Abstract
Purpose
Intraoperative imaging of parathyroid glands (PGs) has been developed in order to reduce the risk of unintentional parathyroidectomy during total thyroidectomy. This novel modality is based on their intrinsic characteristic of autofluorescence (AF) after near-infrared light exposure. The aim of this study was to assess the effect of this method on the risk of unintentional PG excision (total or partial) during total thyroidectomy.
Methods
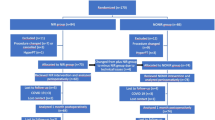
This was a single-blind, randomized-controlled trial including adult patients who underwent scheduled total thyroidectomy between December 2019 and March 2020. These patients were randomly allocated to two groups: one in which near-infrared autofluorescence imaging (NIRAF) was applied (NIR group) and one without NIRAF (NONIR group). Hormonal and biochemical assessment was performed pre- and 24-h postoperatively. AF findings and the number of PGs autotransplanted were recorded.
Results
One-hundred and eighty patients were eligible. Unintentional (total or partial) PG excision rates during total thyroidectomy in the NONIR (n = 90) and NIR (n = 90) groups were 28.9% [95% confidence interval (CI) 19.8–39.4%] and 14.4% (95% CI 7.7–22.1%), respectively (p = 0.02). Furthermore, NIR reduced the risk of parathyroid tissue presence in the specimen sent for pathology (relative risk 0.51, 95% CI 0.28–0.92; p = 0.02). However, the number of PGs identified by NIR could not predict the risk of postoperative hypoparthyroidism.
Conclusions
NIRAF imaging during total thyroidectomy led to a significant reduction in PG excision rates. However, this modality did not result in the reduction of postoperative hypoparathyroidism or hypocalcemia risk.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thyroidectomy is a widely performed operation characterized by low morbidity and mortality [1]. The modern thyroid surgeon aims at providing surgery with the lowest complication rate [1]. Refinement of surgical techniques has decreased the rate of both hypoparathyroidism and vocal cord palsy [2]. However, even when performed by the most experienced surgeons, parathyroid secretion could be impaired [3]. The estimated rates of temporary and permanent hypoparathyroidism following total thyroid surgery are 19–38% and 0–3%, respectively [4]. Postoperative hypoparathyroidism can be caused by various mechanisms including partial or total, excision of the parathyroid glands (PGs). The recovery of PGs, in cases of injury or devascularization, is indicated by the regain of their function and, in the case of inadvertent excision, by that ability of the remaining tissue to compensate the functional postoperative deficit [5]. The incidence of inadvertent parathyroidectomy during thyroidectomy ranges from 8 to 50% and may result in postoperative hypocalcemia [6, 7].
Conventional measures to reduce the risk of postoperative hypoparathyroidism were mainly based on surgeon-dependent identification and preservation of the PGs and their vascular pedicles [8]. Limitations of naked eye inspection and subjectivity of palpation are imposing challenges for all surgeons. Real-time intraoperative identification and functional maintenance of structures are largely dependent on the surgeon’s experience. Several methods are available to detect PGs during surgery, such as the intravenous administration of methylene blue, frozen section analysis, intact parathyroid hormone (PTH) monitoring, oral 5-aminolaevulinic acid administration and indocyanine green (ICG) fluorescence imaging have been reported [9,10,11,12,13]. In 2011, Paras et al. first reported that the PGs emit autofluorescence (AF) when exposed to near-infrared light (NIR) [14]. The technique, based on the use of AF to detect the PGs intraoperatively, was attractive as it provided an opportunity to lower the incidence of postoperative hypoparathyroidism. In addition, this technique needs no drugs, thus avoiding adverse effects [14].
The primary aim of the present study was to evaluate the effect of intraoperative AF imaging (AFI) in reducing the unintentional PG excision (total or partial) rates during total thyroidectomy (TT). Secondary endpoints were: (i) to estimate the association between the number of PGs identified with AF and the risk of 24-h postoperative hypoparathyroidism and hypocalcemia; (ii) to identify the cutoff points that could predict low PTH levels (i.e., <20 pg/ml) postoperatively.
Material and methods
Study design
This was a single-blind randomized-controlled trial including adult patients who underwent a scheduled TT at the AHEPA University Hospital of Thessaloniki and the Interbalkan Medical Center of Thessaloniki during a 4-month period (between December 2019 and March 2020).
After acquiring approval from the scientific board of AHEPA University Hospital of Thessaloniki, the patients who signed the information and consent form (ICF) after thorough explanation of the purposes of the study were randomly allocated to two groups by computer software (https://www.randomizer.org/): (i) patients operated without near-infrared imaging (NONIR group) and (ii) patients operated with near-infrared imaging (NIR group).
The study was performed in accordance with relevant guidelines and regulations. The trial was registered in clinicaltrials.gov with registration number NCT04204317.
All patients underwent preoperative direct laryngoscopy to assess vocal cord motility. All procedures were performed by a surgical team dedicated to thyroid surgery. Anesthesia was standardized following the protocol proposed by Andrieu et al. [15]. Data for all patients (results of AF and number of PGs that were autotransplanted) were collected prospectively and recorded in a database. Parathyroid fragment in the specimen was considered as any parathyroid tissue identified in it (ranging from the whole gland to a small part of it).
Preoperative blood samples were collected and biochemical analysis was performed. Twenty-four hours postoperatively, new blood samples were drawn and biochemical analysis was performed. In that way, baseline albumin, 25-hydroxy-vitamin D [25(OH)D], pre- and postoperative serum calcium, phosphorus and PTH concentrations were determined. All patients with a postoperative calcium level <8.0 mg/dL (normal range 8.2–10.6 mg/dL) were considered as having hypocalcemia. All patients with PTH < 20 pg/ml (normal range 14–72 pg/ml) were considered as having hypoparathyroidism. All patients with hypoparathyroidism received oral calcium carbonate and vitamin D analogs. The length of hospital stay was the same for the two groups (1 day).
Inclusion and exclusion criteria
Inclusion criteria were: (i) age > 18 years, (ii) written ICF for TT and (iii) ICF for the patient to be included into the prospectively formed databank. Exclusion criteria were: (i) participation in another clinical trial which could affect this study’s outcomes, (ii) prior neck operation, (iii) primary hyperparathyroidism, (iv) vitamin D deficiency at the time of operation, (v) use of drugs that influence calcium metabolism (i.e., vitamin D analogs, oral calcium supplements, bisphosphonates, teriparatide, thiazide diuretics and aromatase inhibitors), (vi) previous radiation of the neck.
Surgical technique and AF monitoring
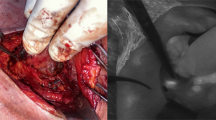
For the purposes of the study, TT was performed with the use of energy devices (Focus Harmonic Scalpel®, LigasureTM Small Jaw, Thunderbeat®, or LigasureTM Exact). The comparison between them has been demonstrated to give an equivalent impact concerning postoperative PG dysfunction [16]. In order to minimize the impact that energy devices have on PG function, the dissection of PGs (when needed) was performed by the use of clamp-and-ties [3]. PGs were not actively searched during thyroidectomy. The operating team had an image of the operative field once the upper pole was dissected, then one when the lower pole was dissected and, finally, one when the thyroid gland was laterally viewed and suspended from the ligament of Berry. The same procedure was performed contralaterally. Finally, two images of the dissected specimen were taken one anteriorly and one posteriorly. NIR was performed with a Fluobeam® LX (Fluoptics, Grenoble, France) that emits NIR light with a class 1 laser system which provides an irradiance of 5 mW/cm2 at 750 nm, in order to visualize the fluorescence intensity of different tissues. The intraoperative use during imaging was performed with the surgical lights OFF, but the room lights ON. The technique uses the intrinsic AF properties of the PGs. Infrared light is absorbed by PGs, which emits fluorescent light at a different wavelength. A special filter captures this light, allowing visualization of PGs in real time.
Statistical analysis
All data were presented as mean values ± SD (standard deviation) or SEM (standard error of the mean) as implied. Student’s t-test or ANOVA for repeated measures was used as applicable. For correlations between measured values, Pearson or Spearman’s analyses were performed as appropriate. For all values, p < 0.05 was considered as significant. The statistical software packages SPSS 20.0 for OsX (SPSS Inc., Chicago, IL) and GraphPad Prism 4 (GraphPad Software, Inc., La Jolla, CA) were used.
Results
Two hundred and thirty-six consecutive patients scheduled for TT were assessed for eligibility. According to the inclusion criteria, 180 patients were eligible to participate. A flow diagram of the study population is presented in Fig. 1. The epidemiologic characteristics of all participants, as well as the various parameters measured (pre- and postoperative PTH, calcium, and phosphorus concentrations), together with the number of PGs identified are presented in Table 1.
The unintentional (total or partial) excision rate of PGs during TT was higher in the NONIR (n = 26; total excision in 20) compared with the NIR (n = 14; total excision in 8) group [28.9% (95% CI 19.8–39.4%), 14.4% (95% CI 7.7–22.1%; p = 0.02)]. The risk of parathyroid tissue identification in the specimen sent for pathology was significantly reduced with the NIR method (relative risk 0.51; 95% CI 0.28–0.92, p = 0.02)). The existence of parathyroid tissue in the specimen independently predicted postoperative PTH levels, which were 8.9 pg/ml (95% CI 1.6–16.1) lower than those detected in patients with absence of parathyroid fragments in the specimen (p = 0.017) (Fig. 2).
With regard to the secondary endpoint, there was no difference in the percentage of patients with temporary hypoparathyroidism (at 24 h) between NIR and NONIR groups (27.7% vs. 25.9%, respectively; p = 0.79). This was also the case for hypocalcemia incidence (3.2% and 5.6%, respectively; p = 0.43). As expected, there was no crude association either between postoperative calcium decrease (ΔCa = preoperative calcium − postoperative calcium) (p = 0.15) or postoperative phosphorus increase (ΔP = preoperative phosphorus − postoperative phosphorus) and the existence of fragments in the specimen (p = 0.19).
Moreover, there was no association between postoperative hypocalcemia and the number of PGs spontaneously identified (p = 0.97). Similarly, this was also the case for postoperative hypoparathyroidism (p = 0.61). However, when <2 PGs were spontaneously identified by Fluobeam, no cases of postoperative hypocalcemia (p = 0.01) or hypoparathyroidism were observed (p < 0.001). This cut-off point was positively associated with the postoperative decrease in serum calcium and PTH levels, as well with the increase in serum phosphorus concentrations (p = 0.046, p = 0.046, and p = 0.049 respectively).
Discussion
This study showed that NIRAF imaging during TT may lead to a significant reduction in PG excision rates. However, this modality does not seem to be efficacious in reducing the risk of temporal hypoparathyroidism and/or hypocalcemia.
It is well-known that TT may impair parathyroid function [3, 16], leading to hypoparathyroidism (either classical or partial) that can be detected clinically and/or biochemically with classic (static) or dynamic parathyroid testing [3, 5]. During the last decade, various efforts have been made in order to minimize the impact of thyroidectomy on the PGs, by using either colorant or fluorophores. In this way, the intraoperative PG identification is easier and their damage may be limited.
In the present study, the surgical technique was not driven by the need to identify the PGs, but to perform the same TT with fewer complications. In most of the studies, that try to evaluate the value of the AF properties of the parathyroids in vivo, surgeons actively searched for all four (if possible) PGs [17,18,19,20,21,22]. In this study, PGs were not searched actively, but only in view of the thyroid gland and its bed. Moreover, a final view of the specimen was done at the end of the operation. When the PGs were detected on the specimen, they were re-implantated (in one case). We decided to use the fluoroscope in this way, because it did not significantly alter the way we operate on thyroid on an everyday basis. Actually, this use of the fluoroscope was more convenient for the surgeons. Moreover, these results were comparable with those obtained with the active detection of PGs with ICG, in accordance with previous studies (i.e., 77–100% of PGs are detectable with AF and 68% even before dissection) [17, 22,23,24,25,26].
As far as unintentional PG excision (total or partial) is concerned, AF identification of the PGs led to a significant reduction of parathyroid fragments in the surgical specimen. This finding is in complete agreement with those reported in other studies, despite the fact that we did not actively searched for PGs. Furthermore, the inadvertent resection of PGs without NIR in the present study was within the acceptable rate of 1.1–30%, depending on the definition used, whereas a significant decrease of inadvertent parathyroidectomy was observed by the use of NIR as for the others [7, 17, 19]. Only the study by DiMarco et al. stated that the percentage of inadvertent parathyroidectomy remains unchanged with NIR imaging, but we strongly believe that this is not the case [27]. Moreover, we strongly believe that despite the decrease in inadvertent parathyroidectomy rates with the use of NIR, this is not zeroed. This is probably due to two effects described by Idogawa et al., notably the “white out effect” and the “black out effect” [28]. In the first case, the background of the thyroid may present strong fluorescence (e.g., in cases of cancers or toxic adenomas) and therefore the PG is not detected. In the second case, PG itself shows low fluorescent levels and cannot be pointed out.
One would expect that a significant decrease in inadvertent PG excision rates with NIR would result in a consequent decrease in both postoperative hypoparathyroidism and hypocalcemia risk. However, these parameters seem to be unaffected by AFI. With regard to this point, conflicting data exist in the literature, indicating either a decrease or no impact on postthyroidectomy hypocalcemia rates after introduction of AFI [8, 17, 27, 29]. The fact that there is only a handful of studies examining this phenomenon requires more research in order to reach a safe conclusion.
Postoperative hypoparathyroidism and hypocalcemia are caused by (total or partial) unintentional excision of the PGs, but can also be due to injury, stretching or compression, devascularization, and/or ischemia [5]. It is therefore understandable that when AFI is used in a standard mode (not searching PGs all the time), most of the factors leading to temporary hypoparathyroidism/hypocalcemia are still existing. In this direction, two issues should be considered: (i) the continuous use of AFI (adapted on glasses for example) is probably going to improve postoperative parathyroid function, due to the fact that PGs could be handled more gently (structural maintenance) and with better vascular identification (functional maintenance) and (ii) the key point when treating patients is not to induce permanent harm. In this context, despite the fact that temporary postoperative hypoparathyroidism/hypocalcemia rates remain unchanged with or without AFI, it is our belief that AFI is going to drastically decrease the rates of permanent hypoparathyroidism.
The risk of PG damage is increased when their anatomic relation with the thyroid capsule is tight and when PGs tend to get up with the thyroid when it is medially retracted during TT. These may be the reasons for the good correlation between hypoparathyroidism/hypocalcemia and the number of PGs observed. When <2 PGs are detected during a routine operation (either visually or with AFI), then the probability of having an intrathyroidal parathyroid is far less than the probability of a PG away from the thyroid gland (this is an assumption based on the probability of having an intrathyroidal adenoma, which is actually only 1–3%) [30].
The present study has certain limitations. First, whether PGs emitted fluorescence signals or not, this was decided on the basis of the surgeon’s visual assessment. Thus, it was not a quantified evaluation. This may lead to two mistakes: (i) one concerning the intensity of the signal received by the stimulated tissue and (ii) one concerning the contrast between the stimulated tissue and the adjacent structures. This could explain the fact that despite the use of AFI, there are still fragments in the specimens. Second, like with every technology, the way it is used influences its results. In the case of AFI, we suppose that it is different to use it by having as target to spot all PGs, rather than excising the thyroid.
Conclusions
This study demonstrated that AFI may reduce the rate of unintentional PG excision (either total or partial) during a TT. However, this event does not correlate with a reduction in temporal hypoparathyroidism and/or hypocalcemia rates. Nonetheless, we noted than when <2 PGs are spotted in the surgical field with AFI, these risks may be decreased. In any case, more studies are needed in order to determine (i) the best way to intraoperatively use AFI so that we can take advantage of an early detection of the PGs and (ii) the effect of AFI on permanent hypoparathyroidism risk.
Change history
24 May 2021
A Correction to this paper has been published: https://doi.org/10.1007/s12020-021-02765-3
References
T.S. Papavramidis, K. Sapalidis, N. Michalopoulos, K. Triantafillopoulou, G. Gkoutzamanis, I. Kesisoglou, S.T. Papavramidis, UltraCision harmonic scalpel versus clamp-and-tie total thyroidectomy: a clinical trial. Head Neck 32, 723–727 (2010)
P. Anagnostis, I. Pliakos, S. Panidis, A. Chorti, V. Stelmach, A. Michalopoulos, T.S. Papavramidis, Should total thyroidectomies be performed by high-volume endocrine surgeons? A cost-effectiveness analysis. Endocrine 67, 131–135 (2020)
T.S. Papavramidis, O. Anastasiou, I. Pliakos, G. Kotsovolis, S. Panidis, A. Michalopoulos, Parathyroid function after total thyroidectomy: a randomized clinical trial concerning the influence of the surgical technique. Endocr. Pract. 24, 150–155 (2018)
O. Edafe, R. Antakia, N. Laskar, L. Uttley, S.P. Balasubramanian, Systematic review and meta-analysis of predictors of post-thyroidectomy hypocalcaemia. Br. J. Surg. 101, 307–320 (2014)
O.E. Anastasiou, M.P. Yavropoulou, T.S. Papavramidis, C. Tzouvara, K. Triantafyllopoulou, S. Papavramidis, J.G. Yovos, Secretory capacity of the parathyroid glands after total thyroidectomy in normocalcemic subjects. J. Clin. Endocrinol. Metab. 97, 2341–2346 (2012)
S. Gourgiotis, P. Moustafellos, N. Dimopoulos, G. Papaxoinis, S. Baratsis, E. Hadjiyannakis, Inadvertent parathyroidectomy during thyroid surgery: the incidence of a complication of thyroidectomy. Langenbecks Arch. Surg. 391, 557–560 (2006)
A. Sitges-Serra, S. Ruiz, M. Girvent, H. Manjon, J.P. Duenas, J.J. Sancho, Outcome of protracted hypoparathyroidism after total thyroidectomy. Br. J. Surg. 97, 1687–1695 (2010)
F. Benmiloud, G. Godiris-Petit, R. Gras, J.C. Gillot, N. Turrin, G. Penaranda, S. Noullet, N. Chereau, J. Gaudart, L. Chiche, S. Rebaudet, Association of autofluorescence-based detection of the parathyroid glands during total thyroidectomy with postoperative hypocalcemia risk: results of the parafluo multicenter randomized clinical trial. JAMA Surg. 155, 106–112 (2020)
N.E. Dudley, Methylene blue for rapid identification of the parathyroids. Br. Med. J. 3, 680–681 (1971)
W.S. Duke, W.I. Omesiete, N.J. Walsh, D.J. Terris, Baseline intraoperative intact parathyroid hormone levels in parathyroid surgery. Head Neck 4, 592–597 (2019)
N.D. Perrier, P. Ituarte, S. Kikuchi, A.E. Siperstein, Q.Y. Duh, O.H. Clark, R. Gielow, T. Hamill, Intraoperative parathyroid aspiration and parathyroid hormone assay as an alternative to frozen section for tissue identification. World J. Surg. 24, 1319–1322 (2000)
W. Stummer, S. Stocker, S. Wagner, H. Stepp, C. Fritsch, C. Goetz, A.E. Goetz, R. Kiefmann, H.J. Reulen, Intraoperative detection of malignant gliomas by 5-aminolevulinic acid-induced porphyrin fluorescence. Neurosurgery 42(3), 518–525 (1998)
J. van den Bos, L. van Kooten, S.M.E. Engelen, T. Lubbers, L.P.S. Stassen, N.D. Bouvy, Feasibility of indocyanine green fluorescence imaging for intraoperative identification of parathyroid glands during thyroid surgery. Head Neck 41, 340–348 (2019)
C. Paras, M. Keller, L. White, J. Phay, A. Mahadevan-Jansen, Near-infrared autofluorescence for the detection of parathyroid glands. J. Biomed. Opt. 16, 067012 (2011)
G. Andrieu, H. Amrouni, E. Robin, B. Carnaille, J.M. Wattier, F. Pattou, B. Vallet, G. Lebuffe, Analgesic efficacy of bilateral superficial cervical plexus block administered before thyroid surgery under general anaesthesia. Br. J. Anaesth. 99, 561–566 (2007)
T.S. Papavramidis, I. Pliakos, A. Chorti, S. Panidis, G. Kotsovolis, V. Stelmach, D. Koutsoumparis, S. Bakkar, A. Michalopoulos, Comparing LigasureTM Exact dissector with other energy devices in total thyroidectomy: a pilot study. Gland Surg. 9, 271–277 (2020)
F. Benmiloud, S. Rebaudet, A. Varoquaux, G. Penaranda, M. Bannier, A. Denizot, Impact of autofluorescence-based identification of parathyroids during total thyroidectomy on postoperative hypocalcemia: a before and after controlled study. Surgery 163, 23–30 (2018)
H. Idogawa, T. Sakashita, T. Yagi, K. Segawa, A. Homma, Pathological evaluation of the accuracy of a fluorescence spectroscopy system for detecting parathyroid glands. Eur. Arch. Otorhinolaryngol. 277, 3145–3147 (2020).
B. Kahramangil, F. Dip, F. Benmiloud, J. Falco, M. de La Fuente, S. Verna, R. Rosenthal, E. Berber, Detection of parathyroid autofluorescence using near-infrared imaging: a multicenter analysis of concordance between different surgeons. Ann. Surg. Oncol. 25(4), 957–962 (2018)
E. Kose, B. Kahramangil, H. Aydin, M. Donmez, E. Berber, Heterogeneous and low-intensity parathyroid autofluorescence: patterns suggesting hyperfunction at parathyroid exploration. Surgery 165, 431–437 (2019)
M.A. McWade, C. Paras, L.M. White, J.E. Phay, A. Mahadevan-Jansen, J.T. Broome, A novel optical approach to intraoperative detection of parathyroid glands. Surgery 154, 1371–1377 (2013)
M.A. McWade, M.E. Sanders, J.T. Broome, C.C. Solorzano, A. Mahadevan-Jansen, Establishing the clinical utility of autofluorescence spectroscopy for parathyroid detection. Surgery 159, 193–202 (2016)
F. De Leeuw, I. Breuskin, M. Abbaci, O. Casiraghi, H. Mirghani, A. Ben Lakhdar, C. Laplace-Builhe, D. Hartl, Intraoperative near-infrared imaging for parathyroid gland identification by auto-fluorescence: a feasibility study. World J. Surg. 40, 2131–2138 (2016)
J. Falco, F. Dip, P. Quadri, M. de la Fuente, R. Rosenthal, Cutting edge in thyroid surgery: autofluorescence of parathyroid glands. J. Am. Coll. Surg. 223, 374–380 (2016)
R. Ladurner, S. Sommerey, N.A. Arabi, K.K.J. Hallfeldt, H. Stepp, J.K.S. Gallwas, Intraoperative near-infrared autofluorescence imaging of parathyroid glands. Surg. Endosc. 31, 3140–3145 (2017)
T.S. Papavramidis, P. Anagnostis, A. Chorti, I. Pliakos, S. Panidis, D. Koutsoumparis, A. Michalopoulos, Do near-infrared intra-operative findings by the use of indocyanine green correlate with post-thyroidectomy parathyroid function? - the ICGPREDICT study. Endocr. Pract. https://doi.org/10.4158/EP-2020-0119 (2020). Online ahead of print
A. DiMarco, R. Chotalia, R. Bloxham, C. McIntyre, N. Tolley, F.F. Palazzo, Does fluoroscopy prevent inadvertent parathyroidectomy in thyroid surgery? Ann. R. Coll. Surg. Engl. 101, 508–513 (2019)
H. Idogawa, T. Sakashita, A. Homma, A novel study for fluorescence patterns of the parathyroid glands during surgery using a fluorescence spectroscopy system. Eur. Arch. Otorhinolaryngol. 277, 1525–1529 (2020)
F. Dip, J. Falco, S. Verna, M. Prunello, M. Loccisano, P. Quadri, K. White, R. Rosenthal, Randomized controlled trial comparing white light with near-infrared autofluorescence for parathyroid gland identification during total thyroidectomy. J. Am. Coll. Surg. 228, 744–751 (2019)
T.S. Papavramidis, M. Polyzonis, I. Pliakos, N. Michalopoulos, G. Goutzamanis, G. Karayannopoulou, A. Ziakas, S. Papavramidis, Intrathyroid parathyroid adenoma: should preoperative imaging tests help guiding scheduled operation. Report of two cases. Ann. Thyroid Res. 1, 23–26 (2014)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Papavramidis, T.S., Chorti, A., Tzikos, G. et al. The effect of intraoperative autofluorescence monitoring on unintentional parathyroid gland excision rates and postoperative PTH concentrations—a single-blind randomized-controlled trial. Endocrine 72, 546–552 (2021). https://doi.org/10.1007/s12020-020-02599-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-020-02599-5