c 6.5%. The effect estimates were reported as relative risks (RRs). RR was defined as either odds ratios (ORs), risk ratios, or hazard ratios (HRs).
Risk of bias assessment was performed independently by two authors using the Newcastle–Ottawa Scale (NOS). Arising discrepancies were resolved by discussion.
Statistical analysis
Comparison between the highest versus lowest TyG categories in terms of incidence of T2DM was performed using DerSimonian-Laird random-effects meta-analysis regardless of heterogeneity, and the effects estimate was reported as RR. P value was considered significant if below 0.05. Assessment of interstudy heterogeneity was based on the I2 statistics and Cochran Q test; in which, I2 of >50% or p value < 0.10 indicates significant heterogeneity. A two-stage random-effects dose–response meta-analysis was performed using the generalized least-squares regression trend estimation method based on logRRs across TyG index intervals in studies reporting at least three quantitative classifications. Potential for non-linear relationship based on RRs of each quantitative TyG index was evaluated using the restricted cubic splines with seven-knots model. A Wald-type test was performed to assess non-linearity by testing the regression coefficient of the second spline. Funnel-plot and Egger’s test were performed to assess the risk of publication bias and small-study effects. Non-parametric trim-and-fill analysis using Linear 0 estimator was performed. STATA 16.0 (StataCorp LLC, TX, USA) was used to perform meta-analysis. Restricted-maximum likelihood random-effects meta-regression was performed using the potential effect modifiers for (1) incidence of diabetes and (2) association between TyG index and incidence of T2DM. Subgroup analysis for prospective and retrospective studies was performed. Diagnostic test meta-analysis was performed for the TyG index cut-off value between 8.7 and 8.9 (rounding to one decimal place) to generate the sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and area under the curve (AUC).
Results
Baseline characteristics
270,229 subjects from 14 studies were included in this systematic review and meta-analysis [2, 3, 9,10,11,12,13,14,15,16,17,18,19,20] (Fig. 1). The baseline characteristics of the included studies and the risk of bias assessment can be found in Table 1 and Table 2. The pooled incidence of T2DM was 9% [7–10%]. Meta-regression analysis indicates that baseline age (coefficient: 0.67, p = 0.026), drinking (coefficient: 0.03, p = 0.035), HDL (coefficient: Ftable −0.89, p = 0.035), and length of follow-up (coefficient: 0.16, p = 0.010) affect the incidence of T2DM in future, but not male gender (p = 0.896), BMI (p = 0.058), smoking (p = 0.872), and LDL (p = 0.213). Family history of T2DM and hypertension were not included because less than ten studies were reporting these variables.
Triglyceride-glucose index and incidence of type 2 diabetes mellitus (unadjusted model)
High TyG index was associated with increased incidence of T2DM in pooled unadjusted model (RR 4.68 [3.01, 7.29], p < 0.001; I2: 96.6%, p < 0.001) (Fig. 2). Subgroup analyses provide detailed effect estimate for prospective cohorts (RR 3.62 [2.83, 4.62], p < 0.001; I2: 84.2%, p < 0.001) and retrospective (RR 7.49 [2.71, 20.66], p < 0.001; I2: 95.4%, p < 0.001).
Triglyceride-glucose index and incidence of type 2 diabetes mellitus (adjusted model)
In the pooled adjusted model, high TyG was also associated with increased incidence of T2DM (adjusted RR 3.54 [2.75, 4.54], p < 0.001; I2: 83.7%, p < 0.001) (Fig. 3). Subgroup analyses provide detailed effect estimate for prospective cohorts (adjusted RR 3.21 [2.53, 4.07], p < 0.001; I2: 73.8%, p < 0.001) and retrospective (adjusted RR 4.29 [2.83, 6.48], p < 0.001; I2: 70.8%, p = 0.008).
Dose–response meta-analysis
Dose–response meta-analysis for the adjusted RR showed that the linear association analysis was not significant per 0.1 increase in TyG index (adjusted RR 1.01 [0.99, 1.03], p = 0.223). There is a non-linear trend (p < 0.001) for the association between TyG index and incidence of T2DM (Fig. 4a). The dose–response curve became increasingly steeper at TyG index above 8.6. Using TyG 7.2 as reference value, the RR for TyG of 7.9, 8, 8.3, 8.6, 9, and 9.5 were (adjusted RR 1.05 [1.00, 1.10]), (adjusted RR 1.06 [1.00, 1.12]), (adjusted RR 1.10 [1.02, 1.18]), (adjusted RR 1.19 [1.08, 1.31]), (adjusted RR 1.51 [1.33, 1.71]), and (adjusted RR 2.62 [2.17, 3.17]).
Dose–response meta-analysis between triglyceride-glucose index and incidence of type 2 diabetes mellitus with restricted cubic splines. Adjusted relative risk [solid line] with 95% confidence interval (95% CI) [long dashed lines] for the association of the triglyceride-glucose index and type 2 diabetes mellitus. Full model (a) and prospective cohorts (b)
Dose–response meta-analysis for the adjusted RR of prospective cohorts showed that the linear association analysis was not significant per 0.1 increase in TyG index (adjusted RR 1.01 [0.99, 1.03], p = 0.327). The curve was non-linear (p < 0.001) (Fig. 4b).
Publication bias
The funnel plots of unadjusted (Fig. 5a) and adjusted (Fig. 5b) models were asymmetrical. The pooled unadjusted (p = 0.200) and adjusted models (p = 0.411) were not statistically significant for small-study effects. Non-parametric trim-and-fill analysis showed that by the imputation of 5 studies (Linear L0) in the left side of the plot, the RRs were 2.60 [2.01, 3.36] (Fig. 5c) and 2.93 [2.25, 3.83] (Fig. 5d), for the unadjusted and adjusted models, respectively.
Meta-regression analysis
Meta-regression analysis indicates that the association between TyG index and incidence of T2DM was affected by baseline age (coefficient: −0.05, p = 0.015), but not male gender (p = 0.183), BMI (p = 0.490), smoking (p = 0.572), drinking (p = 0.366), HDL (p = 0.112), LDL (p = 0.446), and length of follow-up (p = 0.288). The family history of T2DM and hypertension was not included because fewer than ten studies reported these variables.
Diagnostic test meta-analysis
Diagnostic test meta-analysis indicates that the TyG index with cut-off points between 8.7 and 8.9 has a 54% sensitivity, 71% specificity, PLR of 1.9, NLR of 0.64, and AUC of 0.66 [0.62–0.70] for incident T2DM (Fig. 6).
Discussion
This meta-analysis showed that a high TyG index was associated with the incidence of T2DM in both unadjusted and adjusted models. The dose–response relationship between TyG index and the incidence of T2DM was non-linear. A TyG index cut-off point between 8.7 to 8.9 has a 54% sensitivity and 71% specificity for predicting incident T2DM.
The pooled analysis of both unadjusted and adjusted effect estimates has high heterogeneity. We can observe that the inconsistency in the pooled effect estimate persists even after subgroup analyses. Meta-regression analysis was performed twice, first for exploring factors associated with the incidence of T2DM and second for the exploration of the heterogeneity of pooled unadjusted estimate. In the first meta-regression analysis, higher baseline age, drinking, and lengthier follow-up were associated with the incidence of T2DM while higher HDL was protective. A cohort of 5.1 million people in South Korea found that low HDL was an independent predictor of the incidence of T2DM in a stepwise manner [21]. This study reflects the findings of the Framingham study regarding the relationship between low HDL levels and incidence of T2DM [22]. Evidence suggests that glucose metabolism is directly and indirectly affected by HDL [21]. HDL reduces lipid accumulation in the pancreatic cells, attenuating inflammation and apoptosis associated with lipid deposition [23, 24]. Controversies surround the alcohol consumption and incidence of T2DM, Atherosclerosis Risk in Communities study showed that drinking was inversely associated with the incidence of T2DM [25]. Finnish Twin Cohort Study indicates that moderate drinking may reduce T2DM risk, while high alcohol consumption increases the incidence of T2DM [26]. A Korean cohort indicates a J-shaped association between alcohol drinking and incidence of T2DM.
Exploration of heterogeneity for the unadjusted effect estimate showed that higher baseline age attenuates the effect of TyG index on the incidence of T2DM. In the first analysis, we observe that age increases the risk of T2DM, one of the possible explanations is that as age increases, TyG index became less influential for determining the risk of T2DM. Thus, varying mean age among the included studies contributes to heterogeneity in the pooled effect estimate. However, meta-regression analysis for the unadjusted outcome has several caveats. First, the highest category versus the lowest categories has different TyG index, although the numbers are usually close to each other.
The RR was lower in the prospective subgroup than the retrospective subgroup; one of the main reasons is the higher risk of bias in retrospective cohorts. Baseline medical data in retrospective studies were collected through medical records or history taking, this may not represent the actual baseline characteristics. Meanwhile, in prospective studies, data were uniformly collected at the start of the study. In the pooled adjusted analysis, we observe significant small-study effects and asymmetry in the funnel plot, subsequent non-parametric trim-and-fill analysis indicates that the effect estimate was lower with the imputation of new studies on the left side of the plot. Thus, even in the presence of studies lower than the current pooled effect estimate, it is unlikely to change the direction of the overall effect.
In this pooled analysis, we observe a large magnitude of the effect estimate and the dose–response gradient, increasing the certainty of evidence. In individuals with IR, insulin cannot function properly to stimulate glucose uptake. This condition is also associated with obesity and metabolic syndrome, characterized by central, visceral, and ectopic fat accumulation [27]. The storage of TG in non-adipose tissues, such as skeletal muscle, liver, pancreas, and heart, causes dysfunction at the cellular level and disruption of organ function [28]. Excess lipids may accumulate intercellularly and interfere with organ function through the paracrine effects of the released adipokines, whereas intracellular lipid deposition is associated with reduced insulin sensitivity. Adipocyte dysfunction enhanced lipolysis, and free fatty acid (FFA) oxidation impairment results in a continuous oversupply of FFA and consequently lipid accumulation intracellularly [27, 29]. The FFA metabolites, including ceramides, diacylglycerol, and long-chain acyl-CoA, have deleterious effects on the cell and cause defects in insulin signaling [30].
Adipocytes and adipose tissue play a pivotal role in glucose and lipid metabolism. During IR, adipose, muscle, liver, and other tissues contribute to hyperglycemia and hyperlipidemia, which are toxic to the pancreatic β cells. High TG concentrations reduce glucokinase activity and glucose-stimulated insulin secretion in pancreatic islets, and high glucose levels cause islet cell destruction due to continuous oxidative stress [31]. Intramuscular TG deposition leads to translocation of glucose transporter type 4 (GLUT-4) to the cell membrane, which eventually reduces glucose uptake by skeletal muscle. Hepatic TG accumulation causes a decrease in insulin-stimulated hepatic glucose uptake and reduces insulin-suppressed hepatic glucose production, in the same way as it does in skeletal muscle [27, 32]. In the heart, elevated FFA supply and diminished oxidation result in lipid intermediates which disrupt insulin signaling, and therefore GLUT-4 involved glucose uptake. The ectopic fat significantly contributed to the development of IR and consequently T2DM and other metabolic conditions [27, 28].
Among the traditional risk factors, serum levels of fasting TG and glucose could justify the risk of developing IR and T2DM. These metabolic components are frequently found to be elevated and are associated with several chronic diseases. In this systematic review and meta-analysis, we found that the TyG index was significantly associated with an increased incidence of T2DM. TyG index is a low-cost, feasible, and applicable biomarker in daily practice. This parameter is much simpler and more affordable than other IR evaluation methods, including clamp-IR, HOMA-IR, quantitative insulin sensitivity check index, continuous infusion of glucose with model assessment fasting plasma insulin, glucose-to-insulin ratio, and oral glucose tolerance test [5, 33,34,35]. In multiple studies, including those with American, European, and Asian populations, a higher TyG index was significantly associated with IR, even though ectopic fat deposition is affected by ethnicity [2, 3, 31, 36,37,38,39].
Clinical Implications
Triglyceride and glucose are often measured in a medical check-up, whether in an apparently healthy population or patients with comorbidities. This means that IR can be screened without additional cost and applied in developed and developing countries. The association between the TyG index and T2DM was non-linear, indicating that in patients with high TyG index, an intensive lifestyle change becomes increasingly important. Although the effectiveness of lifestyle and pharmacological intervention in people with a high TyG index is yet to be defined, it is advisable to do so as diet control and exercise are encouraged in patients with prediabetes. Since T2DM may be asymptomatic, the TyG index may help anticipate incident T2DM by increasing the frequency of blood glucose check-ups, facilitating early detection and prevention of complications. The cut-off point between 8.7 and 8.9 might be useful in classifying people at risk of developing incident T2DM, however, the non-linearity of the association should also be considered. At the public health level, measuring TyG index does not add costs and may effectively curb the incidence of T2DM or its associated complications.
Limitations
The studies were mainly from Asia, there are no studies from North America, South America, Africa, and Australia. There was only one study from European continent. In addition, the samples from Chinese studies potentially overlap due to DATADRYAD database use. Several studies were retrospective and are prone to bias. Several studies inadequately reported several important variables such as family history of T2DM. Several included studies have a high percentage of missing data or dropouts, which may potentially cause bias. The studies have different follow-up lengths, which may contribute to inconsistency, although it is not demonstrated in the regression analysis. Finally, whether any of the subjects took lipid-lowering drugs or anti-diabetic medications was not sufficiently addressed.
Conclusion
TyG index was associated with the incidence of T2DM in a non-linear fashion. In addition, the association was significantly affected by age.
Data availability
Associated data are available upon reasonable request.
References
Internation Diabetes Federation (2019) International Diabetes Federation; Diabetes Atlas 9th edition (Internation Diabetes Federation, Brussels 2019)
P. Chamroonkiadtikun, T. Ananchaisarp, W. Wanichanon, The triglyceride-glucose index, a predictor of type 2 diabetes development: a retrospective cohort study. Prim. Care Diabetes 14, 161–167 (2020). https://doi.org/10.1016/j.pcd.2019.08.004
B. Park, H.S. Lee, Y.J. Lee, Triglyceride glucose (TyG) index as a predictor of incident type 2 diabetes among nonobese adults: a 12-year longitudinal study of the Korean Genome and Epidemiology Study cohort. Transl. Res. 228, 42–51 (2021). https://doi.org/10.1016/j.trsl.2020.08.003
E. Bonora, G. Formentini, F. Calcaterra et al. HOMA-estimated insulin resistance is an independent predictor of cardiovascular disease in type 2 diabetic subjects: prospective data from the Verona Diabetes Complications Study. Diabetes Care 25, 1135–1141 (2002). https://doi.org/10.2337/diacare.25.7.1135
C.S. Tam, W. Xie, W.D. Johnson et al. Defining insulin resistance from hyperinsulinemic-euglycemic clamps. Diabetes Care 35, 1605 LP–1601610 (2012). https://doi.org/10.2337/dc11-2339
A. Sánchez-García, R. Rodríguez-Gutiérrez, L. Mancillas-Adame et al. Diagnostic accuracy of the triglyceride and glucose index for insulin resistance: a systematic review. Int. J. Endocrinol. 2020, 1–7 (2020). https://doi.org/10.1155/2020/4678526
K. Park, C.W. Ahn, S.B. Lee et al. Elevated TyG index predicts progression of coronary artery calcification. Diabetes Care 42, 1569–1573 (2019). https://doi.org/10.2337/dc18-1920
A. da Silva, A.P.S. Caldas, D.M.U.P. Rocha, J. Bressan, Triglyceride-glucose index predicts independently type 2 diabetes mellitus risk: a systematic review and meta-analysis of cohort studies. Prim. Care Diabetes 14, 584–593 (2020). https://doi.org/10.1016/j.pcd.2020.09.001
C. Chen, L. Liu, K. Lo et al. Association between triglyceride glucose index and risk of new-onset diabetes among chinese adults: findings from the China Health and Retirement Longitudinal Study. Front. Cardiovasc. Med. 7, 1–8 (2020). https://doi.org/10.3389/fcvm.2020.610322
M. Janghorbani, S.Z. Almasi, M. Amini, The product of triglycerides and glucose in comparison with fasting plasma glucose did not improve diabetes prediction. Acta Diabetol. 52, 781–788 (2015). https://doi.org/10.1007/s00592-014-0709-5
D.Y. Lee, E.S. Lee, J.H. Kim et al. Predictive value of triglyceride glucose index for the risk of incident diabetes: A 4-year retrospective longitudinal study. PLoS ONE 11, 1–14 (2016). https://doi.org/10.1371/journal.pone.0163465
J.W. Lee, N.K. Lim, H.Y. Park, The product of fasting plasma glucose and triglycerides improves risk prediction of type 2 diabetes in middle-aged Koreans. BMC Endocr. Disord. 18, 1–10 (2018). https://doi.org/10.1186/s12902-018-0259-x
S.H. Lee, H.S. Kwon, Y.M. Park et al. Predicting the development of diabetes using the product of triglycerides and glucose: The chungju metabolic disease cohort (CMC) study. PLoS ONE 9 (2014). https://doi.org/10.1371/journal.pone.0090430
X. Li, G. Li, T. Cheng et al. Association between triglyceride-glucose index and risk of incident diabetes: a secondary analysis based on a Chinese cohort study: TyG index and incident diabetes. Lipids Health Dis. 19, 1–11 (2020). https://doi.org/10.1186/s12944-020-01403-7
E.Q. Liu, Y.P. Weng, A.M. Zhou, C.L. Zeng, Association between triglyceride-glucose index and type 2 diabetes mellitus in the Japanese population: a secondary analysis of a retrospective cohort study. Biomed. Res. Int. (2020). https://doi.org/10.1155/2020/2947067
S. Low, K.C.J. Khoo, B. Irwan et al. The role of triglyceride glucose index in development of Type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 143, 43–49 (2018). https://doi.org/10.1016/j.diabres.2018.06.006
D. Navarro-González, L. Sánchez-Íñigo, J. Pastrana-Delgado et al. Triglyceride-glucose index (TyG index) in comparison with fasting plasma glucose improved diabetes prediction in patients with normal fasting glucose: The Vascular-Metabolic CUN cohort. Prev. Med. 86, 99–105 (2016). https://doi.org/10.1016/j.ypmed.2016.01.022
B. Wang, M. Zhang, Y. Liu et al. Utility of three novel insulin resistance-related lipid indices for predicting type 2 diabetes mellitus among people with normal fasting glucose in rural China. J. Diabetes 10, 641–652 (2018). https://doi.org/10.1111/1753-0407.12642
Z. Wang, L. Zhao, S. He. Triglyceride–glucose index as predictor for future type 2 diabetes mellitus in a Chinese population in southwest China: a 15-year prospective study. Endocrine (2021). https://doi.org/10.1007/s12020-020-02589-7
M. Zhang, B. Wang, Y. Liu et al. Cumulative increased risk of incident type 2 diabetes mellitus with increasing triglyceride glucose index in normal-weight people: The Rural Chinese Cohort Study. Cardiovasc. Diabetol. 16, 1–11 (2017). https://doi.org/10.1186/s12933-017-0514-x
S.-H. Lee, H.-S. Kim, Y.-M. Park et al. HDL-cholesterol, its variability, and the risk of diabetes: a nationwide population-based study. J. Clin. Endocrinol. Metab. 104, 5633–5641 (2019). https://doi.org/10.1210/jc.2019-01080
P.W.F. Wilson, J.B. Meigs, L. Sullivan et al. Prediction of incident diabetes mellitus in middle-aged adults: The framingham offspring study. Arch. Intern. Med. 167, 1068–1074 (2007). https://doi.org/10.1001/archinte.167.10.1068
A.L. Siebel, S.E. Heywood, B.A. Kingwell, HDL and glucose metabolism: Current evidence and therapeutic potential. Front. Pharmacol. 6 (2015). https://doi.org/10.3389/fphar.2015.00258
B.G. Drew, K.A. Rye, S.J. Duffy et al. The emerging role of HDL in glucose metabolism. Nat. Rev. Endocrinol. 8, 237–245 (2012). https://doi.org/10.1038/nrendo.2011.235
X. He, C.M. Rebholz, N. Daya et al. Alcohol consumption and incident diabetes: the Atherosclerosis Risk in Communities (ARIC) study. Diabetologia 62, 770–778 (2019). https://doi.org/10.1007/s00125-019-4833-1
S. Carlsson, N. Hammar, V. Grill, J. Kaprio, Alcohol consumption and the incidence of type 2 diabetes: a 20-year follow-up of the Finnish Twin Cohort Study. Diabetes Care 26, 2785–2790 (2003). https://doi.org/10.2337/diacare.26.10.2785
M. Snel, J.T. Jonker, J. Schoones et al. Ectopic fat and insulin resistance: pathophysiology and effect of diet and lifestyle interventions. Int J. Endocrinol. 2012, 983814 (2012). https://doi.org/10.1155/2012/983814
D. Ferrara, F. Montecucco, F. Dallegri, F. Carbone, Impact of different ectopic fat depots on cardiovascular and metabolic diseases. J. Cell Physiol. 234, 21630–21641 (2019). https://doi.org/10.1002/jcp.28821
V.T. Samuel, G.I. Shulman, The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J. Clin. Investig. 126, 12–22 (2016). https://doi.org/10.1172/JCI77812
K. Morino, K.F. Petersen, G.I. Shulman, Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes 55(Suppl 2), S9–S15 (2006). https://doi.org/10.2337/db06-S002
E.-Q. Liu, Y.-P. Weng, A.-M. Zhou, C.-L. Zeng, Association between triglyceride-glucose index and type 2 diabetes mellitus in the Japanese population: a secondary analysis of a retrospective cohort study. Biomed. Res. Int. 2020, 2947067 (2020). https://doi.org/10.1155/2020/2947067
B. Gustafson, S. Hedjazifar, S. Gogg et al. Insulin resistance and impaired adipogenesis. Trends Endocrinol. Metab. 26, 193–200 (2015). https://doi.org/10.1016/j.tem.2015.01.006
A. Katsuki, Y. Sumida, E.C. Gabazza et al. Homeostasis model assessment is a reliable indicator of insulin resistance during follow-up of patients with type 2 diabetes. Diabetes Care 24, 362 LP–362365 (2001). https://doi.org/10.2337/diacare.24.2.362
Y. Wang, P. Fedorcsák, P.O. Dale et al. Simplification of continuous infusion of glucose with model assessment in the evaluation of insulin resistance in women with PCOS. Gynecol. Endocrinol. 15, 192–197 (2001). https://doi.org/10.1080/gye.15.3.192.197
L.U. Monzillo, O. Hamdy, Evaluation of insulin sensitivity in clinical practice and in research settings. Nutr. Rev. 61, 397–412 (2003). https://doi.org/10.1301/nr.2003.dec.397-412
J.C. Locateli, W.A. Lopes, C.F. Simões et al. Triglyceride/glucose index is a reliable alternative marker for insulin resistance in South American overweight and obese children and adolescents. J. Pediatr. Endocrinol. Metab. 32, 1163–1170 (2019). https://doi.org/10.1515/jpem-2019-0037
E. Dikaiakou, E.A. Vlachopapadopoulou, S.A. Paschou et al. Τriglycerides-glucose (TyG) index is a sensitive marker of insulin resistance in Greek children and adolescents. Endocrine 70, 58–64 (2020). https://doi.org/10.1007/s12020-020-02374-6
H. Van Minh, H.A. Tien, C.T. Sinh et al. Assessment of preferred methods to measure insulin resistance in Asian patients with hypertension. J. Clin. Hypertens. (2021). https://doi.org/10.1111/jch.14155
V. Calcaterra, C. Montalbano, A. de Silvestri et al. Triglyceride Glucose Index as a Surrogate Measure of Insulin Sensitivity in a Caucasian Pediatric Population. J. Clin. Res. Pediatr. Endocrinol. (2019). https://doi.org/10.4274/jcrpe.galenos.2019.2019.0024
Author contributions
R.P.: conceptualization, methodology, data curation, formal analysis, investigation, validation, writing—original draft, writing—review & editing. I.H.: data curation, investigation, writing - original draft, writing—review & editing. Irvan: data curation, investigation, writing—original draft. M.A.L.: data curation, investigation, writing—original draft. R.V.: investigation, validation, writing—review and editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pranata, R., Huang, I., Irvan et al. The association between triglyceride-glucose index and the incidence of type 2 diabetes mellitus—a systematic review and dose–response meta-analysis of cohort studies. Endocrine 74, 254–262 (2021). https://doi.org/10.1007/s12020-021-02780-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-021-02780-4