Abstract
Purpose
We aimed to investigate the relation between preoperative serum thyrotrophin (TSH) and clinicopathological features in patients with papillary thyroid carcinoma (PTC) and microcarcinoma (PTMC).
Methods
Patients who underwent thyroidectomy and diagnosed to have benign nodular disease or PTC/PTMC in our clinic were evaluated retrospectively. Patients with a previous history of thyroid surgery, patients using antithyroid medications or thyroid hormone and patients with tumors known to be unresponsive to TSH were excluded.
Results
Data of 1632 patients were analyzed. Histopathological diagnosis was benign in 969 (59.4%) and malignant in 663 (40.6%) patients. Preoperative median serum TSH was significantly higher in malignant compared to benign group (1.41 IU/dL vs. 0.98 IU/dL, p < 0.001). Malignancy risk increased gradually as going from hyperthyroidism to euthyroidism and hypothyroidism (20, 40.6, and 59.1%, respectively, p < 0.05). Serum TSH was lowest in benign nodular disease, higher in PTMC and highest in PTC (p < 0.001). This was also true when patients with positive antithyroid peroxidase/antithyroglobulin and with lymphocytic thyroiditis were excluded from the analysis (p < 0.001). Serum TSH was higher in patients with bilateral tumor, capsular invasion and lymph node metastasis (LNM) compared to patients with unilateral tumor, without capsule invasion and without LNM, respectively (p = 0.036, p = 0.002, and p = 0.001, respectively). Patients with aggressive variant PTC had higher serum TSH than nonaggressive ones (p < 0.05).
Conclusion
Preoperative serum TSH is associated with PTMC, PTC and LNM. Serum TSH seems to be related with thyroid cancer regardless of autoimmunity. With the present study, for the first time, we showed an association between serum TSH and aggressive variants of PTC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thyroid cancer which accounts for about 1% of all neoplasms is the most common malignancy of the endocrine system. The incidence is gradually increasing in both men and women owing to various reasons such as increased use of imaging methods and early detection of tumors ≤ 1 cm which are called microcarcinomas [1]. The prognosis of differentiated thyroid cancer (DTC) is generally good, while a small ratio of patients might have persistent or recurrent disease, and a minority die from thyroid cancer [2]. Thus, accurate risk stratification is important to determine the patients who needs more aggressive therapy [2, 3]. Because thyrotrophin (TSH) is a well known stimulant for thyroid cells, TSH suppression is recommended in many patients with DTC after thyroidectomy and radioiodine ablation treatment [1, 4]. This approach was shown to reduce recurrence [5] and increase disease-specific survival [6].
In many previous studies, serum TSH was presented as an independent predictor for the diagnosis of thyroid malignancy [4]. Higher TSH values, even within normal ranges, were reported to be associated with a higher risk of thyroid cancer and more advanced disease [7]. However, the role of TSH in the development and/or the progression of thyroid cancer is not clear, yet [1, 8]. More evidence is required to display the possible effects of serum TSH as a risk factor for thyroid carcinoma.
In this study, we firstly aimed to determine the association between preoperative serum TSH and papillary thyroid cancer (PTC) including microcarcinomas. Additionaly, we tried to investigate whether a relation between serum TSH and clinicopathological features and variants of PTC exists.
Materials and methods
Medical records of patients who underwent thyroidectomy and had a histopathological diagnosis of benign thyroid disease or PTC between December 2006 and September 2014 were reviewed retrospectively. Patients with unilateral resection, patients with TSH unresponsive thyroid neoplasms (medullary and anaplastic thyroid cancer and thyroid lymphoma) and patients with a history of radiotherapy to head and neck region were excluded as well as patients with a history of antithyroid or thyroid hormone replacement therapy. The data of 1632 patients with preoperative nodular thyroid disease were analyzed. Local ethical committee approval was obtained in accordance with the ethical standards of Helsinki declaration.
Sex, age, preoperative serum TSH, free triiodothyronine (fT3), free thyroxine (fT4), anti-thyroglobulin antibody (anti-TgAb), and antithyroid peroxidase antibody (anti-TPOAb), presence of histopathologically confirmed lymphocytic thyroiditis were evaluated in each patient. Chemiluminescence methods (Immulite 2000, Diagnostic Products Corp., Los Angeles, CA, USA and UniCel DXI 800, Beckman Coulter, Brea, CA) were used for measurement of serum TSH, fT3 and fT4, anti-TPOAb, and anti-TGAb levels. The normal ranges for TSH, fT3, fT4, anti-TPOAb, and anti-TgAb were 0.4–4 μIU/mL, 1.57–4.71 pg/mL, 0.85–1.78 ng/dl, 0–35 IU/mL, and 0–40 IU/mL, respectively. The thyroid antibody levels over the upper range were accepted as positive.
Patients were classified as benign and malignant histopathologically. In patients with PTC, primary tumor size, bilaterality, the number of tumor foci, cervical lymph node metastasis (LNM), extrathyroidal extension, capsular and vascular invasion, and histological variants were recorded. Central lymph node dissection is not routinely performed in our center for patients undergoing thyroidectomy. However, central and/or lateral lymph node dissection is performed when suspicous lymph nodes are detected during preoperative or intraoperative evaluation. Multifocality was defined as two or more tumor foci within the thyroid gland. Greatest tumor diameter in histopathological examination was accepted as tumor size. For multifocal tumors, the diameter of the greatest tumor focus was taken as the primary tumor. When there were tumor foci both in the right and left lobes of the thyroid gland, the patient was defined to have bilateral disease. PTC that is measuring 1 cm or less in diameter was classified as papillary thyroid microcarcinoma (PTMC) and that is measuring higher than 1 cm as PTC.
Histological variants of PTC were classified as nonaggressive (classical/conventional and follicular variants), aggressive (tall cell, diffuse sclerosing and columnar variants) and other variants. In 13 patients, tumor had features of more than one variant. In seven of these patients, aggressive and nonaggressive variants were concomitant in the same patient and excluded from the analysis, while remaining six had two aggressive or two nonaggressive variants and were not excluded.
Staging was performed according to the seventh edition of TNM classification by the AJCC. In 215 patients with a follow up period of at least 5 years, dynamic risk stratification was made as defined by Momesso et al. in 2014 [9]. Accordingly, patients with excellent response, indeterminate response, biochemical incomplete response, or structural incomplete response to therapy were determined.
Statistical analysis
Statistical analysis, calculations and ROC curve analysis was made with IBM SPSS Statistics 21.0 (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version21.0. Armonk, NY: IBM Corp.). Microsoft Office Excel 2013 program was used to prepare graphics. Distribution of age, TSH, fT3 and fT4 were determined by Shapiro-Wilk test and normality graphics. These variables were defined as median (range) since they were not normally distributed. Categorical variables were presented as n (%). Mann–Whitney U-test was used to compare age, TSH, fT3 and fT4 in benign and malignant patients and to compare TSH in subgroups of malignant patients. Serum TSH in different variants was compared by Kruskal–Wallis analysis. Pairwise comparisons were made by Bonferroni correction. Sex, anti-TgAb and anti-TPOAb positivity, histopathological results in euthyroid, hypothyroid, and hyperthyroid patients were examined by Pearson chi-square test. Logistic regression analysis was performed to analyze effect of TSH on malignancy and LNM. Variable selection criteria were p < 0.05 for inclusion and p > 0.10 for exclusion. Since age has a clinical significance in malignant patients, it was included in the model although not statisticallysignificant. G statistics was calculated to determine the importance of TSH compared to other factors. ROC curve analysis was performed to examine the role of TSH in predicting malignancy. Youden index was used to determine cut-off value. A p-value < 0.05 was accepted to indicate statistical significance.
Results
Among 1632 patients, histopathological diagnosis was benign in 969 (59.4%) and PTC/PTMC in 663 (40.6%). Median ages were 49 (range:19–83) in benign and 49 (range:19–84) in malignant groups (p = 0.182). There were 195 (20.1%) and 126 (19.0%) male patients in benign and malignant groups, respectively (p = 0.576) (Table 1).
Median TSH was significantly lower in benign patients compared to malignant ones [0.98 IU/dL (0.002–37) vs. 1.41 IU/dL (0.005–24), p < 0.001]. There was no significant difference in serum fT4 between benign and PTC patients (p = 0.539), while fT3 was significantly higher in benign patients (p < 0.001). Both anti-TgAb and anti-TPOAb positivity and histopathologically confirmed lympocytic thyroiditis were higher in malignant patients (p = 0.003, p < 0.001, and p < 0.001, respectively) (Table 1).
Thyroid hormones were normal in 1553 patients (95.2%) and there were 44 patients (2.7%) with newly diagnosed subclinical or overt hypothyroidism and 35 patients (2.1%) with newly diagnosed subclinical or overt hyperthyroidism. 7 (20.0%) of patients with hyperthyroidism, 630 (40.6%) of patients with euthyroidism and 26 (59.1%) of patients with hypothyroidism had malignancy (p < 0.05).
There were 279 (42.1%) patients with PTC and 384 (57.9%) with PTMC. Mean age of patients with PTMC was significantly higher than patients with PTC (50.36 ± 11.45 vs. 46.61 ± 12.84, p < 0.001). Median serum TSH was lowest in benign patients, higher in patients with PTMC and highest in patients with PTC (p < 0.05 for all comparisons) (Table 2). When only euthyroid patients with negative anti-TPOAb and/or anti-TgAb and without lymphocytic thyroiditis were analyzed, patients with benign histopathology had significantly lower TSH compared to patients with PTMC and PTC (p < 0.05 for each), while patients with PTMC and PTC had similar TSH (p > 0.05).
Age, anti-TPOAb and/or anti-TgAb positivity, lypmphocytic thyroiditis, and TSH were included in multivariate analysis after univariate analysis (Table 3). Accordingly, 1 IU/dL increase in TSH was increasing malignancy risk by 1.221 (%95 GA:1.099–1.356) (p < 0.001). When lymphocytic thyroiditis was excluded from the analysis, and age and anti-TPOAb and/or anti-TgAb positivity were included, malignancy risk increased by 1.247 with each 1 IU/dL increase in TSH (%95 GA:1.123–1.385) (p < 0.001). In model 3, anti-TPOAb and/or anti-TgAb positivity was also excluded and malignancy risk increased by 1.303 as TSH increased 1 IU/dL (%95 GA:1.115–1.524) (p < 0.001). When the effect of variables in model were examined by G statistics, the most important variable was serum TSH (p < 0.001).
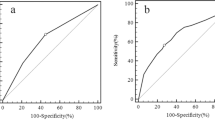
Predictive value of TSH to discriminate benign and malignant lesions was determined by ROC analysis and area under the curve (AUC) was found as 0.633 (95% CI: 0.605–0.660) (p < 0.001) (Fig. 1). Among 2 cut-off points, the one with the higher value was chosen because the aim was to differentiate malignant patients. A cut off value of 1.245 IU/dL for TSH had a sensitivity of 57% and a specifity of 65%.
Among malignant patients, 549 (83.2%) had stage 1, 27 (4.1%) had stage 2, 75 (11.4%) had stage 3, and 9 (1.3%) had stage 4 disease. 233 (35.1%) of malignant patients had multifocal and 148 (22.3%) had bilateral disease. Capsular invasion, vascular invasion, extrathyroidal extension, and LNM were observed in 171 (25.8%), 25 (3.8%), 60 (9.0%), and 60 (9.0%) patients, respectively.
Preoperative serum TSH was significantly higher in patients with bilateral compared to unilateral tumor, patients with capsular invasion compared to without capsular invasion and patients with LNM compared to without LNM (p = 0.036, p = 0.002, and p = 0.001, respectively) (Table 4). There were 497 (75.0%) classical variant and 140 (21.1%) follicular variant PTC. Tall cell, columnar and diffuse sclerosing variants were observed in 11 (1.7%), 4 (0.65%), and 3 (0.5%) of PTCs. 23 (3.5%) tumors were diagnosed as other variants. In patients with aggressive variant PTC, preoperative TSH was higher than patients with nonaggressive variants (p < 0.05). There was not any difference in serum TSH in patients with unifocal and multifocal tumor. Patients with stages 1–2 tumor also had higher preoperative TSH than patients with stages 3–4 tumor, and patients with extrathyroidal extension and vascular invasion had higher preoperative TSH compared to patients without these features, but the differences were not significant (Table 4).
Dynamic risk stratification in 215 patients revealed excellent response in 201 (93.4%), biochemical incomplete response in 7 (3.3%) and structural incomplete response in 7 (3.3%) patients. Although preoperative serum TSH was higher in patients with biochemical and structural incomplete response compared to patients with excellent response, the difference was not statistically significant.
In multivarite analysis model I, age, sex, preoperative TSH, lymphocytic thyroiditis, tumor size, multifocality and extrathyroidal extension were significant factors for LNM (Table 5). When anti-TPO positivity was excluded from the model, we observed that LNM risk increased by 1.529 (95% CI: 1.156–2.022) with each 1 U/mL increase in TSH. Preoperative TSH was the second most important predictive factor for LNM after tumor size (G = 9.122, p = 0.003).
Discussion
Serum TSH concentration even within normal ranges was shown to be related with increased malignancy risk in patients with nodular thyroid disease [10]. In the study by Boelaert et al. a serum TSH of 0.9 IU/L was determined as a cut-off value that was predictive for malignancy [11]. TSH was found to be an independent risk factor for DTC regardless of the presence of thyroid nodules in another study [12]. In the present study, we also found that preoperative serum TSH was higher in malignant patients compared to benign ones. In contrary to these findings, Kim et al. and Castro et al. did not find a significant relation between serum TSH and thyroid malignancy [13, 14]. However in the study by Kim et al. there were only 249 (18.7%) patients with benign disease compared to 1080 (81.3%) patients with PTC. This imbalance between two groups of patients might be responsible for the lack of association between TSH and thyroid malignancy [13]. In the study by Castro et al., a relatively small series of 462 patients operated with suspicious cytological diagnosis was included [14].
In our study, optimal TSH value that can be used to differentiate benign and malignant thyroid lesions was 1.245 IU/dL with an AUC of 0.63. This cut-off value was not very reliable for the detection of thyroid cancer since the sensitivity was 57% and specificity was 65%. In accordance with ours, the optimal TSH concentration for thyroid cancer prediction was 1.59 mIU/L with an AUC of 0.58 (95% CI 0.53– 0.62) and sensitivity of 74% and specificity of 57% in the study by He et al. [1]. Similarly, low sensitivity and specifity were also reported in other previous studies that tried to determine a cut-off value of TSH that can discriminate benign and malignant thyroid nodules [15, 16].
Another contradictory issue is whether TSH cause development or progression or both development and progression of thyroid cancer. In a mouse model, BRAF knock-in and TSH receptor knockout mice developed low-grade, smaller, and less invasive PTC with a longer latency. This was an important experimental study supporting the relation between TSH and thyroid cancer [17]. Gerschpacher et al. did not find any significant difference in TSH concentration in patients with PTMC. However, there was a positive trend in correlation between nodule size and TSH levels in patients with PTMC. The authors concluded that TSH might be involved in the progression rather than development of existing thyroid cancer [18]. Some other studies reported similar preoperative TSH levels in patients with PTMC and patients with benign nodular diseases [19,20,21]. However, some limitations should be pointed in these studies. The sample sizes were very small in some, patients with medullary thyroid carcinoma which is known to be unresponsive to TSH were chosen as control group or the diagnosis was based on cytological results in a considerable number of patients. In the study by Zafon et al. patients with benign lesions had the lowest, PTMC (n = 36) had intermediate, and DTC had the highest levels of TSH. However, the differences in TSH were only significant between benign and thyroid cancer of larger size groups and this was partly explained by small number of cancer cases [8]. In a recent metaanalysis of 9 studies and 6523 patients, high TSH concentration was presented as an independent risk factor for differentiated thyroid microcarcinoma (odds ratio = 1.23, 95% CI = 1.03–1.46, p = 0.001) in patients with a nodule. This led to a hypothesis that TSH might influence the development of differentiated malignancy [22]. Another metanalysis revealed an association between increased serum TSH and thyroid cancer both in nodules > 1 and <1 cm [23]. This suggested that TSH had similar effect on tumor pathogenesis regardless of tumor size. Association between serum TSH and thyroid cancer was confirmed in our study and we further showed lowest TSH in benign, intermediate in PTMC and highest in PTC patients.
The relationship between thyroid autoimmunity and thyroid cancer was suggested previously. It is not clear whether increased serum TSH due to autoimmune disease or autoimmunity itself cause an increase in PTC risk in these patients. Thyroid antibody positivity and lymphocytic thyroiditis were significantly higher in PTC patients in our study. To avoid the possible effect of autoimmunity on serum TSH, we made further analysis after excluding patients with positive thyroid autoantibodies, lymphocytic thyroiditis and thyroid dsyfunctions. We observed that the relation between TSH and malignancy was still present in euthyroid patients without autoimmune thyroiditis. Additionaly, in this group of patients, serum TSH was significantly higher in PTMC than benign group. Fiore et al. reported significantly higher serum TSH in patients with PTC compared to patients with benign disease whether thyroid autoimmunity was present or not [24]. As a result, although a possible association between autoimmunity and thyroid cancer can not be excluded, some other different mechanisms seem to play role in the association between serum TSH and malignancy.
There are studies showing a relation between preoperative serum TSH and more advanced cancer stage at diagnosis. A meta-analysis of 28 studies reported a correlation of TSH with higher disease grade even at normal and subnormal levels [25]. Zheng et al. included 56 studies in another metaanalysis and showed that high TSH was significantly related with tumor size and LNM [23]. All these findings suggest a role of TSH on thyroid cancer promotion and aggressiveness. In contrary, Kim et al. did not demonstrate higher TSH levels in advanced tumor stage [12]. Similarly, another study did not find association between serum TSH concentration and the prevalence of LNM, extrathyroidal invasion, diffusion, and advanced stages in DTC patients [1]. Preoperative serum TSH was significantly higher in patients with LNM in our study. Although patients with advanced tumor stage and incomplete response had higher TSH than their counterparts, the differences did not reach statistical significance. There may be some explanations for these results. The number of patients with microcarcinoma was high in our study and a majority of patients were in low tumor stage group, with only 9 patients in stage 4. In addition, 5 year follow-up might be considered as a short period to evaluate recurrence.
Several different histologic variants of PTC with different prognosis have been described in the literature [26, 27]. To our knowledge, for the first time in the present study, we showed higher preoperative TSH levels in aggressive variants of PTC compared to nonaggressive ones. However, number of patients with aggressive variants was small and we think this result needs to be confirmed by further large scale studies.
The relationship between TSH and PTC was demonstrated in several studies with different methodologies and conflicting results. These were generally retrospective, cross-sectional studies open to bias associated with their design. Some studies [11, 18] included TSH independent tumors and some were based on small patient populations [19, 28]. We had relatively larger sample size in our study. Also, we have excluded TSH independent tumors and incidental microcarcinomas to avoid bias. In addition, we repeated the analysis in euthyroid patients without thyroid autoimmunity.
The most important limitation of this study is that it was retrospective and performed in a single center. Secondly, only patients with nodular thyroid disease were included.
In conclusion, high TSH was associated with increased risk of thyroid cancer and LNM regardless of autoimmune thyroid disease. This study supports the hypothesis that TSH has an important role in both the development and progression of thyroid malignancy. In addition, for the first time, we showed higher preoperative TSH in aggressive variants of PTC compared to nonaggressive ones. It seems that it is time to consider using TSH in the diagnosis and follow up malignant thyroid lesions, however the main concern is when and how. Further prospective studies might help to find answers to these questions and clarify the relation between TSH and aggressive cancer variants.
References
L.Z. He, T.S. Zeng, L. Pu, S.X. Pan, W.F. Xia, L.L. Chen, Thyroid hormones, autoantibodies, ultrasonography, and clinical parameters for predicting thyroid cancer. Int. J. Endocrinol. 2016, 8215834 (2016)
C. Li, W. Yu, J. Fan et al., Thyroid functional parameters and correlative autoantibodies as prognostic factors for differentiated thyroid cancers. Oncotarget 7, 49930–49938 (2016)
D.S. McLeod, D.S. Cooper, P.W. Ladenson et al.,Prognosis of differentiated thyroid cancer in relation to serum thyrotropin and thyroglobulin antibody status at time of diagnosis. Thyroid 24, 35–42 (2014)
K. Boelaert, The association between serum TSH concentration and thyroid cancer. Endocr. Relat. Cancer 16, 1065–1072 (2009)
P. Pujol, J.P. Daures, N. Nsakala et al., Degree of thyrotropin suppression as a prognostic determinant in differentiated thyroid cancer. J. Clin. Endocrinol. Metab. 81, 4318–4323 (1996)
E.L. Mazzaferri, S.M. Jhiang, Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am. J. Med. 97, 418–428 (1994)
E. Fiore, P. Vitti, Serum, TSH and risk of papillary thyroid cancer in nodular thyroid disease. J. Clin. Endocrinol. Metab. 97, 1134–1145 (2012)
C. Zafon, G. Obiols, J.A. Baena et al., Preoperative thyrotropin serum concentrations gradually increase from benign thyroid nodules to papillary thyroid microcarcinomas then to papillary thyroid cancers of larger size. J. Thyroid. Res. 2012, 530721 (2012)
D.P. Momesso, R.M. Tuttle, Update on differentiated thyroid cancer staging. Endocrinol. Metab. Clin. North Am. 43, 401–421 (2014)
M.R. Haymart, D.J. Repplinger, G.E. Leverson et al., Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J. Clin. Endocrinol. Metab. 93, 809–814 (2008)
K. Boelaert, J. Horacek, R.L. Holder et al., Serum thyrotropin concentration as a novel predictor of malignancy in thyroid nodules investigated by fine-needle aspiration. J. Clin. Endocrinol. Metab. 91, 4295–4301 (2006)
H.K. Kim, J.H. Yoon, S.J. Kim et al., Higher TSH level is a risk factor for differentiated thyroid cancer. Clin. Endocrinol. 78, 472–477 (2013)
K.W. Kim, Y.J. Park, E.H. Kim et al., Elevated risk of papillary thyroid cancer in Korean patients with Hashimoto’s thyroiditis. Head. Neck. 33, 691–695 (2011)
M.R. Castro, R.P. Espiritu, R.S. Bahn et al., Predictors of malignancy in patients with cytologically suspicious thyroid nodules. Thyroid 21, 1191–1198 (2011)
S. Rinaldi, M. Plummer, C. Biessy et al., Thyroid-stimulating hormone, thyroglobulin, and thyroid hormones and risk of differentiated thyroid carcinoma: the EPIC study. J. Natl. Cancer Inst. 106, dju097 (2014)
A. Dorange, S. Triau, S. Mucci-Hennekinne et al., An elevated level of TSH might be predictive of differentiated thyroid cancer. Ann. Endocrinol. 72, 513–521 (2011)
A.T. Franco, R. Malaguarnera, S. Refetoff et al., Thyrotrophin receptor signaling dependence of Braf‐induced thyroid tumor initiation in mice. Proc. Natl. Acad. Sci. USA 108, 1615–1620 (2011)
M. Gerschpacher, C. Göbl, C. Anderwald et al., Thyrotropin serum concentrations in patients with papillary thyroid microcancers. Thyroid 20, 389–392 (2010)
R. Negro, R. Valcavi, F. Riganti et al., Thyrotropin values in patients with micropapillary thyroid cancer versus benign nodular disease. Endocr. Pract. 19, 651–655 (2013)
L. Shi, Y. Li, H. Guan et al., Usefulness of serum thyrotropin for risk prediction of differentiated thyroid cancers does not apply to microcarcinomas: results of 1,870 Chinese patients with thyroid nodules. Endocr. J. 59, 973–980 (2012)
J.S. Choi, C.M. Nam, E.K. Kim et al., Evaluation of serum thyroid-stimulating hormone as indicator for fine-needle aspiration in patients with thyroid nodules. Head. Neck. 37, 498–504 (2015)
R.L. Shi, T. Liao, N. Qu et al., The usefulness of preoperative thyroid stimulating hormone for predicting differentiated thyroid microcarcinoma. Otolaryngol. Head. Neck. Surg. 154, 256–262 (2016)
J. Zheng, C. Li, W. Lu et al., Quantitative assessment of preoperative serum thyrotropin level and thyroid cancer. Oncotarget 7, 34918–34929 (2016)
E. Fiore, T. Rago, M.A. Provenzale et al., Lower levels of TSH are associated with a lower risk of papillary thyroid cancer in patients with thyroid nodular disease: Thyroid autonomy may play a protective role. Endocr. Relat. Cancer 16, 1251–1260 (2009)
D.S. McLeod, K.F. Watters, A.D. Carpenter et al., Thyrotropin and thyroid cancer diagnosis: a systematic review and dose-response meta-analysis. J. Clin. Endocrinol. Metab. 97, 2682–2692 (2012)
C.E. Silver, R.P. Owen, J.P. Rodrigo et al., Aggressive variants of papillary thyroid carcinoma. Head. Neck. 33, 1052–1059 (2011)
X. Shi, R. Liu, F. Basolo et al., Differential clinicopathological risk and prognosis of major papillary thyroid cancer variants. J. Clin. Endocrinol. Metab. 101, 264–274 (2016)
J. Jonklaas, H. Nsouli-Maktabi, S.J. Soldin, Endogenous thyrotropin and triiodothyronine concentrations in individuals with thyroid cancer. Thyroid 18, 943–952 (2008)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Tam, A.A., Ozdemir, D., Aydın, C. et al. Association between preoperative thyrotrophin and clinicopathological and aggressive features of papillary thyroid cancer. Endocrine 59, 565–572 (2018). https://doi.org/10.1007/s12020-018-1523-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-018-1523-6