Abstract
The evaluation and management of hypothyroidism in children are similar to adults, but there are important differences based on the dependence on normal thyroid function for neurocognitive and physical development. In the pediatric population, hypothyroidism is frequently categorized as congenital or acquired hypothyroidism, depending on the age of presentation and the underlying etiology. The evaluation and management of children and adolescents with hypothyroidism are determined by the etiology as well as by the age at diagnosis, severity of the hypothyroidism, and the response to thyroid hormone replacement therapy. Children and adolescents require higher weight-based doses for thyroid hormone replacement than do adults, likely due to a shorter half-life of thyroxine (T4) and triiodothyronine (T3) in children, but weight-based dose requirements decrease as the child advances into adulthood. Multiple gaps in knowledge remains regarding how to optimize the treatment of hypothyroidism in pediatric patients, including (but not limited to) the selection of patients with subclinical hypothyroidism for treatment, and the potential benefit of combined LT3/LT4 therapy for patients with persistent symptoms and/or low T3 on LT4 monotherapy. The life-long impact on growth and development, and potentially on long-term cardiovascular and psychosocial health, are significant and highlight the importance of future prospective studies in pediatric patients to explore these areas of uncertainty.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thyroid hormone production, transport, and action
During the first 10–12 weeks of embryogenesis, the primordial thyroid gland migrates inferiorly to its normal position, transforms into its bilobed shape, and develops the capacity to trap iodine and secrete thyroid hormones (THs) [1]. Pituitary control of the thyroid by thyroid-stimulating hormone (TSH) is asserted beginning in the second trimester. The identification of transcription factors critical for thyroid gland formation—including NKX2–1, FOXE1, HHEX, and PAX8—has improved our understanding of thyroid gland development and function. Several of these transcription factors are also involved in nonthyroid related organogenesis. As an example, expression of NKX2–1 is critical in the development and function of interneurons in the central nervous system, surfactant producing cells in the lungs, and expression of thyroid peroxidase and thyroglobulin. Mutations in NKX2–1 are associated with brain–lung–thyroid syndrome characterized by hereditary chorea, respiratory distress syndrome, and congenital hypothyroidism (CH) secondary to thyroid dysgenesis [2]. More salient for our review, introduction of Nkx2–1 and Pax8 into embryonic or induced pluripotent stem cells can regenerate functioning thyroid follicular cells in mice rendered hypothyroid by radioactive iodine ablation [3].
Within hours after birth, a healthy, term infant experiences a surge in the neonatal serum TSH to a mean of ~80 mIU/L, with a subsequent, rapid decline in the TSH level over the next 2 days [4]. By 2–4 weeks of age, the TSH falls within the normal range for children and adults, although a smaller cohort of infants normalize their TSH by 1–3 months of age, with slight variation based on sex and age [5, 6]. In preterm or very low-birth weight infants (<1500 grams), there is a delayed and attenuated surge in TSH, which may be further accentuated by acute illness and medications [7, 8].
Approximately 80% of the TH secreted by the normal thyroid is T4, with T3 comprising the remaining 20%. T4 is a prohormone with a sixfold–sevenfold longer half-life than T3. T3 is the biologically active hormone, having a 15-fold greater affinity than T4 for TH nuclear receptors (TRs). TRs have variable expression in different tissues. TRalpha is highly expressed in the myocardium, skeletal muscle, intestine, bone, and central nervous system, whereas TRbeta is highly expressed in the hypothalamus, pituitary, liver, and kidney [9]. To reach the nuclear receptors, TH is actively transported across the cell membrane via transporter proteins. The most specific transport protein is monocarboxylate transporter 8 (MCT8), which plays a key role in T4 secretion from the thyroid gland and transport of TH into the brain [10]. Because of the variable tissue expression of MCT8 and TRs and variable reliance of different organs on these factors, inactivating mutations in these genes are associated with mixed clinical and biochemical forms of thyroid disease characterized by features of both hypothyroidism and hyperthyroidism and, unfortunately, less response to TH replacement therapy (see Groeneweg et al. for a review of TH transport disorders [10] and Dumitrescu et al. for a review of resistance to TH [9]).
Diagnosis and treatment of hypothyroidism
Hypothyroidism occurs when the concentration of circulating TH is inadequate to maintain normal TH signaling at the tissue level. In primary thyroid disease (disease impacting the thyroid gland), the TSH (normal range 0.5–5.5 mIU/L) is the most sensitive marker of inadequate TH levels, reflected by an increase in TSH to between 5.5 and 10 mIU/L (subclinical hypothyroidism) or >10 mIU/L (overt hypothyroidism) [11]. A low T4 or freeT4 in the setting of an elevated TSH may further distinguish a condition where LT4 replacement would be beneficial [12]. Therefore, the goal of treatment for primary hypothyroidism is normalization of TSH. In central hypothyroidism, the TSH is low or inappropriately normal despite low T4 levels, secondary to abnormalities in the hypothalamus (thyrotropin-releasing hormone, TRH) or pituitary (TSH). The goal of treatment in central hypothyroidism is to achieve serum T4 or free T4 concentrations in the upper half of the age-specific normal range. Because TH modulates the function of nearly every organ system, the hypothyroidism has important adverse effects on cardiovascular, neurologic, gastrointestinal, and metabolic function. In children, TH also plays a critical role in normal growth and development, and hypothyroidism results in significant impairments in these processes. Therefore, prompt recognition and treatment of hypothyroidism in infants and children are essential to optimizing physical and neurodevelopmental outcomes.
History of thyroid hormone replacement formulations
The first use of thyroid replacement therapy in the modern era was reported by Bettencourt and Serrano in 1890 with transplantation of animal thyroid tissue into a patient with myxedema. The use of intravenous thyroid extract was associated with a high risk of overdosage, which led to the transition to oral ingestion of fresh or desiccated thyroid extracts. The dose of the medications was titrated to clinical endpoints, initially resolution of hypothyroid symptoms, and later measurement of basal metabolic rate or “protein-bound iodine” (a proxy for TH). Synthetic sodium salts of levothyroxine (LT4) and liothyronine (LT3) were introduced in the United States in the 1950s, and marked improvement in the biochemical assessment of dose adequacy occurred with the introduction of radioimmune assays for measuring TH levels [13].
In 1906, the United States Food and Drug Administration (FDA) was formed. In 1962, in response to thalidomide-associated fetal malformations, the Kefauver–Harris amendment required that all medications introduced into the U.S. market since 1938 be subject to efficacy and safety standards. After the establishment of standards for bioequivalence and therapeutic equivalence, an “AB” code designation allowed for free substitution of the same medication from different manufacturers, in spite of dose differences of up to 10–12% between manufacturers for the same dose-labeled tablet. Within each manufacturer, the LT4 content in a given tablet must be within 5% of the designated dose. As of 2017, there are five FDA-approved LT4 tablet manufacturers in the United States, and one gel-cap product (Tirosint®), that also has an approved solution form (Tirosint®-SOL). Desiccated TH extracts were introduced into the market prior to 1938 and therefore are not regulated by the FDA [14].
Congenital hypothyroidism
Etiology
The incidence of CH is reported to be between 1:2000 and 1:4000 births, with the higher incidence related to lowering of the TSH diagnostic level for diagnosis [15]. The most common cause of primary CH is failure of normal thyroid gland development (dysgenesis) or failure of a eutopic thyroid gland to produce TH normally (dyshormonogenesis). Thyroid dysgenesis accounts for ~85% permanent CH while dyshormonogenesis accounts for the remainder [2]. Despite the frequency of thyroid dysgenesis, and an increasing number of identified causative genes, the majority of cases are sporadic and a genetic etiology is found in only 2–5% [16]. In contrast, dyshormonogenesis is most commonly associated with defects in TH synthesis, including mutations of thyroglobulin (TG), thyroid peroxidase (TPO), dual oxidase 2 (DUOX2) and its associated protein (DUOXA2), the sodium-iodide symporter (SLC5A5), the apical iodide transporter pendrin (SLC26A4), and iodotyrosine deiodinase (IYD) [17].
Congenital central hypothyroidism is rare, with an incidence between 1:16,000 and 1:20,000, and is usually caused by inborn structural or functional defects that may be associated with mutations in genes involved in hypothalamic and pituitary development [18]. Because these transcription factors regulate expression of multiple pituitary cell types, multiple pituitary hormone deficiencies are present in about 75% of infants with congenital central hypothyroidism [19].
Last, several extrinsic factors can cause hypothyroidism in neonates. Iodine deficiency remains the most common cause of neonatal hypothyroidism worldwide. Preterm infants are at increased risk of iodine deficiency, particularly because of the low iodine content of preterm infant formulas and of the parental nutrition commonly used in intensive care nurseries [20]. Iodine excess can also cause hypothyroidism and may occur secondary to exposure to topical iodine based antiseptics [21], exposure to high content iodine medications, such as amiodarone [22], radiographic contrast agents [23, 24], or high maternal dietary intake of iodine that is passed through breast milk [25]. Other extrinsic causes include transplacental passage of antithyroid medications used to treat hyperthyroidism (methimazole, carbimazole, or propylthiouracil), transfer of maternal IgG antibodies that block activation of the TSH receptor [26] and many additional medications that can alter TH levels (Table 2) [27, 28].
Diagnosis
Infants with severe congenital hypothyroidism may present with hypothermia, bradycardia, poor feeding, hypotonia, large fontanelles, myxedema, macroglossia, and umbilical hernia. This presentation is most common when both fetal and maternal hypothyroidism are present, as in iodine deficiency or untreated maternal hypothyroidism. However, many neonates manifest few or no symptoms even with significant hypothyroidism, making clinical diagnosis difficult in this age group. Implementation of universal newborn screening has nearly eradicated severe intellectual impairment due to CH in areas where screening is practiced, but CH remains a leading cause of preventable intellectual impairment in areas without newborn screening programs.
Screening protocols vary regionally but generally begin with measurement of TSH and/or T4 in a dried blood spot collected from each infant within a few days after delivery. Prompt diagnosis and treatment of CH are critical to optimize developmental outcome, so any abnormal newborn screen result should prompt immediate confirmation of TSH and free T4 concentrations in a serum sample [29]. The initial sample generally should be obtained at least 24 h after delivery to avoid false-positive results due to the physiologic TSH surge [4, 30]. If a repeat newborn screen is obtained after the first few days of life—whether to confirm an abnormal result, or as standard practice in all or selected newborns—gestational age- and postnatal age-specific reference ranges must be used to avoid missing the diagnosis CH by misinterpreting an elevated TSH as normal in the context of the higher TSH reference range that is applicable only to the first few days after birth [15]. An example of an evaluation and treatment algorithm can be found on the American College of Medical Genetics webpage; https://www.ncbi.nlm.nih.gov/books/NBK55827/.
Determining the etiology of the CH via radiological imaging rarely alters initial management but may provide insight into prognosis. Ultrasound (US) or thyroid scintigraphy (using 99mTc or 123I) can assess the presence or absence of a normally located thyroid gland, which can distinguish between thyroid dysgenesis and dyshormonogenesis [31]. While hypothyroidism due to dysgenesis is usually permanent, about 35% of patients with a eutopic thyroid gland have transient disease and will not require lifelong therapy [32, 33].
Evaluation for TSH receptor-blocking antibodies should be considered in patients with a eutopic thyroid gland on US even if there is no maternal history of autoimmune thyroid disease. If TSH receptor antibodies are documented, they indicate a transient hypothyroidism that usually resolves within 3–4 months. Nevertheless, if the mother had unrecognized hypothyroidism during gestation, neurodevelopment still may be impaired even if postnatal treatment is instituted promptly [26].
Treatment
In infants whose screening whole blood TSH is ≥40 mIU/L, LT4 should be initiated as soon as the confirmatory serum sample is obtained, without awaiting the results. In infants with screening TSH <40 mIU/L, LT4 should be initiated if the confirmatory serum TSH >20 mIU/L, or between 6 and 20 mIU/L with a low free T4 concentration [29]. The management of infants with mild TSH elevation (serum TSH 6–20 mIU/L) and normal free T4 levels is controversial. Although such patients are frequently identified by more stringent newborn screening thresholds, the neurodevelopmental risks posed by untreated mild disease remain uncertain [34,35,36,37]. Many practitioners elect to treat infants in this subclinical CH range in an effort to prevent any possible adverse developmental effects, but close monitoring of thyroid function without treatment may be reasonable in some cases, after careful discussion with the family.
The initial dose of LT4 for CH is 10–15 μg/kg daily. There is on-going discussion if doses at the high end of this range should be used for patients with significantly elevated TSH (>100 mIU/L) secondary to concerns that overtreatment may also be associated with altered neurodevelopmental outcome [38,39,40]. Serum thyroid function testing is monitored every 1–2 weeks until normal, and then every 1–2 months during the first year of life and every 2–4 months during the 2nd and 3rd year of life with LT4. The LT4 dose is adjusted to maintain the serum TSH in the mid-normal range and the serum free T4 in the mid- to upper-half of the normal range (Table 1). A subgroup of infants with CH display variable degrees of TH resistance with persistently elevated TSH levels despite high-normal or frankly elevated free T4 concentrations [41]. For these patients, the addition of LT3 to LT4 therapy can facilitate normalization of the TSH, but whether this improves outcomes is unknown [42]. For a detailed review of evidence and recommendations for the diagnosis and management of CH, the reader is referred to comprehensive consensus guidelines on this topic [31].
LT4 tablets should be crushed, suspended in a small volume of water, breast milk, or non-soy based infant formula, and administered via a syringe or teaspoon (not in a bottle). LT4 should not be administered with multivitamins containing calcium or iron. Although LT4 formulations produced by different manufacturers may vary slightly [despite reported bioequivalence as defined by the FDA], it remains uncertain whether—or under what circumstances—clinically significant differences exist between generic and brand-name LT4 preparations in children. Limited data suggest that brand-name LT4 may be superior to generic in children with severe congenital hypothyroidism, but not in those with equally severe acquired hypothyroidism [29, 43]. Compounded LT4 solutions do not provide reliable dosing and should not be used. Tirosint®-SOL is a stable liquid form of levothyroxine that is available in Europe and the United States with reported unaltered absorption when administered with milk (and other breakfast beverages, in adults) [44]; however, optimal dosing of liquid LT4 preparations in neonates may differ from that of tablet forms [45, 46].
Prenatal treatment of CH may be considered in rare cases of dyshormonogenesis that present with a large fetal goiter, which can cause polyhydramnios as well as airway compromise that may obstruct breathing after birth. Intra-amniotic injection of LT4 may help decrease fetal goiter size to prevent these complications. Although there is no consensus on intra-amniotic LT4 dose or schedule, several reports have employed 150–500 μg/dose (or 10 μg/kg estimated fetal weight) every 2 weeks, with adjusted frequency based on fetal goiter response [47,48,49]. Intrauterine demise and need for intubation at birth are potential complications in such cases [50].
Outcome
Transient CH should be considered in patients without thyroid dysgenesis who have a borderline elevated TSH at the initiation of therapy (between 6 and 20 mIU/L with a normal T4) and no need for an increase in LT4 dose despite normal growth and development. An LT4 dose of <2 μg/kg daily at age 2–3 years is associated with a greater likelihood of transient disease [33]. A US should be performed prior to the trial off of LT4 to confirm a normal, eutopic thyroid gland.
In general, the prognosis for children born with CH is excellent and the majority achieve normal neurocognitive and physical development. However, despite optimal postnatal treatment, children with severe CH (often associated with athyreosis) may have mild deficits in motor development, verbal skills, attention, or memory [51, 52]. Hearing deficits are also present in about 10% of patients with congenital hypothyroidism [53]. Patients and families should be counseled about these issues, and careful monitoring of development and academic progress is important to identify and address problems early.
Acquired hypothyroidism
Etiology
The etiology of hypothyroidism is related to the iodine status of the population. In countries or regions without iodine supplementation programs, iodine deficiency is the most common cause of acquired hypothyroidism. Autoimmune (or Hashimoto) thyroiditis is the most common etiology in iodine-sufficient regions, and its incidence has been increasing, including early-onset presentations [54, 55]. The incidence of autoimmune thyroid disease is also increased in patients with Down syndrome [56, 57], Turner syndrome [58, 59], type 1 diabetes mellitus [60, 61], or other forms of autoimmune disease [62]. Obesity can also be associated with a mild increase in TSH (between 5 and 10 mIU/L), but weight loss leads to normalization of TSH levels, indicating that this is likely an adaptation of the hypothalamic–pituitary–thyroid axis to, rather than a cause of, obesity [12].
Additional causes of acquired hypothyroidism include central hypothyroidism associated with trauma or tumors of the central nervous system, and primary hypothyroidism after exposure to ionizing irradiation (i.e., total body irradiation for bone marrow transplant, treatment of Hodgkin’s lymphoma and others) [63, 64] or after definitive treatment of Graves’ disease, thyroid nodules, or thyroid cancer. Consumptive hypothyroidism is a rare disorder associated with vascular (usually hepatic) tumors in children, as well as vascular, fibrous, and gastrointestinal stromal tumors in adults [65]. The disorder is caused by expression of type-3 deiodinase by the tumor and subsequent inactivation of circulating T4 (to reverse T3) and T3 (to T2) [66]. Consumptive hypothyroidism should be considered in patients that present with refractory hypothyroidism despite extraordinary amounts of TH replacement [67]. Beta-blocker therapy is associated with involution of the hemangiomas and coincident resolution of the consumptive hypothyroidism [68]. Last, numerous medications can cause hypothyroidism through a variety of mechanisms (Table 2). Many of these cases are transient, resolving after discontinuation of the medication [27, 28].
Diagnosis
The diagnosis of acquired hypothyroidism is based on history, physical examination, and radiologic data, interpreted in the context of thyroid function laboratory data. The symptoms commonly associated with hypothyroidism, including brittle hair, cold intolerance, slowed thinking, weight gain, constipation, and others are often not useful in isolation as a trigger for testing because they lack specificity having significant cross over with common, nonthyroid related complaints. However, after diagnosis, these symptoms may be followed longitudinally to aid in the adjustment of TH replacement therapy [69]. Some clinical signs may be of greater utility, including puffiness (myxedema), slowed Achilles reflex time, and goiter. The latter is most easily detected by visual inspection with the patient moving from a neutral neck position to neck extension with swallowing (see https://www.youtube.com/watch?v=Z9norsLPKfU) [2]. Although autoimmune thyroiditis is the most common etiology of acquired hypothyroidism, a goiter may not be present in cases of severe and rapid autoimmune-mediated destruction of the thyroid (atrophic thyroiditis) [70, 71].
In pediatric patients, severe and prolonged primary hypothyroidism (TSH typically >100 mIU/L) may present with growth failure and pubertal delay, or rarely with pseudo-precocious puberty (breast development in girls or testicular enlargement in boys, both without virilization) [70]. In both situations there is concomitant bone age (BA) delay, and, for the majority of patients, decreased adult height (>2 SD below mid-parental height prediction), although some patients may achieve mid-parental target height with combined use of LT4, gonadotropins-releasing hormone agonist, and growth hormone (GH) therapy [72, 73]. Additional features associated with pseudo-precocious puberty (designated Van Wyk–Grumbach syndrome) include galactorrhea, vaginal bleeding, and ovarian cysts associated with elevated prolactin, normal to high estradiol, elevated follicle stimulating hormone (FSH) with suppressed luteinizing hormone (LH) [70]. The ovarian cysts may be large with an increased risk of torsion and may be associated with elevated tumor markers including CA-125, lactate dehydrogenase (LDH) and inhibin, all of which normalize with LT4 therapy [74].
Prolonged elevation in TRH may also lead to anterior pituitary enlargement with expansion of the sella turcica caused by marked thyrotroph hyperplasia, which may be mistaken for a pituitary macroadenoma [75, 76]. GH and insulin-like growth factor-1 levels are often reduced in hypothyroidism, and if tested patients would fail provocative GH testing [77]. However, in contrast to a true pituitary macroadenoma, the pituitary mass resolves with L-T4 replacement therapy (Fig. 1) [71].
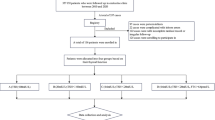
Laboratory assessment of suspected hypothyroidism includes serum TSH and T4 concentrations. Autoantibodies to thyroglobulin (TgAb) and thyroid peroxidase (TPOAb) can be included to assess for autoimmune thyroiditis. In patients with a TSH in the subclinical range (between 5 and 10 mIU/L), the presence of TgAb and/or TPOAb, a history of an additional autoimmune disease (celiac disease, type 1 diabetes mellitus, vitiligo, and others) or chromosomopathy (Turner or Down syndrome), and the presence of goiter on physical examination are all associated with an increased risk of progression to overt hypothyroidism (TSH >10 mIU/L) [12, 69]. In a 5-year prospective study of 234 children, 50% of pediatric patients with euthyroid autoimmune thyroiditis at diagnosis (elevated TPOAb or TgAb) remained euthyroid; however, in patients with subclinical hypothyroidism at presentation, 40% reverted to euthyroidism, 31% progressed to overt hypothyroidism, and 25% remained with subclinical hypothyroidism [78]. Prepubertal children (<10 years of age) with elevated TPOAb or TgAb are more likely to present with overt hypothyroidism and to progress from subclinical to overt hypothyroidism compared with pubertal children [55, 79]. In patients with acquired central hypothyroidism, function of the other anterior pituitary hormone axes should be assessed.
Another potential explanation for an elevated TSH with a normal T4 is chronic poor compliance with LT4 followed by adherence to taking daily LT4 several days prior to laboratory testing. In this situation, an observed LT4 absorption test may be performed to ensure normal LT4 absorption (Fig. 2, modified from reference [28]). Last, patients with acquired central hypothyroidism may have an inappropriately normal to minimally elevated TSH with a low T4. Patients being evaluated for central hypothyroidism should have other anterior pituitary hormone axes assessed, with replacement of hydrocortisone prior to initiation of LT4 in patients found to have concomitant ACTH deficiency.
Thyroid US may be helpful in confirming the diagnosis of autoimmune thyroiditis. Common US features of autoimmune thyroiditis include parenchymal heterogeneity and hypoechogenicity and the presence of hypoechoic micro- and macro-nodules (‘cobblestone appearance’ from lymphatic follicle formation). Unfortunately, these features appear to have high specificity for Hashimoto’s but low sensitivity for identifying thyroid gland dysfunction [80]. The addition of conventional color and power Doppler vascular imaging (CDI), as well as the more recent imaging technique of superb microvascular imaging, improves the sensitivity with increased blood flow being a marker for the early phase of the thyroiditis and showing a positive correlation to TSH levels in pediatric patients with Hashimoto’s thyroiditis [81, 82]. Thyroid US may also be informative for diagnoses of other etiologies of hypothyroidism, including obesity [83], postradiation, and chemotherapy for the treatment of nonthyroid malignancy [84], as well as abnormalities in thyroid gland formation [85].
Treatment and prognosis of acquired hypothyroidism
Treatment of acquired hypothyroidism consists of replacing the deficiency of TH to improve symptoms and prevent the adverse consequences of hypothyroidism. As in adults, levothyroxine (LT4) is the recommended treatment for hypothyroidism in children and adolescents. In primary hypothyroidism, serum TSH is the most sensitive measure of thyroid status, and the goal of therapy is to maintain serum TSH within the age-specific normal range [86]. At present, there are no data confirming a clinical benefit of maintaining TSH within a lower range (e.g., 0.5–2 mIU/L). For central hypothyroidism, in which by definition serum TSH does not reflect systemic thyroid status, serum free T4 levels should be maintained in the upper half of the reference range [86].
The dose of LT4 required to restore euthyroidism depends on patient age and on the severity of hypothyroidism. The dose required per kilogram body mass to fully replace thyroid function is significantly higher in children than in adults and decreases with age (Table 1). Thyroid function should be assessed 4–6 weeks after initiation or any change in LT4 dose, and the dose should be adjusted to achieve and maintain consistent euthyroidism. Once stable, thyroid function testing should be performed every 6 months through adolescence. For patients with profound hypothyroidism (TSH >100 mIU/L), initiation of treatment may be associated with changes in behavior [87] and, rarely, pseudotumor cerebri [88].
As in adults, there is considerable controversy in the pediatric literature over whether subclinical hypothyroidism should be treated [89,90,91]. To date there are no clear data showing short- or long-term negative consequences associated with untreated, idiopathic subclinical hypothyroidism, including no apparent adverse effect on linear growth, cardiovascular risk, behavior, or cognition [90,91,92]. However, because the risks of LT4 replacement therapy are low, many clinicians consider it reasonable to initiate treatment to avoid any potential negative impact on growth and development. Treatment may also be stratified based on risk, with patients selected for LT4 treatment if they have a history of a nonthyroid autoimmune disease (celiac, type 1 diabetes, vitiligo, and others), chromosomopathy (Down syndrome or Turner syndrome), goiter, elevated TPOAb or TgAb, or US findings suggestive of autoimmune thyroiditis. Subclinical hypothyroidism may also be associated with an increase in total and low-density lipoprotein cholesterol which may serve as a marker for tissue-level hypothyroidism as well as be related to increased cardiovascular risk in adults [93]. Thus, while there is no indication that patients with subclinical hypothyroidism benefit in general from LT4 replacement therapy, an individualized approach to treatment may be warranted [12, 69, 89].
Adherence to LT4 may be influenced by multiple factors, including who administers the medication (parent/care-giver, patient, school nurse), the location where the medication is stored (kitchen vs. bedroom vs. bathroom), and the time of day when the medication is administered. Although the majority of providers and pharmacists recommend administration of LT4 prior to breakfast, bedtime administration may be more convenient and does not appear to be associated with worse control of hypothyroidism in children and adolescents [94]. A pill box may aid in reminding and tracking medication adherence, and newer, Bluetooth-enabled pill boxes and bottle caps can track dose administration and send an electronic reminder to both the patient and caregiver when a dose has been missed or to prevent a double-dosing. Additional interventions to improve adherence include phone-based applications that can alarm or text patients to remind them to take their medication. Ongoing age-appropriate education about the disease and its treatment is critical to optimize adherence, and interventions can be combined with rewards to incentivize adherence [95].
In patients for whom adherence is in question after assessing possible confounding medical, dietary, and medicinal factors, a supervised LT4 absorption test can be performed. After an overnight fast, the patient ingests—under direct observation in the outpatient clinic—the cumulative equivalent of 3 days of LT4 followed by 100–200 ml of water (modified protocol from Koulouri et al.) [28]. An LT4 is mainly absorbed in the duodenum, jejunum, and ileum, with maximum absorption in a euthyroid patient occurring ~2-h after ingestion [96]. T4 levels are measured every 30 min for 3–4 h to assess for a rise in serum T4 levels. Supervised observation of LT4 dosing is continued at home for the next 4–5 weeks with weekly TSH and T4 measurements to assess for TSH normalization. Adjustments in LT4 dosing and lifestyle can then be pursued.
Outcome
The goal of TH replacement in children and adolescence is similar to adults, to reduce or eliminate hypothyroidism-related signs and symptoms, with the additional target of achieving normal growth and development. For many pediatric patients, LT4 replacement is anticipated to be life-long; however, there is potential to discontinue LT4 in patients with drug-induced hypothyroidism (amiodarone, lithium, and others) and even in some patients with autoimmune thyroiditis [97]. There are no data supporting that dietary supplements or a gluten-free diet can help achieve remission of autoimmune thyroid disease, but this is a topic of interest for many patients and families, and future research in this area is warranted [98,99,100].
Summary
Pediatric hypothyroidism is common, and while there are many similarities between children and adults with respect to its evaluation and treatment, the importance of optimizing thyroid health is amplified in children and adolescents because of the impact of hypothyroidism on neurocognitive development and physical growth. Universal salt iodization programs and routine newborn screening facilitate prevention and prompt diagnosis and treatment of infants with CH, and these programs have been highly effective at reducing preventable and irreversible neurocognitive deficits for the vast majority of patients. In children and adolescents, autoimmune thyroiditis remains the dominant cause of acquired hypothyroidism, but many other etiologies are common in clinical practice. Multiple gaps in knowledge remains regarding how to optimize the treatment of hypothyroidism in pediatric patients, including (but not limited to) the selection of patients with subclinical hypothyroidism for treatment, and the potential benefit of combined LT3/LT4 therapy for patients with persistent symptoms and/or low T3 on LT4 monotherapy. The life-long impact on growth and development, and potentially on long-term cardiovascular and psychosocial health, are significant and highlight the importance of future prospective studies in pediatric patients to explore these areas of uncertainty.
References
L.P. Fernandez, A. Lopez-Marquez, P. Santisteban, Thyroid transcription factors in development, differentiation and disease. Nat. Rev. Endocrinol. 11(1), 29–42 (2015). https://doi.org/10.1038/nrendo.2014.186
P. Hanley, K. Lord, A.J. Bauer, Thyroid disorders in children and adolescents: a review. JAMA Pedia. 170(10), 1008–1019 (2016). https://doi.org/10.1001/jamapediatrics.2016.0486
A.A. Kurmann, M. Serra, F. Hawkins, S.A. Rankin, M. Mori, I. Astapova, S. Ullas, S. Lin, M. Bilodeau, J. Rossant, J.C. Jean, L. Ikonomou, R.R. Deterding, J.M. Shannon, A.M. Zorn, A.N. Hollenberg, D.N. Kotton, Regeneration of thyroid function by transplantation of differentiated pluripotent stem cells. Cell Stem Cell 17(5), 527–542 (2015). https://doi.org/10.1016/j.stem.2015.09.004
D.A. Fisher, W.D. Odell, Acute release of thyrotropin in the newborn. J. Clin. Investig. 48(9), 1670–1677 (1969). https://doi.org/10.1172/JCI106132
G. Gunapalasingham, C. Frithioff-Bojsoe, M.A.V. Lund, P.L. Hedley, C.E. Fonvig, M. Dahl, O. Pedersen, M. Christiansen, T. Hansen, U. Lausten-Thomsen, J.C. Holm, Reference values for fasting serum concentrations of thyroid-stimulating hormone and thyroid hormones in healthy Danish/North-European white children and adolescents. Scand. J. Clin. Lab. Investig. 79(1-2), 129–135 (2019). https://doi.org/10.1080/00365513.2019.1581945
A.J. Lem, Y.B. de Rijke, H. van Toor, M.A. de Ridder, T.J. Visser, A.C. Hokken-Koelega, Serum thyroid hormone levels in healthy children from birth to adulthood and in short children born small for gestational age. J. Clin. Endocrinol. Metab. 97(9), 3170–3178 (2012). https://doi.org/10.1210/jc.2012-1759
J. Simpson, F.L. Williams, C. Delahunty, H. van Toor, S.Y. Wu, S.A. Ogston, T.J. Visser, R. Hume, Scottish Preterm Thyroid, G.: Serum thyroid hormones in preterm infants and relationships to indices of severity of intercurrent illness. J. Clin. Endocrinol. Metab. 90(3), 1271–1279 (2005). https://doi.org/10.1210/jc.2004-2091
F.L. Williams, J. Simpson, C. Delahunty, S.A. Ogston, J.J. Bongers-Schokking, N. Murphy, H. van Toor, S.Y. Wu, T.J. Visser, R. Hume,Collaboration from the Scottish Preterm Thyroid, G, Developmental trends in cord and postpartum serum thyroid hormones in preterm infants. J. Clin. Endocrinol. Metab. 89(11), 5314–5320 (2004). https://doi.org/10.1210/jc.2004-0869
A.M. Dumitrescu, S. Refetoff, S., in Impaired Sensitivity to Thyroid Hormone: Defects of Transport, Metabolism and Action. ed. by K.R. Feingold, B. Anawalt, A. Boyce, G. Chrousos, K. Dungan, A. Grossman, J.M. Hershman, G. Kaltsas, C. Koch, P. Kopp, M. Korbonits, R. McLachlan, J.E. Morley, M. New, L. Perreault, J. Purnell, R. Rebar, F. Singer, D.L. Trence, A. Vinik, D.P. Wilson (Endotext, South Dartmouth, MA, 2000)
S. Groeneweg, W.E. Visser, T.J. Visser, Disorder of thyroid hormone transport into the tissues. Best practice & research. Clin. Endocrinol. Metab. 31(2), 241–253 (2017). https://doi.org/10.1016/j.beem.2017.05.001
I. Onsesveren, M. Barjaktarovic, L. Chaker, Y.B. de Rijke, V.W.V. Jaddoe, H.M. van Santen, T.J. Visser, R.P. Peeters, T.I.M. Korevaar, Childhood thyroid function reference ranges and determinants: a literature overview and a prospective cohort study. Thyroid. 27(11), 1360–1369 (2017). https://doi.org/10.1089/thy.2017.0262
M. Salerno, D. Capalbo, M. Cerbone, F. De Luca, Subclinical hypothyroidism in childhood—current knowledge and open issues. Nat. Rev. Endocrinol. 12(12), 734–746 (2016). https://doi.org/10.1038/nrendo.2016.100
J.V. Hennessey, The emergence of levothyroxine as a treatment for hypothyroidism. Endocrine 55(1), 6–18 (2017). https://doi.org/10.1007/s12020-016-1199-8
J.V. Hennessey, Historical and current perspective in the use of thyroid extracts for the treatment of hypothyroidism. Endocr. Pract. 21(10), 1161–1170 (2015). https://doi.org/10.4158/EP14477.RA
M.J. Kilberg, I.R. Rasooly, S.H. LaFranchi, A.J. Bauer, C.P. Hawkes, Newborn Screening in the US May Miss Mild Persistent Hypothyroidism. J. Pediatr. 192, 204–208 (2018). https://doi.org/10.1016/j.jpeds.2017.09.003
R. Abu-Khudir, S. Larrivee-Vanier, J.D. Wasserman, J. Deladoey, Disorders of thyroid morphogenesis. Best practice & research. Clin. Endocrinol. Metab. 31(2), 143–159 (2017). https://doi.org/10.1016/j.beem.2017.04.008
M.J. Kwak, Clinical genetics of defects in thyroid hormone synthesis. Ann. Pediatr. Endocrinol. Metab. 23(4), 169–175 (2018). https://doi.org/10.6065/apem.2018.23.4.169
C. Peters, A.S.P. van Trotsenburg, N. Schoenmakers, O.F. DIAGNOSIS, Endocrine disease: congenital hypothyroidism: update and perspectives. Eur. J. Endocrinol. 179(6), R297–R317 (2018). https://doi.org/10.1530/EJE-18-0383
D.A. van Tijn, J.J. de Vijlder, B. Verbeeten Jr., P.H. Verkerk, T. Vulsma, Neonatal detection of congenital hypothyroidism of central origin. J. Clin. Endocrinol. Metab. 90(6), 3350–3359 (2005). https://doi.org/10.1210/jc.2004-2444
M.B. Belfort, E.N. Pearce, L.E. Braverman, X. He, R.S. Brown, Low iodine content in the diets of hospitalized preterm infants. J. Clin. Endocrinol. Metab. 97(4), E632–E636 (2012). https://doi.org/10.1210/jc.2011-3369
J.E. Pinsker, K. McBayne, M. Edwards, K. Jensen, D.F. Crudo, A.J. Bauer, Transient hypothyroidism in premature infants after short-term topical iodine exposure: an avoidable risk? Pediatr. Neonatol. 54(2), 128–131 (2013). https://doi.org/10.1016/j.pedneo.2012.10.005
A. Creo, H. Anderson, P. B. Cannon, A. Lteif, S. Kumar, P. Tebben, A.M. Iqbal, A. Ramakrishna, S. Pittock, Patterns of amiodarone-induced thyroid dysfunction in infants and children. Heart Rhythm (2019). https://doi.org/10.1016/j.hrthm.2019.03.015
M.L. Barr, H.K. Chiu, N. Li, M.W. Yeh, C.M. Rhee, J. Casillas, P.J. Iskander, A.M. Leung, Thyroid dysfunction in children exposed to iodinated contrast media. J. Clin. Endocrinol. Metab. 101(6), 2366–2370 (2016). https://doi.org/10.1210/jc.2016-1330
S.S. Jick, M. Hedderson, F. Xu, Y. Cheng, P. Palkowitsch, A. Michel, Iodinated contrast agents and risk of hypothyroidism in young children in the United States. Investig Radio. 54(5), 296–301 (2019). https://doi.org/10.1097/RLI.0000000000000541
J. Farebrother, M.B. Zimmermann, M. Andersson, Excess iodine intake: sources, assessment, and effects on thyroid function. Ann. N. Y Acad. Sci. 1446(1), 44–65 (2019). https://doi.org/10.1111/nyas.14041
R.S. Brown, C.A. Alter, A. Sadeghi-Nejad, Severe unsuspected maternal hypothyroidism discovered after the diagnosis of thyrotropin receptor-blocking antibody-induced congenital hypothyroidism in the neonate: failure to recognize and implications to the fetus. Horm. Res. Paediatr. 83(2), 132–135 (2015). https://doi.org/10.1159/000368671
B.R. Haugen, Drugs that suppress TSH or cause central hypothyroidism. Best practice & research. Clin. Endocrinol. Metab. 23(6), 793–800 (2009). https://doi.org/10.1016/j.beem.2009.08.003
O. Koulouri, C. Moran, D. Halsall, K. Chatterjee, M. Gurnell, Pitfalls in the measurement and interpretation of thyroid function tests. Best practice & research. Clin. Endocrinol. Metab. 27(6), 745–762 (2013). https://doi.org/10.1016/j.beem.2013.10.003
J. Leger, A. Olivieri, M. Donaldson, T. Torresani, H. Krude, G. van Vliet, M. Polak, G. Butler, European Society for Paediatric Endocrinology consensus guidelines on screening, diagnosis, and management of congenital hypothyroidism. J. Clin. Endocrinol. Metab. 99(2), 363–384 (2014). https://doi.org/10.1210/jc.2013-1891
D.A. Fisher, W.D. Odell, C.J. Hobel, R. Garza, Thyroid function in the term fetus. Pediatrics 44(4), 526–535 (1969)
J. Leger, A. Olivieri, M. Donaldson, T. Torresani, H. Krude, G. van Vliet, M. Polak, G. Butler, European Society for Paediatric Endocrinology consensus guidelines on screening, diagnosis, and management of congenital hypothyroidism. Horm. Res. Paediatr. 81(2), 80–103 (2014). https://doi.org/10.1159/000358198
N. McGrath, C.P. Hawkes, P. Mayne, N.P. Murphy, Permanent decompensated congenital hypothyroidism in newborns with whole-blood thyroid-stimulating hormone concentrations between 8 and 10 mU/L: the case for lowering the threshold. Horm. Res. Paediatr. 89(4), 265–270 (2018). https://doi.org/10.1159/000488288
S. Rabbiosi, M.C. Vigone, F. Cortinovis, I. Zamproni, L. Fugazzola, L. Persani, C. Corbetta, G. Chiumello, G. Weber, Congenital hypothyroidism with eutopic thyroid gland: analysis of clinical and biochemical features at diagnosis and after re-evaluation. J. Clin. Endocrinol. Metab. 98(4), 1395–1402 (2013). https://doi.org/10.1210/jc.2012-3174
S.D. Grosse, G. Van Vliet, Prevention of intellectual disability through screening for congenital hypothyroidism: how much and at what level? Arch. Dis. Child. 96(4), 374–379 (2011). https://doi.org/10.1136/adc.2010.190280
S.J. Lain, J.P. Bentley, V. Wiley, C.L. Roberts, M. Jack, B. Wilcken, N. Nassar, Association between borderline neonatal thyroid-stimulating hormone concentrations and educational and developmental outcomes: a population-based record-linkage study. Lancet Diabetes Endocrinol. 4(9), 756–765 (2016). https://doi.org/10.1016/S2213-8587(16)30122-X
C. Trumpff, J. De Schepper, J. Vanderfaeillie, N. Vercruysse, H. Van Oyen, R. Moreno-Reyes, J. Tafforeau, S. Vandevijvere, Neonatal thyroid-stimulating hormone concentration and psychomotor development at preschool age. Arch. Dis. Child. 101(12), 1100–1106 (2016). https://doi.org/10.1136/archdischild-2015-310006
S. Lain, C. Trumpff, S.D. Grosse, A. Olivieri, G. Van Vliet, Are lower TSH cutoffs in neonatal screening for congenital hypothyroidism warranted? Eur. J. Endocrinol. 177(5), D1–D12 (2017). https://doi.org/10.1530/EJE-17-0107
J.J. Bongers-Schokking, W.C. Resing, Y.B. de Rijke, M.A. de Ridder, S.M. de Muinck Keizer-Schrama, Cognitive development in congenital hypothyroidism: is overtreatment a greater threat than undertreatment? J. Clin. Endocrinol. Metab. 98(11), 4499–4506 (2013). https://doi.org/10.1210/jc.2013-2175
J.J. Bongers-Schokking, W.C.M. Resing, W. Oostdijk, Y.B. de Rijke, S.de Muinck Keizer-Schrama, Relation between early over- and undertreatment and behavioural problems in preadolescent children with congenital hypothyroidism. Horm. Res. Paediatr. 90(4), 247–256 (2018). https://doi.org/10.1159/000494056
P.E. Aleksander, M. Bruckner-Spieler, A.M. Stoehr, E. Lankes, P. Kuhnen, D. Schnabel, A. Ernert, W. Stablein, M.E. Craig, O. Blankenstein, A. Gruters, H. Krude, Mean high-dose l-thyroxine treatment is efficient and safe to achieve a normal iq in young adult patients with congenital hypothyroidism. J. Clin. Endocrinol. Metab. 103(4), 1459–1469 (2018). https://doi.org/10.1210/jc.2017-01937
D.A. Fisher, E.J. Schoen, S. La Franchi, S.H. Mandel, J.C. Nelson, E.I. Carlton, J.H. Goshi, The hypothalamic-pituitary-thyroid negative feedback control axis in children with treated congenital hypothyroidism. J. Clin. Endocrinol. Metab. 85(8), 2722–2727 (2000). https://doi.org/10.1210/jcem.85.8.6718
L. Paone, A.F. Fleisch, H.A. Feldman, R.S. Brown, A.J. Wassner, Liothyronine improves biochemical control of congenital hypothyroidism in patients with central resistance to thyroid hormone. J. Pediatr. 175, 167–172 e161 (2016). https://doi.org/10.1016/j.jpeds.2016.04.022
J.M. Carswell, J.H. Gordon, E. Popovsky, A. Hale, R.S. Brown, Generic and brand-name l-thyroxine are not bioequivalent for children with severe congenital hypothyroidism. J. Clin. Endocrinol. Metab. 98(2), 610–617 (2013). https://doi.org/10.1210/jc.2012-3125
A. Bernareggi, E. Grata, M.T. Pinorini, A. Conti, Oral liquid formulation of levothyroxine is stable in breakfast beverages and may improve thyroid patient compliance. Pharmaceutics 5(4), 621–633 (2013). https://doi.org/10.3390/pharmaceutics5040621
A. Cassio, S. Monti, A. Rizzello, I. Bettocchi, F. Baronio, G. D’Addabbo, M.O. Bal, A. Balsamo, Comparison between liquid and tablet formulations of levothyroxine in the initial treatment of congenital hypothyroidism. J. Pediatr. 162(6), 1264–1269 (2013). https://doi.org/10.1016/j.jpeds.2012.11.070. 1269 e1261-1262
E. Peroni, M.C. Vigone, S. Mora, L.A. Bassi, C. Pozzi, A. Passoni, G. Weber, Congenital hypothyroidism treatment in infants: a comparative study between liquid and tablet formulations of levothyroxine. Horm. Res. Paediatr. 81(1), 50–54 (2014). https://doi.org/10.1159/000356047
A. Corbacioglu Esmer, A. Gul, H. Dagdeviren, I. Turan Bakirci, O. Sahin, Intrauterine diagnosis and treatment of fetal goitrous hypothyroidism. J. Obstet. Gynaecol. Res 39(3), 720–723 (2013). https://doi.org/10.1111/j.1447-0756.2012.02003.x
K.M. Davidson, D.S. Richards, D.A. Schatz, D.A. Fisher, Successful in utero treatment of fetal goiter and hypothyroidism. N. Engl. J. Med. 324(8), 543–546 (1991). https://doi.org/10.1056/NEJM199102213240807
M. Polak, D. Luton, Fetal thyroidology. Best practice & research. Clin. Endocrinol. Metab. 28(2), 161–173 (2014). https://doi.org/10.1016/j.beem.2013.04.013
P. Vasudevan, C. Powell, A.K. Nicholas, I. Scudamore, J. Greening, S.M. Park, N. Schoenmakers, Intrauterine death following intraamniotic triiodothyronine and thyroxine therapy for fetal goitrous hypothyroidism associated with polyhydramnios and caused by a thyroglobulin mutation. Endocrinol. Diabetes Metab. Case Rep. 2017 (2017). https://doi.org/10.1530/EDM-17-0040
A. Hauri-Hohl, N. Dusoczky, A. Dimitropoulos, R.H. Leuchter, L. Molinari, J. Caflisch, O.G. Jenni, B. Latal, Impaired neuromotor outcome in school-age children with congenital hypothyroidism receiving early high-dose substitution treatment. Pediatr. Res 70(6), 614–618 (2011). https://doi.org/10.1203/PDR.0b013e3182321128
J. Leger, Congenital hypothyroidism: a clinical update of long-term outcome in young adults. Eur. J. Endocrinol. 172(2), R67–R77 (2015). https://doi.org/10.1530/EJE-14-0777
L. Lichtenberger-Geslin, S. Dos Santos, Y. Hassani, E. Ecosse, T.Van Den Abbeele, J.Leger, Factors associated with hearing impairment in patients with congenital hypothyroidism treated since the neonatal period: a national population-based study. J. Clin. Endocrinol. Metab. 98(9), 3644–3652 (2013). https://doi.org/10.1210/jc.2013-1645
C.J. Eastman, M.B. Zimmermann, in The Iodine Deficiency Disorders. ed. by K.R. Feingold, B. Anawalt, A. Boyce, G. Chrousos, K. Dungan, A. Grossman, J.M. Hershman, G. Kaltsas, C. Koch, P. Kopp, M. Korbonits, R. McLachlan, J. E. Morley, M. New, L. Perreault, J. Purnell, R. Rebar, F. Singer, D.L. Trence, A. Vinik, D.P. Wilson (Endotext, South Dartmouth, MA, 2000)
M. Wasniewska, A. Corrias, M. Salerno, A. Mussa, D. Capalbo, M.F. Messina, T. Aversa, S. Bombaci, F. De Luca, M. Valenzise, Thyroid function patterns at Hashimoto’s thyroiditis presentation in childhood and adolescence are mainly conditioned by patients’ age. Horm. Res. Paediatr. 78(4), 232–236 (2012). https://doi.org/10.1159/000343815
P.A. Gibson, R.W. Newton, K. Selby, D.A. Price, K. Leyland, G.M. Addison, Longitudinal study of thyroid function in Down’s syndrome in the first two decades. Arch. Dis. Child. 90(6), 574–578 (2005). https://doi.org/10.1136/adc.2004.049536
S. McGowan, J. Jones, A. Brown, L. Reynolds, K. Leyland, P. Charleton, M. Rahim, M. Mansor, S. Ritha, M. Donaldson, Capillary TSH screening programme for Down’s syndrome in Scotland, 1997–2009. Arch. Dis. Child. 96(12), 1113–1117 (2011). https://doi.org/10.1136/archdischild-2011-300124
J.L. Frias, M.L. Davenport, Health supervision for children with Turner syndrome. Pediatrics 111(3), 692–702 (2003)
A. Gawlik, T. Gawlik, A. Januszek-Trzciakowska, H. Patel, E. Malecka-Tendera, Incidence and dynamics of thyroid dysfunction and thyroid autoimmunity in girls with Turner’s syndrome: a long-term follow-up study. Horm. Res. Paediatr. 76(5), 314–320 (2011). https://doi.org/10.1159/000331050
E. Piatkowska, M. Szalecki, Autoimmune thyroiditis in children and adolescents with type 1 diabetes. Pediatr. Endocrinol., diabetes, Metab. 17(4), 173–177 (2011)
S. Severinski, S. Banac, N.S. Severinski, V. Ahel, K. Cvijovic, Epidemiology and clinical characteristics of thyroid dysfunction in children and adolescents with type 1 diabetes. Coll. Antropol. 33(1), 273–279 (2009)
A. Rojas-Villarraga, J. Amaya-Amaya, A. Rodriguez-Rodriguez, R.D. Mantilla, J.M. Anaya, Introducing polyautoimmunity: secondary autoimmune diseases no longer exist. Autoimmune Dis. 2012, 254319 (2012). https://doi.org/10.1155/2012/254319
W. Chemaitilly, C.A. Sklar, Endocrine complications in long-term survivors of childhood cancers. Endocr.-Relat. Cancer 17(3), R141–R159 (2010). https://doi.org/10.1677/ERC-10-0002
M. Demirkaya, B. Sevinir, H. Saglam, L. Ozkan, O. Akaci, Thyroid functions in long-term survivors of pediatric Hodgkin’s lymphoma treated with chemotherapy and radiotherapy. J. Clin. Res. Pediatr. Endocrinol. 3(2), 89–94 (2011). https://doi.org/10.4274/jcrpe.v3i2.18
M. Weber Pasa, R. Selbach Scheffel, A. Borsatto Zanella, A.L. Maia, J.M. Dora, Consumptive hypothyroidism: case report of hepatic hemangioendotheliomas successfully treated with vincristine and systematic review of the syndrome. Eur. Thyroid J. 6(6), 321–327 (2017). https://doi.org/10.1159/000481253
S.A. Huang, H.M. Tu, J.W. Harney, M. Venihaki, A.J. Butte, H.P. Kozakewich, S.J. Fishman, P.R. Larsen, Severe hypothyroidism caused by type 3 iodothyronine deiodinase in infantile hemangiomas. N. Engl. J. Med. 343(3), 185–189 (2000). https://doi.org/10.1056/NEJM200007203430305
N. Jassam, T.J. Visser, T. Brisco, D. Bathia, P. McClean, J.H. Barth, Consumptive hypothyroidism: a case report and review of the literature. Ann. Clin. Biochem. 48(Pt 2), 186–189 (2011). https://doi.org/10.1258/acb.2010.010170
M.C. Vigone, F. Cortinovis, S. Rabbiosi, M. Di Frenna, A. Passoni, L. Persani, G. Chiumello, C. Gelmetti, G. Weber, Difficult treatment of consumptive hypothyroidism in a child with massive parotid hemangioma. J. Pediatr. Endocrinol. Metab. 25(1-2), 153–155 (2012)
J. Jonklaas, A.C. Bianco, A.J. Bauer, K.D. Burman, A.R. Cappola, F.S. Celi, D.S. Cooper, B.W. Kim, R.P. Peeters, M.S. Rosenthal, A.M. Sawka, American Thyroid Association Task Force on Thyroid Hormone, R.: guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid. 24(12), 1670–1751 (2014). https://doi.org/10.1089/thy.2014.0028
S.M. Cabrera, L.A. DiMeglio, E.A. Eugster, Incidence and characteristics of pseudoprecocious puberty because of severe primary hypothyroidism. J. Pediatr. 162(3), 637–639 (2013). https://doi.org/10.1016/j.jpeds.2012.10.043
N.S. Larson, J.E. Pinsker, Primary hypothyroidism with growth failure and pituitary pseudotumor in a 13-year-old female: a case report. J. Med Case Rep. 7, 149 (2013). https://doi.org/10.1186/1752-1947-7-149
T.D. Nebesio, M.D. Wise, S.M. Perkins, E.A. Eugster, Does clinical management impact height potential in children with severe acquired hypothyroidism? J. Pediatr. Endocrinol. Metab. 24(11-12), 893–896 (2011)
J.B. Quintos, M. Salas, Use of growth hormone and gonadotropin releasing hormone agonist in addition to L-thyroxine to attain normal adult height in two patients with severe Hashimoto’s thyroiditis. J. Pediatr. Endocrinol. Metab. 18(5), 515–521 (2005)
K.L. Durbin, T. Diaz-Montes, M.B. Loveless, Van wyk and grumbach syndrome: an unusual case and review of the literature. J. Pedia. Adolesc. Gynecol. 24(4), e93–e96 (2011). https://doi.org/10.1016/j.jpag.2010.08.003
K.S. Eom, C. See-Sung, J.D. Kim, J.M. Kim, T.Y. Kim, Primary hypothyroidism mimicking a pituitary macroadenoma: regression after thyroid hormone replacement therapy. Pediatr. Radiol. 39(2), 164–167 (2009). https://doi.org/10.1007/s00247-008-1012-9
A.S. Joshi, P.D. Woolf, Pituitary hyperplasia secondary to primary hypothyroidism: a case report and review of the literature. Pituitary 8(2), 99–103 (2005). https://doi.org/10.1007/s11102-005-3281-8
L. Gaspari, F. Paris, N. Leboucq, A. Bonafe, C. Sultan, Reversible growth failure and complete GH deficiency in a 4-year-old girl with very early Hashimoto’s thyroiditis and subsequent hyperplasia of pituitary thyrotroph cells. Eur. J. Pediatr. 175(8), 1119–1122 (2016). https://doi.org/10.1007/s00431-016-2698-6
T. Aversa, A. Corrias, M. Salerno, D. Tessaris, R. Di Mase, M. Valenzise, D. Corica, F. De Luca, M. Wasniewska, Five-year prospective evaluation of thyroid function test evolution in children with hashimoto’s thyroiditis presenting with either euthyroidism or subclinical hypothyroidism. Thyroid. Assoc. 26(10), 1450–1456 (2016). https://doi.org/10.1089/thy.2016.0080
G. Crisafulli, R. Gallizzi, T. Aversa, G. Salzano, M. Valenzise, M. Wasniewska, F. De Luca, G. Zirilli, Thyroid function test evolution in children with Hashimoto’s thyroiditis is closely conditioned by the biochemical picture at diagnosis. Ital. J. Pediatr. 44(1), 22 (2018). https://doi.org/10.1186/s13052-018-0461-5
R.K. Marwaha, N. Tandon, R. Kanwar, M.A. Ganie, V. Bhattacharya, D.H. Reddy, S. Gopalakrishnan, R. Aggarwal, K. Grewal, S.K. Ganguly, K. Mani, Evaluation of the role of ultrasonography in diagnosis of autoimmune thyroiditis in goitrous children. Indian Pediatr. 45(4), 279–284 (2008)
M.S. Durmaz, N. Akyurek, T. Kara, F. Ates, B. Ozbakir, F. G. Durmaz, S. S. Karaagac, M. Ozturk, Quantitative assessment of thyroid gland vascularization with vascularization index using color superb microvascular imaging in pediatric patients with hashimoto thyroiditis. Ultrasound Q (2019). https://doi.org/10.1097/RUQ.0000000000000430
S.H. Jeong, H.S. Hong, J.Y. Lee, The association between thyroid echogenicity and thyroid function in pediatric and adolescent Hashimoto’s thyroiditis. Med. (Baltim.) 98(14), e15055 (2019). https://doi.org/10.1097/MD.0000000000015055
J. Szczyrski, W. Kosiak, M. Korpal-Szczyrska, M. Mysliwiec, Ultrasound image of the thyroid gland in obese children. J. Ultrason 15(63), 423–428 (2015). https://doi.org/10.15557/JoU.2015.0039
A. Lollert, C. Gies, K. Laudemann, J. Faber, D. Jacob-Heutmann, J. Konig, C. Duber, G. Staatz, Ultrasound evaluation of thyroid gland pathologies after radiation therapy and chemotherapy to treat malignancy during childhood. Int J. Radiat. Oncol. Biol. Phys. 94(1), 139–146 (2016). https://doi.org/10.1016/j.ijrobp.2015.09.016
J.L. Williams, D.L. Paul, G. Bisset 3rd, Thyroid disease in children: part 1: state-of-the-art imaging in pediatric hypothyroidism. Pedia. Radio. 43(10), 1244–1253 (2013). https://doi.org/10.1007/s00247-013-2735-9
J. Jonklaas, A.C. Bianco, A.J. Bauer, K.D. Burman, A.R. Cappola, F.S. Celi, D.S. Cooper, B.W. Kim, R.P. Peeters, M.S. Rosenthal, A.M. Sawka, Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid. 24(12), 1670–1751 (2014). https://doi.org/10.1089/thy.2014.0028
J.F. Rovet, D. Daneman, J.D. Bailey, Psychologic and psychoeducational consequences of thyroxine therapy for juvenile acquired hypothyroidism. J. Pediatr. 122(4), 543–549 (1993). https://doi.org/10.1016/s0022-3476(05)83533-4
C. Van Dop, F.A. Conte, T.K. Koch, S.J. Clark, S.L. Wilson-Davis, M.M. Grumbach, Pseudotumor cerebri associated with initiation of levothyroxine therapy for juvenile hypothyroidism. N. Engl. J. Med. 308(18), 1076–1080 (1983). https://doi.org/10.1056/NEJM198305053081807
H. Gharib, R.M. Tuttle, H.J. Baskin, L.H. Fish, P.A. Singer, M.T. McDermott, Consensus statement #1: subclinical thyroid dysfunction: a joint statement on management from the American Association of Clinical Endocrinologists, the American Thyroid Association, and The Endocrine Society. Thyroid. 15(1), 24–28 (2005). https://doi.org/10.1089/thy.2005.15.24. response 32-23
P.B. Kaplowitz, Subclinical hypothyroidism in children: normal variation or sign of a failing thyroid gland? Int. J. Pediatr. Endocrinol. 2010, 281453 (2010). https://doi.org/10.1155/2010/281453
M.J. O’Grady, D. Cody, Subclinical hypothyroidism in childhood. Arch. Dis. Child. 96(3), 280–284 (2011). https://doi.org/10.1136/adc.2009.181800
M. Cerbone, C. Bravaccio, D. Capalbo, M. Polizzi, M. Wasniewska, D. Cioffi, N. Improda, M. Valenzise, D. Bruzzese, F. De Luca, M. Salerno, Linear growth and intellectual outcome in children with long-term idiopathic subclinical hypothyroidism. Eur. J. Endocrinol. 164(4), 591–597 (2011). https://doi.org/10.1530/EJE-10-0979
L.H. Duntas, L. Wartofsky, Cardiovascular risk and subclinical hypothyroidism: focus on lipids and new emerging risk factors. What is the evidence? Thyroid. 17(11), 1075–1084 (2007). https://doi.org/10.1089/thy.2007.0116
O. Akin, Morning vs. bedtime levothyroxine administration: what is the ideal choice for children? J. Pediatr. Endocrinol. Metab. 31(11), 1249–1255 (2018). https://doi.org/10.1515/jpem-2018-0168
K.D. Coyne, K.A. Trimble, A. Lloyd, L. Petrando, J. Pentz, K. Van Namen, A. Fawcett, C.M. Laing, Interventions to promote oral medication adherence in the pediatric chronic illness population: a systematic review from the Children’s Oncology Group. J. Pedia. Oncol. Nurs. 36(3), 219–235 (2019). https://doi.org/10.1177/1043454219835451
P. Colucci, C.S. Yue, M. Ducharme, S. Benvenga, A review of the pharmacokinetics of levothyroxine for the treatment of hypothyroidism. Eur. Endocrinol. 9(1), 40–47 (2013). https://doi.org/10.17925/EE.2013.09.01.40
R. Nanan, J.R. Wall, Remission of Hashimoto’s thyroiditis in a twelve-year-old girl with thyroid changes documented by ultrasonography. Thyroid. 20(10), 1187–1190 (2010). https://doi.org/10.1089/thy.2010.0102
G.J. Kahaly, D. Schuppan, Celiac disease and endocrine autoimmunity. Dig. Dis. 33(2), 155–161 (2015). https://doi.org/10.1159/000369535
M.I. Liontiris, E.E. Mazokopakis, A concise review of Hashimoto thyroiditis (HT) and the importance of iodine, selenium, vitamin D and gluten on the autoimmunity and dietary management of HT patients.Points that need more investigation. Hell. J. Nucl. Med. 20(1), 51–56 (2017). https://doi.org/10.1967/s002449910507
M.P. Rayman, Multiple nutritional factors and thyroid disease, with particular reference to autoimmune thyroid disease. Proc. Nutr. Soc. 78(1), 34–44 (2019). https://doi.org/10.1017/S0029665118001192
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A.J.B. received financial support and an honoraria from Sandoz AG and is a member of the American Thyroid Association board of directors. A.W. reports no conflicts of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bauer, A.J., Wassner, A.J. Thyroid hormone therapy in congenital hypothyroidism and pediatric hypothyroidism. Endocrine 66, 51–62 (2019). https://doi.org/10.1007/s12020-019-02024-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-019-02024-6