Abstract
Purpose
Metabolic syndrome (MetS) has been extensively studied for its long-term health effects, typically through conventional Cox proportional hazards regression modeling of the overall association of MetS with a single outcome. Such an approach neglects the inherent links between MetS-related disease outcomes and fails to provide sufficient insights into the impact of each component of MetS over time.
Methods
We therefore conducted a retrospective cohort study of 63,680 individuals who received health check-ups at the MJ Health Screening Center in Taiwan from 1997–2005 to study the subsequent risks of hypertension, type 2 diabetes mellitus (T2DM) and chronic kidney disease (CKD) simultaneously for MetS and its components. Multivariate-adjusted hazard ratios (HRs) were calculated using Cox models for multiple failure outcomes.
Results
At baseline, MetS was identified in 7835 participants. Over a median follow-up of 3 years, 8252, 1634, and 6714 participants developed hypertension, T2DM and CKD, respectively. The HR for MetS was 2.41 (95% CI 2.29–2.53) for hypertension, 5.17 (95% CI 4.68–5.71) for T2DM and 1.22 (95% CI 1.15–1.31) for CKD. Three MetS components showed the strongest association with each of the outcomes: elevated blood pressure with hypertension (HR = 3.62, 95% CI 3.46–3.79), raised fasting plasma glucose with T2DM (HR = 8.89, 95% CI 7.86–10.06) and elevated triglycerides with CKD (HR = 1.14, 95% CI 1.08–1.21).
Conclusions
MetS may help identify individuals with metabolic profiles that confer incremental risks for multiple diseases. Additionally, several components of the syndrome should be considered by clinicians, as they show stronger associations with specific diseases than MetS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metabolic syndrome (MetS) is a common complex of metabolic disorders characterized by abdominal obesity, insulin resistance (IR), dyslipidemia, and hypertension [1]. Since MetS was first described in 1988, many definitions have been published by different organizations [2]. Of these, the 2001 Third Report of the National Cholesterol Education Program Adult Treatment Panel (NCEP ATP III) is the most widely used [3].
Although the prevalence of MetS in the same population may vary dramatically depending on the specific diagnostic criteria considered [4], epidemiological data show that MetS is an increasingly important public health issue. Among American adults, the prevalence of MetS increases with age, reaching 18.3% in people aged 20–39 and 46.7% in those 60 or older [5]. In mainland China, its overall prevalence is 19.2% for males and 27.0% for females; this rate is also higher in older age groups [6].
Previous studies have demonstrated that MetS is a constellation of metabolic risk factors for cardiovascular disease, type 2 diabetes mellitus (T2DM), chronic kidney disease (CKD), and all-cause mortality [7,8,9]; however, these outcomes tended to be investigated separately through conventional Cox models, in which the correlations between outcomes were not accounted for. In fact, it is common for MetS-related outcomes to occur in an individual patient during long-term follow-up. Considering that the occurrence of one outcome is very likely to trigger the risk set for subsequent outcomes, failure to control the correlations between different outcomes may lead to a biased estimate of the effect size of MetS. Therefore, utilizing any event times with multiple outcomes in the analysis is more desirable [10].
In addition, the focus of previous studies was more on MetS as a whole than on its components, but for disease prevention, all adverse metabolic traits should be individually and aggressively treated regardless of whether MetS is present. Hence, the disease risk of each MetS component might provide more useful information than the MetS diagnosis itself.
Given previous limitations, this study aimed to simultaneously estimate the future risks of long-term health outcomes (including hypertension, T2DM, and CKD) related to both MetS and its components.
Materials and methods
Study population
This is a retrospective cohort study, and the data were collected from the MJ Health Screening Center in Taiwan. As a membership-oriented private institute with four health check-up clinics (Taipei, Taoyuan, Taichung, and Kaohsiung) around Taiwan, the center provides periodic health examinations to its members. The demographic profiles of the Taiwanese adults who attended the center were similar to those of the general Taiwanese population [11].
The cohort consisted of all participants aged 35–74 who received health check-ups at the center from January 1997–December 2005. Eligible participants required at least one subsequent health examination before December 2006. Participants were excluded if they had hypertension, T2DM or CKD at study entry or had unavailable data for variables used in the analyses, including age, sex, smoking status, alcohol consumption, physical activity, hypertension family history, and T2DM family history.
This study has been ethically approved by the Peking University Institutional Review Board with a waiver of informed consent. The database used in this study was anonymous. All or part of the data used in this research were authorized by and received from the MJ Health Research Foundation (authorization code: MJHRFB2014003C). Any interpretation or conclusion described in this paper does not represent the views of the MJ Health Research Foundation.
Data collection
Participants had a series of biochemical tests for blood and urine, a physical examination, and anthropometric measurements. Overnight fasting blood samples were collected and analyzed (Hitachi 7150 auto-analyzers, Tokyo, Japan), with serum creatinine measured by the uncompensated Jaffe method with the alkaline picrate kinetic test [12]. The Roche Miditron M semi-automated computer-assisted urinalysis system (Combur-10 test M dipstick, Basel, Switzerland) was used for urinary protein; the result was reported as normal, trace, 1+, 2+, 3+, and 4+. Systolic and diastolic blood pressures (BP) were measured twice in the right arm after 10 min rest, and means were calculated. Waist circumference (WC) was measured at the end of a normal expiration at the level of noticeable waist narrowing between the lowest aspect of the rib cage and the highest point of the iliac crest. When narrowing could not be determined, the circumference was measured at the level of the umbilicus. Height was measured to the nearest 0.5 cm and weight to the nearest 0.1 kg. Body mass index (BMI) was calculated as weight in kilograms divided by square of height in meters; participants were classified as underweight (BMI < 18.5 kg/m2), normal (18.5 ≤ BMI < 25 kg/m2), overweight (25 ≤ BMI < 30 kg/m2) and obese (BMI ≥ 30 kg/m2), based on the World Health Organization (WHO) classification system for Asian populations [13].
Each participant completed a self-administered questionnaire at the time of screening to gather information on socio-demographic characteristics, lifestyle features, and antihypertensive and antidiabetic medication, as well as on personal and family history of major chronic diseases. Physical activity was categorized from self-reported data as seldom, sometimes, often and always; smoking status was current, former and non-smoker; alcohol consumption was current, former and non-drinker; and education was schooling for less than 12 years versus ≥ 12 years.
The achievement of data standardization was possible by identical screening procedures with the same model of instruments and formal training and certification of the health practitioners in all four clinics. A more detailed description of the MJ Health Screening data has been documented elsewhere [11, 14, 15].
Definitions of MetS
In this study, MetS was identified based on the modified NCEP ATP III criteria with the Asian cut-off for WC [3, 16]. The diagnosis of MetS was made when three or more of the following criteria were present: (i) central obesity with WC ≥ 80 cm in females and ≥90 cm in males; (ii) triglyceride (TG) ≥ 1.7 mmol/L; (iii) high-density lipoprotein cholesterol (HDL-C) < 1.3 mmol/L in females and <1.04 mmol/L in males; (iv) systolic blood pressure (SBP) ≥ 130 mmHg or diastolic blood pressure (DBP) ≥ 85 mmHg; and (v) fasting plasma glucose (FPG) ≥ 6.1 mmol/L.
Definitions of outcomes
Participants were defined as having T2DM if they had a history of T2DM or if they had positive diabetes screening results (FPG ≥ 7 mmol/L), with the exclusion of type 1 diabetes mellitus [15]. Likewise, hypertension was determined by medical history or positive screening results (SBP ≥ 140 mmHg or DBP ≥ 90 mmHg) [17]. CKD was defined as the presence of either proteinuria (urinary protein ≥ 1+) or the estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73 m2 [18, 19]. eGFR was calculated using the 4-variable Modification of Diet in Renal Disease Study equation (where Scr is serum creatinine level): eGFR (ml/min/1.73 m2) = 186.3 × Scr (mg/dl)−1.154 × age (years)−0.203 × (0.742 if female) [20].
Statistical analysis
Differences in baseline characteristics according to the presence or absence of MetS were compared by the use of two-sided Student’s t-tests and chi-square tests for continuous and categorical variables, respectively. Distribution of MetS individual components among participants with a different number of components was described. Incidence densities of hypertension, T2DM, and CKD were calculated.
Hazard ratios (HRs) and 95% confidence intervals (CIs) from Cox regression of multiple failure times were used to estimate relative risks for incident hypertension, T2DM, and CKD by baseline MetS status. Proportional hazards models for unordered outcomes of different types (i.e., hypertension, T2DM, and CKD) were stratified by outcome and fitted to identify the stratum-specific effects of MetS, accounting for observations of the same patient among strata through the use of robust variance methods [21]. In these models, all participants at baseline were simultaneously assumed to be at risk for all outcomes. Each outcome was allowed to occur only once per participant. When participants experienced one of the outcomes, they were still considered at risk for the other two outcomes. Covariates at baseline considered for adjustment included age, sex, smoking status, alcohol consumption, physical activity, hypertension family history, and T2DM family history. In addition, the associations between individual components of MetS (central obesity, elevated TG, low HDL-C, elevated BP, and elevated FPG) and the development of hypertension, T2DM and CKD were also investigated by performing the above analyses. Proportional hazards assumptions were checked graphically with Kaplan-Meier survival curves, with the crossing of survival curves indicating departure from the assumption. Values of P < 0.05 from 2-sided tests were considered statistically significant. All data analysis was conducted in SAS 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Cohort formation and baseline characteristics
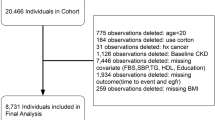
A total of 242,026 participants aged 35–74 underwent health screening during the cohort entry period. Of these participants, 63,680 were included in the final cohort. A flow chart depicting the formation of the cohort is shown in Fig. 1.
Of the cohort, 7835 (12.30%) had MetS at baseline based on the modified NCEP-ATP III criteria. These participants were older, had lower education levels and were more likely to smoke or drink alcohol (Table 1). Generally, the levels of biochemical test results, anthropometric measurements and blood pressure were higher in participants with MetS than in those without; the exceptions were HDL-C and eGFR, which were higher in the latter group.
Prevalence of MetS components
Of the individual MetS components, the prevalence of low HDL-C was the highest in the whole cohort, while elevated BP was the lowest (Table 2). When participants were stratified by sex and MetS status, low HDL-C had the highest prevalence (83.66%) compared with other components among female participants with MetS, whereas elevated TG was the highest (79.16%) in their male counterparts.
Table 3 shows the prevalence of MetS components among participants with different numbers of components. Compared with other components, the prevalence of low HDL-C was the highest across all strata, ranging from 35.89 to 88.24%. Among participants presenting more than one component, the prevalence of elevated BP was the lowest of all components. Central obesity, however, was the lowest when there was only one component.
The relations between individual components of MetS are shown in Table A in Online Resource 1. A total of 48.31% participants with central obesity had a low level of HDL-C at the same time. Approximately 54.94% participants with elevated TG also had a low level of HDL-C. There were 38.14% participants with elevated BP suffering from elevated FPG.
Incidence of hypertension, T2DM, and CKD
Over a median follow-up of 3 years, 8252 (12.96%), 1634 (2.57%), and 6714 (10.54%) participants developed hypertension, T2DM, and CKD, respectively. Incidence densities of the three outcomes, stratified by the presence of MetS and its components, are shown in Table 4. Among participants with MetS, the incidence densities of hypertension, T2DM, and CKD were 88.2, 26.5, and 39.0 per 1000 person-years, respectively, all of which were higher than those among participants without the syndrome. When participants were stratified by MetS components, the highest incidence densities of hypertension and CKD were identified in participants with elevated TG, while the elevated FPG group had the highest incidence density of T2DM.
Associations of MetS with incident hypertension, T2DM, and CKD
Kaplan-Meier survival curves stratified by baseline MetS status are shown in Figure A in Online Resource 1. Proportional hazards assumptions were verified due to the absence of crossing survival curves. Different strength estimates of associations between MetS and incident hypertension, T2DM, and CKD were observed (Table 5). Specifically, HR was strongest for T2DM (Model 1 in Table 5; HR = 6.58, 95% CI 5.97–7.25). The HRs for hypertension and CKD were 3.02 (95% CI 2.88–3.17) and 1.42 (95% CI 1.33–1.51), respectively. These incremental risks decreased but remained significant after adjustments for age, sex, and other classical risk factors (Model 3 in Table 5). Overall, compared with those without MetS, participants with MetS showed a 2.4-fold increased risk for hypertension (HR = 2.41, 95% CI 2.29–2.53), a 5.2-fold increased risk for T2DM (HR = 5.17, 95% CI 4.68–5.71) and a 1.2-fold increased risk for CKD (HR = 1.22, 95% CI 1.15–1.31).
Associations of MetS components with incident hypertension, T2DM, and CKD
Table 6 shows the associations of MetS components with the outcomes of interest. After adjusting for individual components (with the one being studied excluded from the adjustment), three different components of MetS showed the strongest association for each outcome of interest (Model 1 in Table 6): specifically, elevated BP for hypertension (HR = 4.23, 95% CI 4.04–4.42), elevated FPG for T2DM (HR = 9.87, 95% CI 8.74–11.16) and central obesity for CKD (HR = 1.24, 95% CI 1.17–1.32). These relations persisted after adjusting for age, sex, and other classical risk factors, apart from CKD, for which elevated TG presented a greater increased risk (HR = 1.14, 95% CI 1.08–1.21) than central obesity (Model 3 in Table 6).
Discussion
Across the study population, MetS independently contributed to increased risks of hypertension, T2DM, and CKD (2.4-fold, 5.2-fold, and 1.2-fold, respectively). The most significant MetS component associated with each of the outcomes was elevated BP for hypertension, elevated FPG for T2DM and elevated TG for CKD. Individuals at high risk for hypertension, T2DM, and CKD in a general population might be easily recognized using a medical check-up when MetS is presented. Even for those who do not yet satisfy the full MetS criteria, having some of the MetS components would still yield incremental risks of developing these disease outcomes later in life.
According to our study, MetS conferred a considerable risk for developing hypertension in non-diabetic and non-hypertensive individuals; however, elevated BP at baseline was found to be a more powerful predictor than the presence of MetS. This association is consistent with observations from other longitudinal studies conducted among Asian populations [22,23,24]. As elevated BP may represent a status of prehypertension at baseline, it seems natural to have observed its close relationship with hypertension; however, in the present study, all the other components of MetS were also found to be independently associated with an incremental risk of future hypertension. In light of the modifiable nature of the components related to incident hypertension, this finding might have important implications for facilitating hypertension control.
In the present study, MetS had a much greater association with T2DM than with hypertension or CKD, which strongly indicates that MetS originated in the diabetes field and can be used as a predictor of T2DM [25]. Moreover, although elevated FPG was found to be the strongest predictor of T2DM with an 8.9-fold increased risk, central obesity, for which visceral fat is largely responsible, was considered mechanistically as the key driver for T2DM onset [26]. IR, commonly believed to originate from central obesity, has been proposed to play a central role in the pathogenesis of T2DM [27]. Whereas BMI is based on a measurement of total mass, WC reflects central obesity and correlates well with the amount of visceral fat [28]. The current study coherently identifies the high priority of this component by showing a more than doubled risk for T2DM independent of an elevated FPG in both women with a WC ≥ 80 cm and men with a WC ≥ 90 cm. These findings might change clinical practice guidelines and could indicate that WC control should be prioritized to the same extent as plasma glucose level control in T2DM prevention, especially in populations where obesity is epidemic.
Because of the substantial overlap among the clinical features of MetS, hypertension, and T2DM, the CKD risk in individuals with MetS may reflect the presence of hypertension and T2DM, which are known risk factors for CKD, rather than an independent effect [29]. With the elimination of hypertension and T2DM patients at baseline, and with the use of Cox models of multiple failure outcomes, we found that MetS independently contributed to the risk of CKD initiation, confirming the findings of previous studies [29, 30]. However, the mechanisms underlying these associations are not well documented. IR, a prominent trait of MetS, may lead to a progressive loss of renal function by worsening renal hemodynamics through multiple pathways, including activation of the sympathetic nervous system, sodium retention, and down-regulation of the natriuretic peptide system [31].
Unlike previous studies in which central obesity was the MetS component with the strongest association with CKD [32, 33], we found that dyslipidemia (including elevated TG and a low HDL-C level) was the most strongly associated risk factor. Several reasons could explain this variation. First, the prevalence of central obesity was relatively low (16.4%) in the studied population, which could limit our statistical power to detect the contribution of central obesity on the CKD risk. Second, the follow-up time in our study was relatively short, with a median of 3 years, which might not be long enough to demonstrate the correlation effect. Finally, the present study considered potential confounders that could have influenced the relationship between central obesity and CKD and therefore may have obtained a more accurate estimate of HR.
The baseline data showed that MetS is common in Taiwan, with a crude prevalence of 16.16% in males and 8.94% in females; there was also a clustering of metabolic disorders proven to be interrelated. Each MetS component may develop from the common base that is linked to both genetic factors and lifestyle. Thus, the components of the syndrome are not clustered coincidentally but instead form a series of related metabolic disorders tending to co-occur within the same individual [23]. Elevated BP has frequently been the key component cited for the presence of MetS in previous studies [34, 35]. However, we found that low HDL-C had the highest prevalence among all components, which might be a result of the exclusion of hypertensive patients at baseline.
Our study has a number of strengths. To the best of our knowledge, this is the first study to investigate the future risks of hypertension, T2DM, and CKD in relation to MetS and its components simultaneously in the same population, using Cox proportional hazards models of multiple failure outcomes. Such an investigation allows not only a head-to-head comparison over the long-term health effects of different metabolic profiles but also a more precise estimate of the effect size than previous studies examining only one single outcome. In addition, the study population is large and well characterized. With thorough health check-up data, we were able to adjust for a variety of important confounders in the modeling process.
There are also some potential limitations to consider in this study. First, participants in this cohort who underwent regular health exams were relatively healthy, which might lead to a selection bias and subsequent underestimation of risks. Second, given that measurements of proteinuria and serum creatinine suffer from a high degree of intra-individual variability [30], CKD may be misclassified in the present study due to our dependence on proteinuria and a glomerular filtration rate estimated using a serum creatinine-based equation. Such misclassification would have attenuated the relative risk of incident CKD associated with MetS and its components. However, we still observed an incremental risk on CKD development in individuals with MetS, central obesity, and dyslipidemia. Moreover, due to the observational nature of this study, we cannot prove that the observed associations were causal. Therefore, further investigations are needed to determine the effect of the control of metabolic disorders, including MetS and all of its components, on the likelihood of developing hypertension, T2DM, and CKD.
In conclusion, MetS increases the risks of future hypertension, T2DM, and CKD. Moreover, individual components of this syndrome (i.e., elevated BP and FPG) have stronger associations with outcomes of interest (hypertension and T2DM, respectively) than the presence of MetS. Therefore, MetS may help identify individuals with metabolic profiles that confer substantial risks for multiple diseases in general practice and therefore provide auxiliary value in disease prediction and prevention; specific MetS components should also be considered in this process.
References
C.K. Roberts, A.L. Hevener, R.J. Barnard, Metabolic syndrome and insulin resistance: underlying causes and modification by exercise training. Compr. Physiol. 3(1), 1–58 (2013). https://doi.org/10.1002/cphy.c110062
J. Kaur, A comprehensive review on metabolic syndrome. Cardiol. Res. Pract. (2014). https://doi.org/10.1155/2014/943162
National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults: Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 106(25), 3143–3421 (2002)
C. Day, Metabolic syndrome, or what you will: definitions and epidemiology. Diab. Vasc. Dis. Res. 4(1), 32–38 (2007). https://doi.org/10.3132/dvdr.2007.003
M. Aguilar, T. Bhuket, S. Torres, B. Liu, R.J. Wong, Prevalence of the metabolic syndrome in the United States, 2003-2012. JAMA 313(19), 1973–1974 (2015). https://doi.org/10.1001/jama.2015.4260
R. Li, W. Li, Z. Lun, H. Zhang, Z. Sun, J.S. Kanu, S. Qiu, Y. Cheng, Y. Liu, Prevalence of metabolic syndrome in Mainland China: a meta-analysis of published studies. BMC Public Health 16, 296 (2016). https://doi.org/10.1186/s12889-016-2870-y
P.W. Wilson, R.B. D’Agostino, H. Parise, L. Sullivan, J.B. Meigs, Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 112(20), 3066–3072 (2005). https://doi.org/10.1161/CIRCULATIONAHA.105.539528
J. Chen, P. Muntner, L.L. Hamm, D.W. Jones, V. Batuman, V. Fonseca, P.K. Whelton, J. He, The metabolic syndrome and chronic kidney disease in U.S. adults. Ann. Intern. Med. 140(3), 167–174 (2004)
S.H. Wu, Z. Liu, S.C. Ho, Metabolic syndrome and all-cause mortality: a meta-analysis of prospective cohort studies. Eur. J. Epidemiol. 25(6), 375–384 (2010). https://doi.org/10.1007/s10654-010-9459-z
H. Putter, M. Fiocco, R.B. Geskus, Tutorial in biostatistics: competing risks and multi-state models. Stat. Med. 26(11), 2389–2430 (2007). https://doi.org/10.1002/sim.2712
Y.H. Shen, W.S. Yang, T.H. Lee, L.T. Lee, C.Y. Chen, K.C. Huang, Bright liver and alanine aminotransferase are associated with metabolic syndrome in adults. Obes. Res. 13(7), 1238–1245 (2005). https://doi.org/10.1038/oby.2005.147
G.L. Myers, W.G. Miller, J. Coresh, J. Fleming, N. Greenberg, T. Greene, T. Hostetter, A.S. Levey, M. Panteghini, M. Welch, J.H. Eckfeldt, Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin. Chem. 52(1), 5–18 (2006). https://doi.org/10.1373/clinchem.2005.0525144
WHO Expert Consultation: Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 363(9403), 157–163 (2004). https://doi.org/10.1016/S0140-6736(03)15268-3
C.P. Wen, T.Y. Cheng, M.K. Tsai, Y.C. Chang, H.T. Chan, S.P. Tsai, P.H. Chiang, C.C. Hsu, P.K. Sung, Y.H. Hsu, S.F. Wen, All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462 293 adults in Taiwan. Lancet 371(9631), 2173–2182 (2008). https://doi.org/10.1016/S0140-6736(08)60952-6
C.P. Wen, J.P. Wai, M.K. Tsai, Y.C. Yang, T.Y. Cheng, M.C. Lee, H.T. Chan, C.K. Tsao, S.P. Tsai, X. Wu, Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet 378(9798), 1244–1253 (2011). https://doi.org/10.1016/S0140-6736(11)60749-6
C.E. Tan, S. Ma, D. Wai, S.K. Chew, E.S. Tai, Can we apply the National Cholesterol Education Program Adult Treatment Panel definition of the metabolic syndrome to Asians? Diabetes Care 27(5), 1182–1186 (2004)
National Institute for Health and Clinical Excellence: Hypertension: clinical management of primary hypertension in adults. https://www.nice.org.uk/guidance/cg127/chapter/1-Guidance (2011). Accessed 22 Nov 2017
A.S. Levey, J. Coresh, E. Balk, A.T. Kausz, A. Levin, M.W. Steffes, R.J. Hogg, R.D. Perrone, J. Lau, G. Eknoyan, National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann. Intern. Med. 139(2), 137–147 (2003)
A.S. Levey, P.E. de Jong, J. Coresh, M. El Nahas, B.C. Astor, K. Matsushita, R.T. Gansevoort, B.L. Kasiske, K.U. Eckardt, The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 80(1), 17–28 (2011). https://doi.org/10.1038/ki.2010.483
G. Bellomo, S. Venanzi, C. Verdura, P. Saronio, A. Esposito, M. Timio, Association of uric acid with change in kidney function in healthy normotensive individuals. Am. J. Kidney Dis. 56(2), 264–272 (2010). https://doi.org/10.1053/j.ajkd.2010.01.019
L.J. Wei, D.Y. Lin, L. Weissfeld, Regression analysis of multivariate incomplete failure time data by modeling marginal distributions. J. Am. Stat. Assoc. 408(84), 1065–1073 (1989)
B.M. Cheung, N.M. Wat, Y.B. Man, S. Tam, C.H. Cheng, G.M. Leung, J. Woo, E.D. Janus, C.P. Lau, T.H. Lam, K.S. Lam, Relationship between the metabolic syndrome and the development of hypertension in the Hong Kong Cardiovascular Risk Factor Prevalence Study-2 (CRISPS2). Am. J. Hypertens. 21(1), 17–22 (2008). https://doi.org/10.1038/ajh.2007.19
H. Takase, Y. Dohi, T. Toriyama, T. Okado, S. Tanaka, K. Sato, G. Kimura, Metabolic disorders predict development of hypertension in normotensive Japanese subjects. Hypertens. Res. 31(4), 665–671 (2008). https://doi.org/10.1291/hypres.31.665
K. Lu, R. Ding, L. Wang, S. Wu, J. Chen, D. Hu, Association between prevalence of hypertension and components of metabolic syndrome: the data from Kailuan community. Clin. Exp. Hypertens. 37(4), 303–307 (2015). https://doi.org/10.3109/10641963.2014.960973
E.S. Ford, Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care 28(7), 1769–1778 (2005)
K. Dragsbaek, J.S. Neergaard, J.M. Laursen, H.B. Hansen, C. Christiansen, H. Beck-Nielsen, M.A. Karsdal, S. Brix, K. Henriksen, Metabolic syndrome and subsequent risk of type 2 diabetes and cardiovascular disease in elderly women: challenging the current definition. Medicine 95(36), e4806 (2016). https://doi.org/10.1097/MD.0000000000004806
U.J. Jung, M.S. Choi, Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 15(4), 6184–6223 (2014). https://doi.org/10.3390/ijms15046184
O. Hamdy, S. Porramatikul, E. Al-Ozairi, Metabolic obesity: the paradox between visceral and subcutaneous fat. Curr. Diabetes Rev. 2(4), 367–373 (2006)
J.R. Schelling, J.R. Sedor, The metabolic syndrome as a risk factor for chronic kidney disease: more than a fat chance? J. Am. Soc. Nephrol. 15(11), 2773–2774 (2004). https://doi.org/10.1097/01.asn.0000141964.68839.bb
H.T. Cheng, J.W. Huang, C.K. Chiang, C.J. Yen, K.Y. Hung, K.D. Wu, Metabolic syndrome and insulin resistance as risk factors for development of chronic kidney disease and rapid decline in renal function in elderly. J. Clin. Endocrinol. Metab. 97(4), 1268–1276 (2012). https://doi.org/10.1210/jc.2011-2658
B. Spoto, A. Pisano, C. Zoccali, Insulin resistance in chronic kidney disease: a systematic review. Am J Physiol. Renal physiol. (2016). https://doi.org/10.1152/ajprenal.00340.2016
J. Chen, D. Gu, C.S. Chen, X. Wu, L.L. Hamm, P. Muntner, V. Batuman, C.H. Lee, P.K. Whelton, J. He, Association between the metabolic syndrome and chronic kidney disease in Chinese adults. Nephrol. Dial. Transplant. 22(4), 1100–1106 (2007). https://doi.org/10.1093/ndt/gfl759
A. Maleki, M. Montazeri, N. Rashidi, M. Montazeri, E. Yousefi-Abdolmaleki, Metabolic syndrome and its components associated with chronic kidney disease. J. Res. Med. Sci. 20(5), 465–469 (2015)
O. Sidorenkov, O. Nilssen, T. Brenn, S. Martiushov, V.L. Arkhipovsky, A.M. Grjibovski, Prevalence of the metabolic syndrome and its components in Northwest Russia: the Arkhangelsk study. BMC Public Health 10, 23 (2010). https://doi.org/10.1186/1471-2458-10-23
J.V. van Vliet-Ostaptchouk, M.L. Nuotio, S.N. Slagter, D. Doiron, K. Fischer, L. Foco, A. Gaye, M. Gogele, M. Heier, T. Hiekkalinna, A. Joensuu, C. Newby, C. Pang, E. Partinen, E. Reischl, C. Schwienbacher, M.L. Tammesoo, M.A. Swertz, P. Burton, V. Ferretti, I. Fortier, L. Giepmans, J.R. Harris, H.L. Hillege, J. Holmen, A. Jula, J.E. Kootstra-Ros, K. Kvaloy, T.L. Holmen, S. Mannisto, A. Metspalu, K. Midthjell, M.J. Murtagh, A. Peters, P.P. Pramstaller, T. Saaristo, V. Salomaa, R.P. Stolk, M. Uusitupa, P. van der Harst, M.M. van der Klauw, M. Waldenberger, M. Perola, B.H. Wolffenbuttel, The prevalence of metabolic syndrome and metabolically healthy obesity in Europe: a collaborative analysis of ten large cohort studies. BMC Endocr. Disord. 14, 9 (2014). https://doi.org/10.1186/1472-6823-14-9
Acknowledgements
This study was supported by the National Natural Science Foundation of China (grant number: 71673003) and the Interdisciplinary Fund from Peking University (grant number: BMU20160581).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
For this type of study formal consent is not required.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Ding, C., Yang, Z., Wang, S. et al. The associations of metabolic syndrome with incident hypertension, type 2 diabetes mellitus and chronic kidney disease: a cohort study. Endocrine 60, 282–291 (2018). https://doi.org/10.1007/s12020-018-1552-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-018-1552-1