Abstract
Purpose
Near-infrared autofluorescence is a new technology in thyroid surgery to better localize and preserve parathyroid glands. The purpose of this study is to assess if the adoption of NIR-AF can improve in short-, medium-, and long-term post-operative calcium and PTH levels compared to conventional “naked eye” surgery in patients undergoing TT for benign or malignant conditions.
Methods
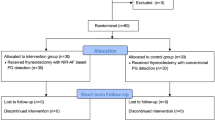
134 patients undergone total thyroidectomy between January 2020 and June 2022; 67 were treated with conventional thyroidectomy, the other 67 underwent surgery adopting an autofluorescence detecting device.
Results
Significant differences were found between the two groups in percentage of patients with short-term hypocalcemia (p = 0.04) and short-term hypoparathyroidism (p = 0.011). Median short-term (p = 0.01) and medium-term (p = 0.03) PTH levels were significantly higher in autofluorescence group, while, short- (p = 0.001), medium- (p < 0.001) and long-term (p = 0.019) percentage variation of PTH levels from baseline were significantly higher in the standard-care group. Finally, the prescription of oral calcium (p < 0.01) after surgery were significantly lower in the autofluorescence group.
Conclusion
The adoption of near-infrared autofluorescence during total thyroidectomy is related to lower short-term hypocalcemia and hypoparathyroidism rates, decreased variation of post-operative PTH levels in short- and medium- and long-term, reducing the necessity of supplementation therapy with oral calcium compared to conventional surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The preservation of parathyroid glands (PGs) in thyroid surgery is of paramount importance to reduce the risk of post-operative hypocalcemia secondary to hypoparathyroidism, the most frequent complication after total thyroidectomy (TT), which occurs in 15–30% of patients and remains permanent in 1–7% of cases [1, 2]. The intraoperative localization of PGs is not always easy due to their similarity to the surrounding adipose tissue, variable position, and small size [3]. Conventional means to reduce the risk of post-operative hypocalcemia are based on the surgeons’ experience to visualize PGs during surgical dissection, but this approach may be subjective and is not always accurate, since it can result in an accidental excision or devascularization of one or more glands [4]. In fact, it has been estimated that the incidence of inadvertent parathyroidectomy in thyroid surgery ranges from 8 to 50% [5], while the autotransplantation rate of at least one PG occurs in 25–50% of patients [6].
Limitations of naked-eye inspection imposed the development of different strategies to aid the intraoperative identification of PGs, such as frozen sections, float or sink, and tissue aspirate parathyroid hormone (PTH) analysis. Nevertheless, these techniques are limited in their applicability due to some disadvantages, such as lack of direct evidence, low sensitivity, and invasiveness [7,8,9].
Near-infrared autofluorescence (NIR-AF) is a new technology available in thyroid surgery for the localization and preservation of PGs [10]. Autofluorescence is a natural phenomenon based on the intrinsic property of the parathyroid tissue to emit a fluorescent signal with a wavelength of 820–830 nm when exposed to a source light of 785 nm [11]. Since the discovery of NIR-AF at Vanderbilt University, different autofluorescence detecting devices were developed and commercialized, such as the Fluobeam (Fluoptics, France), PDE Neo II (Hamamatsu, Japan) and EleVision (Medtronic, Minnesota). At any point during surgery, these devices can provide real-time images of the surgical field on a high-definition screen, highlighting the position of PGs and allowing their early identification and preservation (Fig. 1). Furthermore, NIR-AF is safe, not invasive, and needs no drugs, thus avoiding adverse effects [12].
The purpose of this case–control clinical study was to assess if the application of NIR-AF can lead to an improvement in short-, medium-, and long-term post-operative calcium and PTH levels compared to conventional “naked-eye” surgery in patients undergoing TT for benign or malignant conditions. Other parameters such as the necessity of supplementation with oral calcium and the duration of surgery were analyzed.
Materials and methods
In this case–control study we included patients underwent TT and referred to our institution, Department of Otorhinolaryngology, San Raffaele Hospital, Milan, Italy, between January 2020 and June 2022. Informed consent was obtained from each patient for treatment and use of de-identified clinical data for study purposes. We obtained approval from the Institutional Review Board (IRB) of San Raffaele Hospital for this clinical study (registration number: 116/INT/2021, protocol ID: Autofluorescence PGs), which was conducted according to the ethical standards established in the 1964 Declaration of Helsinki, as revised in 2020.
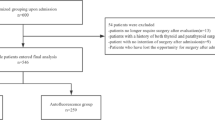
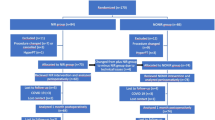
To better evaluate calcemic outcomes following thyroidectomies, only patients who underwent TT with a normal pre-operative parathyroid activity, older than 18 years old, and were mother-tongue Italian or had full capability to understand Italian, were included in this study (Fig. 2). In particular, the selected population was treated with conventional thyroidectomy ± central compartment neck dissection (CCND) (group 1, control group or standard-care group) or thyroidectomy ± CCND assisted with Fluobeam LX® (Fluoptics, France) (group 2, case group or autofluorescence group), according to a step-by-step approach described by our group [13]. All the procedures were performed by a team of thyroid surgeons with similar training level. CCND was performed on 14 patients with thyroid malignancy of the standard-care group (8 unilateral, 6 bilateral); similarly, 13 patients of the autofluorescence group underwent CCND (6 unilateral, 7 bilateral). The following parameters were taken into account and compared between the two groups: (1) preoperative serum calcium, ionized calcium and PTH levels; (2) serum calcium levels and PTH level at different timesteps during both hospital stay and follow-up in clinic, particularly at 1, 2 and 3 post-operative days (which median value has been defined short-term calcemia or PTH level), in the 1st month post-operative routine follow-up (defined medium-term calcemia or PTH level), and 6 months after surgery (defined long-term calcemia or PTH level); (3) surgical times; (4) necessity of supplementation during hospital stay of oral calcium; (5) percentages of short, medium and long-term hypocalcemia and hypoparathyroidism; (6) percentage variation in serum levels of PTH and calcium between the pre-operative and post-operative values in both groups; (7) quantification of the surgeon’s safety and confidence in identifying PGs in the intraoperative context, based on how many glands were detected by autofluorescence and how many were verified by autofluorescence.
To consider a single value of PTH and serum calcium during the hospital stay, we considered the median value of the blood levels of PTH and calcium during hospitalization days for each patient. PTH, serum and ionized calcium levels before surgery were not available in 3, 11 and 5 patients of the autofluorescence group, and in 2, 9 and 3 patients of the standard-care group, respectively. These patients were excluded from the analysis about percentage decrease in PTH and calcium levels.
We defined a patient “hypocalcemic” when total calcium level was <2.1 mmol/L (normal values: 2.1 mmol/L–2.6 mmol/L) and/or ionized calcium level was <1.18 mmol/L (normal values: 1.18 mmol/L–1.3 mmol/L), even if presenting no symptoms of hypocalcemia. Hypoparathyroidism was defined when PTH was <15 pg/ml (normal values 15 – 65 pg/ml) or, according to European Society of Endocrinology Clinical Guideline, in presence of low calcium and inappropriately low (insufficient) circulating PTH levels [14]. According to American Thyroid Association (ATA) statement on postoperative hypoparathyroidism [15], patients whose PTH were <15 pg/mL, serum calcium were <2.1 mmol/L, or ionized calcium were <1.1 mmol/L, were considered for postoperative oral calcium supplementation (1–3 g of calcium carbonate per day). If symptoms or signs of hypocalcemia progress despite treatment, intravenous bolus of calcium (1–2 g of calcium gluconate) was administered.
Statistical analysis
Comparisons between numerical variables were performed using linear regressions; between groups using Kruskal-Wallis rank sum test for numerical variables and Pearson’s Chi square test for categorical variables. Log rank test was used to test differences between groups in terms of insurgence of dysfunction of the clinical variables considered (PTH, total calcium, and ionic calcium). Data were reported as means (±standard deviation) or medians (first quantile, third quantile). Data analysis was performed using programming language R and RSTUDIO integrated development environment. Significance level was set at 0.05.
Results
134 patients fulfilled the inclusion criteria of the study. All patients were followed for 1 to 6 months after surgery. The details of the patient cohort are described in Table 1.
Mean pre-surgical total calcium and ionized calcium levels between the two groups were 2.38 ± 0.19 mmol/L and 1.2 ± 0.04 mmol/L, respectively, with no significant difference (p = 0.874 and p = 0.597, respectively). Considering group 2 (autofluorescence group), among all PGs 54.8% were found by the surgeon and then confirmed by NIR-AF, 33.9% were identified thanks to NIR-AF, and 11.3% were not identified at all. In detail, among the identified PGs, 62% were observed with “naked-eye” and then confirmed by NIR-AF, while 38% were found exclusively thanks to NIR-AF. Regarding surgical times, group 1 (control group) presented a median of 142 min (120.0–169.5 min), and group 2 a median of 153 min (126.0–197.5 min), with no significant difference noted between groups (p = 0.309).
When taken into account post-operative parameters, in terms of PTH levels following surgery, the two-way Anova for time series experiment revealed a significant difference between group 1 and 2 (p = 0.007). In particular, the percentages of patients with short-term hypocalcemia (56.7 vs. 31.3%, p = 0.04) and short-term hypoparathyroidism (56.7 vs. 25.4%, p = 0.011) were significant lower in the group 2 (autofluorescence group), although no differences between the two groups have been found in terms of medium-term hypocalcemia or hypoparathyroidism (19.4 vs. 11.9%, p = 0.05) and long-term hypocalcemia or hypoparathyroidism (11.9 vs. 6.0%, p = 0.4, and 6.0 vs. 6.0%, p = 0.06, respectively) (Table 2). In addition, significant differences have been found between group 1 and 2 regarding the median short-term (16.2 pg/ml vs. 26.8 pg/ml, p = 0.01) and medium-term (31.8 pg/ml vs. 36.5 pg/ml, p = 0.03) PTH levels. Furthermore, short-term (67.3 vs. 30.2%, p = 0.001), medium term (46.0 vs. 7.0%, p < 0.001) and long-term (22.7 vs. 5.2%, p = 0.019) percentage variation (decrease) of PTH levels from baseline was significantly lower in the autofluorescence group (Table 3). Finally, the use of supplementary short-term calcium therapy (p < 0.01) after surgery were significantly higher in the standard-care group (Table 4).
Discussion
In recent years, the application of autofluorescence detecting devices has gained widespread diffusion in helping surgeons to visualize PGs intraoperatively and therefore to reduce the rate of post-operative hypocalcemia, which remains the most frequent complication after TT [16,17,18]. Even if most patients with transient hypocalcemia recover functionality of their PGs, about 1–7% of patients can suffer from permanent hypoparathyroidism, potentially due to the failure of the intraoperative localization of PGs [2].
Our main outcomes demonstrated that patients undergoing TT assisted with autofluorescence detecting devices suffered less frequently for hypocalcemia and hypoparathyroidism the first 3 days after surgery and had higher PTH levels in the short-, medium- and long-term. In fact, the percentage of short-term hypocalcemia and hypoparathyroidism was significantly lower in autofluorescence group if compared to the standard-care group (p = 0.04 and p = 0.011, respectively); in addition the percentage variation of PTH levels from baseline was significantly lower both in the short- (p = 0.001), medium- (p < 0.001) and long-term (p = 0.02) in patients undergone TT under the guidance of NIR-AF compared with the control groups undergone conventional “naked-eye” surgery. On the contrary, no significant differences between the two groups were found in terms of post-operative calcemia.
Higher levels of PTH with the adoption of autofluorescence were detected in a recent case–control study on 542 patients by Kim et al. [19] in which it was described that the incidence of transient (<6 months after surgery) post-operative hypoparathyroidism in patients undergoing surgery with NIR-AF was significantly lower during hospital stay (33.7 vs. 46.6%; p = 0.002) and at 1 month (8.8 vs. 18.9%; p = 0.001) compared to the standard-care group. In fact, regardless of the hypoparathyroidism rates of both groups, patients in the NIR-AF group presented mean values of PTH that were significantly higher than the control group during hospital stay (22.70 vs. 19.17 pg/mL; p = 0.011) and 1 month after surgery (29.27 vs. 25.37 pg/mL; p = 0.001) [19]. Similar to our results, there was no significant difference in the rate of hypocalcemia between the two groups. On the contrary, a systematic review and meta-analysis by our group [20] demonstrated that patients undergoing total or near-total thyroidectomy with the adoption of NIR-AF and/or ICG (indocyanine green) showed better short- and medium-term calcium levels than those operated without (control group), while only numerical trends were appreciable in a short and medium-term hypoparathyroidism between the two groups, with no statistically significant correlations. This data may be explained considering that most of the studies included in our analysis dealt with the incidence of post-operative hypocalcemia, while only a few reported data on short- medium- and long-term post-operative hypoparathyroidism. However, post-operative PTH can be a more reliable parameter to evaluate the efficacy of PG preservation, since calcium levels can be influenced by various conditions, such as state of hydration, drugs, and vitamin D supplementation or deficiency [19]. Better values of PTH demonstrate that there is a decreased risk of impairment of PGs function if autofluorescence detecting devices are employed, thanks to earlier visualization and easier preservation of the glands. In fact, in our study 33.9% of the PGs were localized thanks to autofluorescence, avoiding erroneous excision or devascularization, while the remaining 54.87% of the glands were localized by the surgeon and then confirmed by this technology.
Our secondary outcome showed that the use of oral calcium during hospitalization and at discharge was significantly lower in TT performed with NIR-AF than with conventional means. Avoiding the need to routinely supplement calcium in inpatient or outpatient setting may determinate a decrease of public-health costs and an increase in the patient’s quality of life (QoL) [21,22,23]. Even if there is a lack of data on the relation between the intraoperative use of NIR-AF and calcium supplementation, these outcomes are consistent with a randomized-control study on carried out by Vidal Fortuny et al. which demonstrated that finding at least one well perfused parathyroid gland by adopting ICG angiography was a reliable way of predicting the absence of post-operative hypoparathyroidism, thus avoiding calcium and vitamin D supplementation [24].
The introduction of autofluorescence detecting devices necessarily increases surgical time since they imply additional surgical steps aimed to the search and the preservation of PGs. Furthermore, each time the autofluorescence detecting device is in use, the operative lights must be directed away from the surgical field, which is a time-consuming task especially when conducted the first few times. However, our results showed no significant difference in mean duration of surgery between the two groups (153 vs. 142 min; p = 0.309). A prospective study by Lerchenberger et al. highlighted that the additional time needed for utilization of autofluorescence detecting devices amounts to 5 to 10 min [25]. As mentioned in a previous study by our group, a slight increase in operating times should not necessarily be seen negatively, since NIR-AF forced the surgeon to give more attention to localize PGs and preserve their vascular pedicle [13].
As with any new technology, the adoption of NIR-AF has benefits and drawbacks. A significant advantage is the short learning curve requested. In fact, the device is user-friendly allowing confident and immediate utilization after a few procedures. In addition, even if autofuorescence detecting devices require high initial outlay costs, consumables for each patient consist only of the sterile sheaths that cover the device. Finally, the use of NIR-AF is safe: there are no study in literature that reported any complication arising from the application of this technology. Since it does not require the adoption of dyes or contrast agents, unless ICG angiography is performed, there is no risk of allergic reactions [26]. Nevertheless, the use of ICG is safe, with anecdotic allergic and anaphylactic reactions reported in patients with a history of iodine allergy [26, 27].
The adoption of NIR-AF has some limitations. First, given the absence of penetrative visualization mode more than 2 mm deep, NIR-AF images can be influenced by the presence of adipose tissue surrounding PGs that can reduce fluorescence and their intraoperative visualization [28]; second, and most importantly, this technology is unable to reveal any difference between vascularized and devascularized PGs, which results in the impossibility to predict their vitality. Some intravenous contrast agents such as ICG have been developed to overcome this limit [29]. Third, since in our experience colloid thyroid nodule and patients affected by thyroiditis may have higher levels of fluorescence [30] in some cases autofluorescence could lead to false positive results, and thus this technology is not able to replace the skills and ability of the surgeon. Finally, even if the camera of the device we used is ergonomic and easy to handle, its size is still out of proportion compared to the dimension of the surgical field, which makes it hard for the surgeon to visualize the operating field directly. In fact, during utilization of the autofluorescence detecting device, the surgeon is able to see the surgical field only through the screen.
To the best of our knowledge, this is one of the first studies in our country that assessed the impact of NIR-AF on post-operative hypocalcemia and hypoparathyroidism rates, analyzing data on PTH levels 6 months after surgery, which is not always reported in literature. This underlines functional outcomes of the application of this technology even in a long-term scenario.
Lower percentage variation of PTH levels from the baseline in short-, medium-, and long-term showed that the adoption of autofluorescence has a significant impact on functionality and viability of PGs at even 6 months after surgery. Most of the publications produced on this topic have focused on the improvement of the number of localized PGs and on the sensitivity and specificity of this technology, which can be seen as an anatomic outcome that does not necessarily reflect good function of the PGs [31]. However, our study has some limitations that warrant recognition. The retrospective design and the relatively small number of patients involved are the most important limitations. In addition, our study included only patients undergoing surgery with utilization of autofluorescence without ICG. In our opinion, the adoption of ICG requires a longer learning curve and thus its utilization without a proven experience may introduce a potential bias on post-operative calcium and PTH levels. Finally, as mentioned before, all the procedures were performed by the same surgical team but not by the same surgeon, unavoidably influencing the results.
Conclusion
Post-operative hypocalcemia secondary to hypoparathyroidism accounts for more than half of complications in thyroid surgery, being more frequent than recurrent laryngeal nerve paralysis. The results of this study confirm that the adoption of NIR-AF during total thyroidectomy is related to lower short-term hypocalcemia and hypoparathyroidism rates, lower variation of post-operative PTH levels in short-, medium- and long-term and reduced necessity of supplementation therapy with calcium if compared to conventional naked-eye surgery. Additional randomized controlled trials focusing on the impact of this technology on calcium and PTH levels are necessary.
References
L. Rosato, N. Avenia, P. Bernante et al. Complications of thyroid surgery: analysis of a multicentric study on 14,934 patients operated on in Italy over 5 years. World J. Surg. (2004). https://doi.org/10.1007/s00268-003-6903-1
O. Edafe, R. Antakia, N. Laskar, L. Uttley, S.P. Balasubramanian, Systematic review and meta-analysis of predictors of post-thyroidectomy hypocalcaemia. Br. J. Surg. 101(4), 307–320 (2014). https://doi.org/10.1002/bjs.9384
M.A. McWade, C. Paras, L.M. White et al. Label-free intraoperative parathyroid localization with near-infrared autofluorescence imaging. J. Clin. Endocrinol. Metab. 99(12), 4574–4580 (2014). https://doi.org/10.1210/jc.2014-2503
T.S. Papavramidis, A. Chorti, G. Tzikos et al. The effect of intraoperative autofluorescence monitoring on unintentional parathyroid gland excision rates and postoperative PTH concentrations—a single-blind randomized-controlled trial. Endocrine 72(2), 546–552 (2021). https://doi.org/10.1007/s12020-020-02599-5
S. Gourgiotis, P. Moustafellos, N. Dimopoulos, G. Papaxoinis, S. Baratsis, E. Hadjiyannakis, Inadvertent parathyroidectomy during thyroid surgery: The incidence of a complication of thyroidectomy. Langenbeck’s Arch. Surg. 391(6), 557–560 (2006). https://doi.org/10.1007/s00423-006-0079-8
A. Sitges-Serra, S. Ruiz, M. Girvent, H. Manjón, J.P. Dueñas, J.J. Sancho, Outcome of protracted hypoparathyroidism after total thyroidectomy. Br. J. Surg. 97(11), 1687–1695 (2010). https://doi.org/10.1002/bjs.7219
B. Wang, C.R. Zhu, H. Liu, X.M. Yao, J. Wu. The Accuracy of Near Infrared Autofluorescence in Identifying Parathyroid Gland During Thyroid and Parathyroid Surgery: A Meta-Analysis. Front. Endocrinol. 12(Jun), (2021). https://doi.org/10.3389/fendo.2021.701253
J. Zhu, W. Tian, Z. Xu et al. Expert consensus statement on parathyroid protection in thyroidectomy. Ann. Transl. Med 3(16), 230 (2015). https://doi.org/10.3978/j.issn.2305-5839.2015.08.20
T.S. Papavramidis, P. Anagnostis, A. Chorti et al. Do near-infrared intra-operative findings by the use of indocyanine green correlate with post-thyroidectomy parathyroid function?—the ICGPREDICT study. Endocr. Pract. (2020). https://doi.org/10.4158/EP-2020-0119
C. Paras, M. Keller, L. White, J. Phay, A. Mahadevan-Jansen, Near-infrared autofluorescence for the detection of parathyroid glands. J. Biomed. Opt. 16(6), 067012 (2011). https://doi.org/10.1117/1.3583571
M.A. McWade, C. Paras, L.M. White, J.E. Phay, A. Mahadevan-Jansen, J.T. Broome, A novel optical approach to intraoperative detection of parathyroid glands. Surgery 154(6), 1371–1377 (2013). https://doi.org/10.1016/j.surg.2013.06.046
R. Ladurner, S. Sommerey, N. Al Arabi, K.K.J. Hallfeldt, H. Stepp, J.K.S. Gallwas, Intraoperative near-infrared autofluorescence imaging of parathyroid glands. Surg. Endosc. 31(8), 3140–3145 (2017). https://doi.org/10.1007/s00464-016-5338-3
D. Barbieri, F. Triponez, P. Indelicato, A. Vinciguerra, M. Trimarchi, M. Bussi. Total thyroidectomy with intraoperative neural monitoring and near-infrared fluorescence imaging. Langenbecks Arch. Surg. 0123456789, (2021). https://doi.org/10.1007/s00423-021-02228-3
J. Bollerslev, L. Rejnmark, C. Marcocci et al. European Society of Endocrinology clinical guideline: Treatment of chronic hypoparathyroidism in adults. Eur. J. Endocrinol. 173(2), G1–G20 (2015). https://doi.org/10.1530/EJE-15-0628
L.A. Orloff, S.M. Wiseman, V.J. Bernet et al. American Thyroid Association Statement on Postoperative Hypoparathyroidism: Diagnosis, Prevention, and Management in Adults. Thyroid 28(7), 830–841 (2018). https://doi.org/10.1089/thy.2017.0309
B. Kahramangil, E. Berber, The use of near-infrared fluorescence imaging in endocrine surgical procedures. J. Surg. Oncol. 115(7), 848–855 (2017). https://doi.org/10.1002/jso.24583
M.S. Demarchi, W. Karenovics, B. Bédat, F. Triponez, Intraoperative Autofluorescence and Indocyanine Green Angiography for the Detection and Preservation of Parathyroid Glands. J. Clin. Med. 9(3), 830 (2020). https://doi.org/10.3390/jcm9030830
F. Benmiloud, S. Rebaudet, A. Varoquaux, G. Penaranda, M. Bannier, A. Denizot, Impact of autofluorescence-based identification of parathyroids during total thyroidectomy on postoperative hypocalcemia: a before and after controlled study. Surgery 163(1), 23–30 (2018). https://doi.org/10.1016/j.surg.2017.06.022
D.H. Kim, S.W. Kim, P. Kang et al. Near-infrared autofluorescence imaging may reduce temporary hypoparathyroidism in patients undergoing total thyroidectomy and central neck dissection. Thyroid 31(9), 1400–1408 (2021). https://doi.org/10.1089/thy.2021.0056
D. Barbieri, P. Indelicato, A. Vinciguerra et al. Autofluorescence and Indocyanine Green in Thyroid Surgery: A Systematic Review and Meta-Analysis. Laryngoscope. 1–10 (2020). https://doi.org/10.1002/lary.29297
J. Vidal Fortuny, V. Belfontali, S.M. Sadowski, W. Karenovics, S. Guigard, F. Triponez, Parathyroid gland angiography with indocyanine green fluorescence to predict parathyroid function after thyroid surgery. Br. J. Surg. 103(5), 537–543 (2016). https://doi.org/10.1002/bjs.10101
L. Underbjerg, T. Sikjaer, L. Mosekilde, L. Rejnmark, Cardiovascular and renal complications to postsurgical hypoparathyroidism: a Danish nationwide controlled historic follow-up study. J. Bone Min. Res J. Am. Soc. Bone Min. Res 28(11), 2277–2285 (2013). https://doi.org/10.1002/jbmr.1979
M. Mannstadt, J.P. Bilezikian, R.V. Thakker et al. Hypoparathyroidism. Nat. Rev. Dis. Prim. 3, (2017). https://doi.org/10.1038/nrdp.2017.55
J. Vidal Fortuny, S.M. Sadowski, V. Belfontali et al. Randomized clinical trial of intraoperative parathyroid gland angiography with indocyanine green fluorescence predicting parathyroid function after thyroid surgery. Br. J. Surg. 105(4), 350–357 (2018). https://doi.org/10.1002/bjs.10783
M. Lerchenberger, N. Al Arabi, J.K.S. Gallwas, H. Stepp, K.K.J. Hallfeldt, R. Ladurner. Intraoperative near-infrared autofluorescence and indocyanine green imaging to identify parathyroid glands: A comparison. Int J Endocrinol. 2019 (2019). https://doi.org/10.1155/2019/4687951
M.S. Demarchi, B. Seeliger, J.C. Lifante, P.F. Alesina, F. Triponez, Fluorescence image-guided surgery for thyroid cancer: Utility for preventing hypoparathyroidism. Cancers 13(15), 1–24 (2021). https://doi.org/10.3390/cancers13153792
N. Zaidi, E. Bucak, P. Yazici et al. The feasibility of indocyanine green fluorescence imaging for identifying and assessing the perfusion of parathyroid glands during total thyroidectomy. J. Surg. Oncol. 113(7), 775–778 (2016). https://doi.org/10.1002/jso.24237
A.N. Di Marco, F.F. Palazzo, Near-infrared autofluorescence in thyroid and parathyroid surgery. Gland Surg. 9(Suppl 2), S136–S146 (2020). https://doi.org/10.21037/gs.2020.01.04
H. Jin, Q. Dong, Z. He, J. Fan, K. Liao, M. Cui, Research on indocyanine green angiography for predicting postoperative hypoparathyroidism. Clin. Endocrinol. 90(3), 487–493 (2019). https://doi.org/10.1111/cen.13925
D.H. Kim, S. Lee, J. Jung, S. Kim, S.W. Kim, S.H. Hwang. Near-infrared autofluorescence-based parathyroid glands identification in the thyroidectomy or parathyroidectomy: a systematic review and meta-analysis. Langenbecks Arch Surg. (2021). https://doi.org/10.1007/s00423-021-02269-8
B. Kahramangil, E. Berber, Comparison of indocyanine green fluorescence and parathyroid autofluorescence imaging in the identification of parathyroid glands during thyroidectomy. Gland Surg. 6(6), 644–648 (2017). https://doi.org/10.21037/gs.2017.09.04
Acknowledgements
The authors thank all the staff members of the department of Otolaryngology. The paper was published with written consents of all the patients involved.
Author contributions
D.B., P.I., E.S.: Drafted the article, made a substantial contribution to the concept or design of the work, made a substantial contribution to acquisition, analysis and interpretation of data. A.V., R.A.B., F.D.M., F.L.L., S.B.: made a substantial contribution to acquisition and interpretation of data. Revised the paper critically for important intellectual content. L.G., M.T., M.B.: made a substantial contribution to the concept or design of the work. Approved the version to be published.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
The authors obtained approval from the Institutional Review Board (IRB) of San Raffaele Hospital for this study, which was conducted according to the ethical standards established in the 1975 Declaration of Helsinki, as revised in 1983.
Informed consent
We received informed consent from the patient for all the procedures described in this case report. Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Barbieri, D., Indelicato, P., Vinciguerra, A. et al. The impact of near-infrared autofluorescence on postoperative hypoparathyroidism during total thyroidectomy: a case–control study. Endocrine 79, 392–399 (2023). https://doi.org/10.1007/s12020-022-03222-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-022-03222-5