Abstract
The long-term effect of therapeutic diets in obesity treatment is a challenge at present. The current study aimed to evaluate the long-term effect of a very low-calorie-ketogenic (VLCK) diet on excess adiposity. Especial focus was set on visceral fat mass, and the impact on the individual burden of disease. A group of obese patients (n = 45) were randomly allocated in two groups: either the very low-calorie-ketogenic diet group (n = 22), or a standard low-calorie diet group; (n = 23). Both groups received external support. Adiposity parameters and the cumulative number of months of successful weight loss (5 or 10 %) over a 24-month period were quantified. The very low-calorie-ketogenic diet induced less than 2 months of mild ketosis and significant effects on body weight at 6, 12, and 24 months. At 24 months, a trend to regress to baseline levels was observed; however, the very low-calorie-ketogenic diet induced a greater reduction in body weight (−12.5 kg), waist circumference (−11.6 cm), and body fat mass (−8.8 kg) than the low-calorie diet (−4.4 kg, −4.1 cm, and −3.8 kg, respectively; p < 0.001). Interestingly, a selective reduction in visceral fat measured by a specific software of dual-energy x-ray absorptiometry (DEXA)-scan (−600 g vs. −202 g; p < 0.001) was observed. Moreover, the very low-calorie-ketogenic diet group experienced a reduction in the individual burden of obesity because reduction in disease duration. Very low-calorie-ketogenic diet patients were 500 months with 5 % weight lost vs. the low-calorie diet group (350 months; p < 0.001). In conclusion, a very low-calorie-ketogenic diet was effective 24 months later, with a decrease in visceral adipose tissue and a reduction in the individual burden of disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity, considered by the WHO to be a twenty-first-century pandemic, is a problem worldwide [1, 2]. This severe disease is the main risk factor for cardiovascular morbi-mortality, type 2 diabetes, and even cancer [3, 4], and it is a hugely demanding problem for both patients and health administrations [5, 6]. The only way to protect society and patients from the tremendous toll of obesity and its complications is to find effective medical treatments of prevention and cure [7–10]. Diverse therapeutic strategies have been assayed to decrease body adiposity [11–14]. While the improvement of dietary habits together with an increase in physical activity and behavioral support are the first line of elective treatment to moderate obesity and overweight, the efficacy of such an approach is negligible at long run in the overall population [15]. Nutritional interventions, such as caloric restriction diets, can be an efficient therapeutic approach to promote weight loss in obese patients, although there is no consensus regarding the ideal composition of such interventions [16–18]. A dietetic regimen that mimics fasting by restricting carbohydrates and fat with a moderate increase in protein intake, otherwise known as a very low-calorie-ketogenic (VLCK) diet, has been proposed to achieve rapid weight loss [19]. Ketogenic diets were also able to improve hyperlipidemia and some cardiovascular risk factors [20–22], and were proposed to be safely used as an adjuvant therapy for conventional radiation and chemotherapies and to enhance cancer therapeutic responses [23, 24]. VLCK diets have been shown to be effective, at least in the short to medium term, as a tool to fight obesity [25]. A previous study from our group demonstrated that at 1 year follow-up a VLCK diet was effective in inducing weight loss compared with a standard low-calorie (LC) diet, with good adherence as well as mild and transitory side effects [26]. The current problem with fighting obesity is not only losing weight, but rather maintaining the weight lost. In fact, except in cases of bariatric surgery, all obesity therapies fail in the long run. This issue is especially relevant regarding nutritional intervention studies, since long-term compliance and weight regain are huge concerns [27–34].
The present study was aimed to evaluate the long-term effect of a VLCK diet as part of a commercial weight loss program (Pronokal method), compared with a standard LC diet on decreasing adiposity in obese patients. The main objectives of this study were: (1) evaluate the time-course changes in anthropometric and body composition parameters induced at a follow-up period of 24 months, (2) quantify the effect of these treatments on visceral fat mass evaluated by a sophisticated new software incorporated in the DEXA-scan, and (3) investigate the impact of both dietary treatments on the individual obesity burden through the 24-month follow-up period.
Subjects and methods
Subjects
This study was an open, randomized, controlled, prospective nutritional intervention clinical trial that took place across 2 years in a single center. Patients attending the Obesity Unit at the Hospital Gregorio Marañon of Madrid to receive treatment for obesity were consecutively enrolled in this study. Apart from obesity and prediabetes, participants were generally healthy individuals. The inclusion criteria were: age 18–65 years, body mass index (BMI) >30, stable body weight in the previous 3 months, desire to lose weight, and a history of failed dietary efforts. None of the participants had serious medical conditions (Table 1).
The main exclusion criteria were: type 1 diabetes mellitus or type 2 diabetes mellitus under insulin therapy, obesity induced by other endocrine disorders or by drugs, and use of any weight loss diet or pills in the previous 6 months. Secondary exclusion criteria were: severe depression or any other psychiatric disease, abuse of narcotics or alcohol, severe hepatic insufficiency, any type of renal insufficiency or gout episodes, neoplasia (except basal cell skin cancer), previous events of cardiovascular or cerebrovascular disease, renal lithiasis, uncontrolled hypertension, and hydroelectrolytic alterations. Females with child-bearing potential or those who were pregnant, breast-feeding, intending to become pregnant or not using adequate contraceptive methods were also excluded. All participants provided written informed consent and the Institutional Review Board (Comite Etico de Investigacion Clinica, Hospital Gregorio Marañon, Madrid) approved the study (C.I: 143/09, protocol PRO-PRO 2009-02, Foundation code 143/09 EO). Participants received no monetary incentive. While 79 subjects initially participated in the study, at the 24-month follow-up 45 remained; these 45 subjects are presented in this paper.
Study protocol
Using a controlled, open design, the patients were randomized and allocated to receive either a LC diet (thereafter LC diet; n = 23) or a VLCK diet (n = 22) as part of a commercial weight loss program (Pronokal method), which included lifestyle and behavioral modification support. The intervention for both groups included an evaluation by the specialist physician conducting the study, an assessment by an expert dietician, group meetings, and exercise recommendations. The group meetings and evaluations took place in a hospital setting at 0.5, 2, 4, 6, 8, 10, 12, 18, and 24 months. At 6, 12, and 24 months, the participants’ satisfaction with the diet was assessed. In these meetings, patients received dietary instructions, individual supportive counsel, and encouragement to exercise on a regular basis using a formal exercise program. In addition, a program of telephone reinforcement calls was instituted, and a phone number to address any doubts was provided to all participants. After the 12-month control, follow-up occurred at 18 and 24 months in order to evaluate the patients’ current health situation of the patients as well as their weight and body composition.
Adherence to the diet and exercise recommendations in the VLCK diet group was determined through self-reports, food records, and, in the ketogenic phases, by a urinary ketone qualitative assessment. Interacetonadipstick (CarullaVekar Madrid) were used for ketone analysis, and the patients recorded their analyzed values at each visit to the hospital. Meanwhile, adherence in the LC diet group was assessed according to the self-reports of the patients.
During all visits, the patients completed a questionnaire regarding side effects and dropouts, and the reasons provided were recorded. At 6, 12, and 24 months, a questionnaire of patient satisfaction with their diet, which contained a single question (How satisfied are you with the diet that you are following?) and used a 5-point Likert scale format (1 = very dissatisfied, 2 = dissatisfied, 3 = indifferent, 4 = satisfied, 5 = very satisfied), was also provided to the patients.
Experimental diets
LC diet
The standard LC diet was an equilibrated diet that had a caloric value 10 % below the total metabolic expenditure of each individual. The total metabolic expenditure was calculated from the basal metabolic expenditure (based on the formula FAO/WHO/UN) [35] multiplied by the coefficient of activity, which was calculated according to the physical activity of each participant. The calories provided to this group ranged between 1400 and 1800 kcal/day. The ration of macronutrients provided was 45–55 % carbohydrates, 15–25 % proteins, and 25–35 % fat [36] in addition to a recommended intake of 20–40 g/day of fiber in the form of vegetables and fruits. A ratio exchange model was followed.
VLCK diet
The VLCK diet group followed a diet according to a commercial weight loss program (Pronokal method) based on a high biological value protein preparations diet and natural foods. Each protein preparation contained 15 g of protein, 4 g of carbohydrates, and 3 g of fat, and provided 90–100 kcal [37]. This method has three stages: the active stage, the re-education stage, and the maintenance stage (Fig. 1).
Scheme of the dietary intervention program for the VLCK diet. The duration of the different stages is dependent on the targets and the clinical decision of the physician in charge of the patient. The duration of stage 1, i.e., on ketosis, was less than 2 months, stage 2 ranged from 5–6 months and stage 3 was until 24 months. VLCK very low-calorie-ketogenic diet, LC low-calorie diet
The active stage consists of a very low-calorie diet (600–800 kcal/day) that is low in carbohydrates (<50 g daily from vegetables) and lipids (only 10 g of olive oil per day). The amount of high biological value proteins ranges between 0.8 and 1.2 g per each kg of ideal body weight, to ensure that it meets minimal body requirements and prevents the loss of lean mass. This method produces three ketogenic phases. In phase 1, the patients eat high biological value protein preparations five times a day, and vegetables with a low glycemic index. In phase 2, one of the protein servings is substituted with a natural protein (e.g., meat or fish) either at lunch or at dinner. In the phase 3, a second serving of a low-fat natural protein replaces the second serving of a biological protein preparation. Throughout these ketogenic phases, supplements of vitamins and minerals, such as K, Na, Mg, Ca, and omega-3 fatty acids, were provided in accordance to international recommendations [38]. This active stage is maintained until the patient achieves most of the weight loss target, ideally 80 %. While the ketogenic phases were variable in time depending on the individual and the weight loss target, they lasted between 45–60 days in total.
In the re-education stage, the ketogenic phases were ended by the physician in charge of the patient based on the amount of weight lost, and a low-calorie diet was initiated. At this point, the patients underwent a progressive incorporation of different food groups and participated in a program of alimentary re-education to guarantee the long-term maintenance of the weight lost (Fig. 1). The maintenance stage, which lasted 2 years, consisted of an eating plan balanced in carbohydrates, protein, and fat. Depending on the individual, the calories consumed ranged between 1500 and 2000 kcal/day, and the target was to maintain the lost weight and promote a healthy lifestyle.
Anthropometric measurements
Body weight, BMI, and waist circumference (WC) were the primary outcome measures. At each visit, patients were weighed on the same calibrated scale (Seca 220 scale, Medical Resources, EPI Inc OH, USA) wearing light clothing and no shoes. BMI was calculated as body weight in kg, divided by height in meters squared. WC was recorded with a standard flexible nonelastic metric tape over the midpoint between the last rib and the iliac crest, with the patient standing and exhaling [36].
Body composition analysis
Total body composition analysis
Body composition was measured by DEXA. Total body imaging was acquired using the GE Healthcare Lunar (iDXA Madison, WI, USA). Daily quality control scans were acquired during the study period. No hardware or software changes were made during the course of the trial. During the procedure, patients wore only light clothes.
Subjects were scanned using standard imaging and positioning protocols, while wearing only light clothing. The GE Lunar Body Composition Software option used on the GE Lunar iDXA bone densitometer measured the regional and whole body bone mineral density and lean and fat tissue mass; it also calculated derivative values of bone mineral content, area, soft tissues mass, regional soft tissue mass, total soft tissue mass, fat-free mass, regional/total soft tissue mass ratio, % fat, region % fat, total body % fat, android % fat, and gynoid % fat. The android/gynoid ratio was automatically generated and analyzed using enCORE software version 13.6. For measuring android fat, a region-of-interest was automatically defined as follows: the caudal limit was placed at the top of the iliac crest and its height was set to 20 % of the distance from the top of the iliac crest to the base of the skull to define its cephalad limit of abdominal subcutaneous adipose tissue.
Selective visceral fat mass analysis
Visceral fat (VAT) was calculated using a newly developed fully automated software (CoreScan® GE Healthcare, Madison, WI, USA) for segmenting abdominal fat into subcutaneous fat and visceral fat within the abdominal region using DXA. This method was validated against computed tomography in patient population with a wide range of BMI [39, 40]. DXA-VAT was measured in a 5 cm wide region placed in the abdominal region just above the iliac crest at a level that approximately coincided with the 5th lumbar vertebrae on the whole body DXA scan [40].VAT was estimated by subtracting subcutaneous fat from the total abdominal fat by means of an algorithm described in detail elsewhere and reported in grams [41, 42].
Statistical analysis
This study was an open, randomized, controlled, prospective nutritional intervention clinical trial that took place across 2 years in a single center. Initially, 79 obese subjects were studied; however, at 24-months follow-up, only 45 were still attending the clinic. Of these 45 patients, 22 were in the VLCK diet group, and 23 were in the LC diet group. Weight loss was primarily analyzed in the patients who completed the study (completers), and results were also evaluated with an intention-to-treat (ITT) analysis using the baseline observation carried forward (BOCF). In sensitivity analyses, the last observation carried forward (LOCF) was also used; furthermore, to determine whether the findings were affected by the choice of data-imputation method, multiple imputations (MI) as age, sex, and baseline values were used to predict any missing values at the 24-month time point.
The data were presented as mean ± SD. The differences between the two groups were determined using Fisher’s exact test for categorical variables and Student’s t-test. Alternatively, analysis of variance (ANOVA) was used for comparison of continuous variables between three or more groups. Variables that were not normally distributed were analyzed by nonparametric tests (Mann–Whitney U test or Kruskal–Wallis test). For completers-only analysis, there were no formal imputations; therefore, all the estimations were obtained using all the available data (available data only, or ADO). Analyses were performed using SAS software version 9.1.3 (SAS Institute Inc). The level of significance was set to 5 % (p value < 0.05).
Funding source
The funding for the study as well as the high biological value protein preparations were provided by Protein Supplies, S.L., (Barcelona, Spain) free of charge to the patients. The funding source had no involvement in the study design, recruitment of patients, study interventions, data collection, or interpretation of the results.
Results
Of the 79 participants who were initially enrolled in the study, 26 dropped out of the study in the first 3 months [26]; 8 more dropped out before 24 months. A total of 45 patients completed the study, with 22 in the VLCK diet (aged 47.6 ± 7.8 year, 77 % females, BMI 35.2 ± 4.8, and WC of 112.5 ± 14 cm) and 23 in the LC diet group (age 45.6 ± 9.6 year, 95 % female with BMI of 34.5 ± 5 and WC of 107 ± 11 cm). Their characteristics at enrollment are described in Table 1.
The completers-only analysis revealed that from the beginning (15 days) until the end of the observation, the amount of kg reduction observed in the VLCK diet was double that of the LC diet (Fig. 2). The maximum reduction in weight of around 20 kg of the initial weight was observed at 4, 6, and 12 months, with partial recovery thereafter. The reductions in body weight tended to regress in both dietary intervention groups with no statistically significant differences in the weight recovery pattern between the dietary intervention groups (data not shown). However, at 24 months the effect was still observable, with a reduction in the VLCK diet group of 12.5 kg vs. 5.2 kg in the LC diet group (p < 0.001). When weight loss was analyzed as intention-to-treat with the last observation carried forward (ITT-LOCF), the values or reduction at 24 months were 12.8 kg in the VLCK group and 4.4 kg in the LC group (p < 0.001); similar results were observed when looking at baseline observation carried forward, or ITT-BOCF (Fig. 2). All the analysis regarding body weight changes after 2 years showed that the VLCK diet was more effective than the LC diet in a statistically significant way.
Evolution of weight loss after initiation of treatment and during 24 months of follow-up. Data from the completers groups are presented and the data obtained through the ITT analysis are also shown. LOC last observation carried forward, BOC basal observation carried forward, MI multiple imputations. *p < 0.001 difference between groups; ¥p < 0.001 differences with respect to baseline values
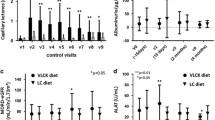
When evaluated under different anthropometric parameters, the reduction in BMI with the VLCK diet was greater than the reduction seen with the LC diet, with a maximal reduction at 6 months of 8.0 and 2.7 units, respectively, and the values of reduction at 24 months of 4.4 and 1.9 units, respectively (p < 0.001; Fig. 3a). Similar observations were found when the excess of weight loss (%) was studied (Fig. 3b). As expected, the remarkable reduction in the waist circumference observed at 6 months, 20.6 cm and 6.0 cm, respectively was partially recovered at 24 months (Fig. 3c). However, the differences were still maintained with a reduction of 11.6 cm in the VLCK diet vs. 4.1 cm in the LC diet (p < 0.05).
When a strict analysis of body composition was undertaken using DEXA (Fig. 4a), lean mass was slightly different between both groups at 4 and 6 months as expected, albeit without differences in the following point of the follow-up visit. Furthermore, although the notable reduction in kg of fat mass observed at 6 months (19.1 kg vs. 6.1; p < 0.001) tended to regress to baseline levels at 24 months, a significant difference was maintained in both groups (8.8 kg for the VLCK diet vs. 3.8 kg for the LC diet; p < 0.001).
Time-course of changes in body composition through 24 months of both nutritional programs’ follow-up. a Changes with respect to baseline in fat and lean body mass. b Changes with respect to baseline in visceral fat mass measured by a specific software of DEXA-scan. *p < 0.001 when comparing fat body mass between groups; **p < 0.05 when comparing lean body mass between groups
Interestingly, the amount of reduction in total fat observed by DEXA was minor compared to what was observed measuring WC. The question raised was if the VLCK diet selectively targeted visceral fat better than total body fat. Therefore, further analysis was performed by sophisticated new software incorporated in the DEXA scan that was able to selectively detect visceral fat. Remarkably, the reduction of visceral mass was more intense in the VLCK diet group than it was in the LC diet group, and this difference was observed even at 24 months, with a reduction of 706 vs. 212 cm3, respectively (p < 0.001)—or, in fat grams, 666 g vs. 200 g, respectively (p < 0.001) (Fig. 4b).
The study of the categories of weight loss percentages (Fig. 5) demonstrated that at 2 years after starting the VLCK diet, 54 % of patients lost more than 10 % of their original weight—in contrast to the LC diet group, in which only 13 % attained such a goal (p < 0.001).
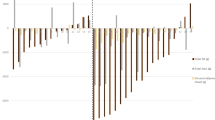
One aim of the present study was to observe whether a reduction in the individual burden of obesity, i.e., the duration of the disease was significantly reduced along the 24-month period, as that concept is more important than the crude value at a fixed time of 2 years. Evaluated as patients with more than 5 % (Fig. 6a) or 10 % (Fig. 6b) reduction of initial weight, the VLCK diet induced a reduction of more than 5 % over the original weight in 100 % of the participants along 18 months, with a slow reduction at 24 months. Similar results were obtained when evaluated as a reduction greater than 10 % of initial body weight. That means that the intervention with the VLCK diet provided 500 cumulative months of reduction in the burden of obesity (loss of 5 % of original weight) in the whole group, or 22 out of 24 months for each patient (Fig. 7).
Distribution of patients who achieved successful weight loss in both dietary groups. a Number of patients who lost at least 5 % of initial body weight. b Number of patients who lost at least 10 % of initial body weight. VLCK very low-calorie-ketogenic diet, LC low-calorie diet. *p < 0.05 between groups
The side effects of the method were mild and mostly occurred in the first months of the treatment as previously reported in the 12 months phase of this trial [26]; they mainly included asthenia, fatigue, headache, constipation, and nausea. None of the dropouts of the study between 1 and 2 years were attributed to collateral side effects.
Discussion
To the best of our knowledge, this is the first study evaluating the long-term effect of a VLCK diet as treatment of obese patients during a 24-month period. Although reduced respect to the first months of treatment, the VLCK diet was significant effective still at 2 years. Notably, the higher beneficial effect of the VLCK diet compared to the LC diet was particularly observed in the visceral fat mass. Moreover, even though reductions in body weight and adiposity tended to regress, the individual burden of the disease excess body weight along the 24 months was lower in the patients with the VLCK diet.
In a previous study from our group, it was shown that a VLCK diet Pronokal Method was significantly more effective than a standard LC diet at one-year follow-up [26]. However, the main drawback of any treatment for obesity is weight regaining in the following years, then long-term evaluation of efficacy is a must [29]. Though the VLCK diet induced a huge loss of adiposity at 6–12 months after dieting, in the current study, a trend to regress to the baseline mean in the anthropometric and body composition parameters was observed at the end of the study. In line with this results, although with a different approach, a previous study evidenced that a low-carbohydrate, high fat, non-restricted-calorie diet achieves the maximum weight loss by 6 months and with a trend to recover the weight loss at 24-months point [43]. Despite this fact, at 24 months, patients who followed the VLCK diet lost 7 kg of body weight, or 2.5 units of BMI, more than patients on the LC diet. These changes were accompanied by a significant reduction of waist circumference, and total body adiposity evaluated by DEXA scan while no relevant changes were observed in lean body mass through the nutritional program.
Although adiposity excess in any body distribution is detrimental for the patient health, solid evidence points toward visceral adipose tissue as the main factor to increase the risk of cardiovascular disease, diabetes, and even several kinds of cancer [44–51]. In fact, this study is novel in that it was observed that a VLCK diet was able to induce a significant reduction in visceral adipose tissue than through the intervention period directly measured by a new DEXA scan software that selectively measures visceral fat mass [39]. This finding, together with the overall lean mass and skeletal bone preservation, is of foremost relevance, reinforcing the beneficial effect of a VLCK diet in obesity treatment [52].
The most powerful demonstration of the beneficial effect of a VLCK diet was the reduction of the time of exposition to an excess adiposity [53, 54]. Similar to smoking or ionizing radiations, recent studies have shown that the risk of all-cause and cause-specific mortality and cancer risk from accumulates with the number of years lived with excess weight [55–57]. Therefore, the reduction in the time exposition to adiposity is a more important data to determine the beneficial effect of a therapeutic program [58]. Our method including the VLCK induced a 5 and 10 % of weight loss in a very large number of patient/month than the LC diet at 24 months. Moreover, patients who follow the VLCK diet were exposed to an excess adiposity during less time with a reduction of 5 % or more from original weight in 22 out of 24 months for each patient. Such a reduction in the individual burden of the disease would be associated with a reduction in the risk of morbidity and mortality.
The beneficial effects of VLCK diet here reported are probably based in the fact of the rapid weight loss in the first 2 months of treatment that increase the patient adherence. The VLCK diet program the time under ketosis last only 30–45 days, but the positive effects are evident even at 2 years.
The sample size is a limitation of this study that does lead to be cautious with the conclusions. However, the statistical significance found when using small populations usually indicates that there is a real difference between the experimental groups, because this outcome is more difficult with a small population. In fact, this trial had over 96.2 % power to detect differences in weight loss at 24 months between both dietary groups. As dropouts from obesity treatment are a constant in all the studies reported, we are not concerned about the relatively low number of patients finally studied. The strength of this study is its long-term design and the direct quantification of visceral fat mass by DEXA, which was also reported for the first time. Its correlation with waist circumference demonstrated the clinical relevance of waist circumference measurement in obesity management as a surrogate of visceral fat mass [59].
Conclusions
In summary, the current study demonstrates for the first time that a VLCK diet can decrease adiposity, in obese individuals at a greater extent than a standard LC diet—and this beneficial effect was maintained 2 years later. It was observed that the ketogenic diet target specifically visceral obesity. Finally, it was observed a reduction of the individual burden of excess adiposity in the obese patients treated with the VLCK diet.
References
K.M. Flegal, M.D. Carroll, B.K. Kit, C.L. Ogden, Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. Jama. 307, 491–497 (2012)
J.L. Gutierrez-Fisac, P. Guallar-Castillon, L.M. Leon-Munoz, A. Graciani, J.R. Banegas, F. Rodriguez-Artalejo, Prevalence of general and abdominal obesity in the adult population of Spain, 2008-2010: the ENRICA study. Obes. Rev. 13, 388–392 (2010)
F.F. Casanueva, B. Moreno, R. Rodriguez-Azeredo, C. Massien, P. Conthe, X. Formiguera, V. Barrios, B. Balkau, Relationship of abdominal obesity with cardiovascular disease, diabetes and hyperlipidaemia in Spain. Clin. Endocrinol. 73, 35–40 (2010)
X. Pi-Sunyer, The medical risks of obesity. Postgrad. Med. 121, 21–33 (2009)
G. Fruhbeck, V. Yumuk. Obesity: a gateway disease with a rising prevalence. Obes. Facts 7 Suppl 2, 33–36 (2014)
E.A. Spieker, N. Pyzocha, Economic impact of obesity. Prim. Care. 43, 83–95 (2016)
J.E. Blundell, J. Hebebrand, J.M. Oppert, What is the value of obesity research? Obes. Facts 3, 279–282 (2010)
G.A. Bray, G. Fruhbeck, D.H. Ryan, J.P. Wilding Management of obesity. Lancet. (2016).
W.T. Cefalu, G.A. Bray, P.D. Home, W.T. Garvey, S. Klein, F.X. Pi-Sunyer, F.B. Hu, I. Raz, L. Van Gaal, B.M. Wolfe, D.H. Ryan, Advances in the science, treatment, and prevention of the disease of obesity: reflections from a diabetes care editors’ expert forum. Diabetes Care 38, 1567–1582 (2015)
G. Di Dalmazi, V. Vicennati, R. Pasquali, U. Pagotto, The unrelenting fall of the pharmacological treatment of obesity. Endocrine 44, 598–609 (2013)
J.P. Despres, A. Golay, L. Sjostrom, Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N. Engl. J. Med. 353, 2121–2134 (2005)
R.V. Dvorak, A.M. Sharma, A. Astrup, Anti-obesity drugs: to be or not to be? Obes. Rev. 11, 833–834 (2010)
M. Hopkins, N.A. King, J.E. Blundell, Acute and long-term effects of exercise on appetite control: is there any benefit for weight control? Curr. Opin. Clin. Nutr. Metab. Care. 13, 635–640 (2010)
L.F. Van Gaal, A.M. Rissanen, A.J. Scheen, O. Ziegler, S. Rossner, Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet 365, 1389–1397 (2005)
G.A. Bray, L.A. Tartaglia, Medicinal strategies in the treatment of obesity. Nature 404, 672–677 (2000)
A. Astrup, T. Meinert Larsen, A. Harper, Atkins and other low-carbohydrate diets: hoax or an effective tool for weight loss? Lancet 364, 897–899 (2004)
A.B. Crujeiras, M.A. Zulet, I. Abete, M. Amil, M.C. Carreira, J.A. Martinez, F.F. Casanueva, Interplay of atherogenic factors, protein intake and betatrophin levels in obese-metabolic syndrome patients treated with hypocaloric diets. Int. J. Obes. 40, 403–410 (2015)
D. Paddon-Jones, E. Westman, R.D. Mattes, R.R. Wolfe, A. Astrup, M. Westerterp-Plantenga, Protein, weight management, and satiety. Am. J. Clin. Nutr. 87, 1558S–1561S (2008)
A. Paoli, Ketogenic diet for obesity: friend or foe? Int. J. Environ. Res. Public Health. 11, 2092–2107 (2014)
A. Al-Khalifa, T.C. Mathew, N.S. Al-Zaid, E. Mathew, H.M. Dashti, Therapeutic role of low-carbohydrate ketogenic diet in diabetes. Nutrition 25, 1177–1185 (2009)
H.M. Dashti, T.C. Mathew, M. Khadada, M. Al-Mousawi, H. Talib, S.K. Asfar, A.I. Behbahani, N.S. Al-Zaid, Beneficial effects of ketogenic diet in obese diabetic subjects. Mol. Cell. Biochem. 302, 249–256 (2007)
M.J. Sharman, W.J. Kraemer, D.M. Love, N.G. Avery, A.L. Gomez, T.P. Scheett, J.S. Volek, A ketogenic diet favorably affects serum biomarkers for cardiovascular disease in normal-weight men. J. Nutr. 132, 1879–1885 (2002)
B.G. Allen, S.K. Bhatia, C.M. Anderson, J.M. Eichenberger-Gilmore, Z.A. Sibenaller, K.A. Mapuskar, J.D. Schoenfeld, J.M. Buatti, D.R. Spitz, M.A. Fath, Ketogenic diets as an adjuvant cancer therapy: history and potential mechanism. Redox. Biol. 2C, 963–970 (2014)
A.F. Branco, A. Ferreira, R.F. Simoes, S. Magalhaes-Novais, C. Zehowski, E. Cope, A.M. Silva, D. Pereira, V.A. Sardao, T. Cunha-Oliveira, Ketogenic diets: from cancer to mitochondrial diseases and beyond. Eur. J. Clin. Invest. 46, 285–298 (2016)
N.B. Bueno, I.S. de Melo, S.L. de Oliveira, T. da Rocha Ataide, Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br. J. Nutr. 110, 1178–1187 (2013)
B. Moreno, D. Bellido, I. Sajoux, A. Goday, D. Saavedra, A.B. Crujeiras, F.F. Casanueva, Comparison of a very low-calorie-ketogenic diet with a standard low-calorie diet in the treatment of obesity. Endocrine 47, 793–805 (2014)
A. Astrup, S. Rossner, Lessons from obesity management programmes: greater initial weight loss improves long-term maintenance. Obes. Rev. 1, 17–19 (2000)
S.G. Camps, S.P. Verhoef, K.R. Westerterp, Weight loss, weight maintenance, and adaptive thermogenesis. Am. J. Clin. Nutr. 97, 990–994 (2013)
A.B. Crujeiras, E. Goyenechea, I. Abete, M. Lage, M.C. Carreira, J.A. Martinez, F.F. Casanueva, Weight regain after a diet-induced loss is predicted by higher baseline leptin and lower ghrelin plasma levels. J. Clin. Endocrinol. Metab. 95, 5037–5044 (2010)
A.B. Crujeiras, M. Pardo, R.R. Arturo, S. Navas-Carretero, M.A. Zulet, J.A. Martinez, F.F. Casanueva, Longitudinal variation of circulating irisin after an energy restriction-induced weight loss and following weight regain in obese men and women. Am. J. Hum. Biol. 26, 198–207 (2014)
A.B. Crujeiras, M.A. Zulet, P. Lopez-Legarrea, R. de la Iglesia, M. Pardo, M.C. Carreira, J.A. Martinez, F.F. Casanueva, Association between circulating irisin levels and the promotion of insulin resistance during the weight maintenance period after a dietary weight-lowering program in obese patients. Metabolism 63, 520–531 (2014)
P.S. Maclean, A. Bergouignan, M.A. Cornier, M.R. Jackman, Biology’s response to dieting: the impetus for weight regain. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301, R581–600 (2011)
R.R. Wing, S. Phelan, Long-term weight loss maintenance. Am. J. Clin. Nutr. 82, 222S–225S (2005)
R.R. Wing, D.F. Tate, A.A. Gorin, H.A. Raynor, J.L. Fava, A self-regulation program for maintenance of weight loss. N. Engl. J. Med. 355, 1563–1571 (2006)
FAO/WHO/UNU. Energy and Protein requirements. Technical Report Series N° 724. World Health Organization, Geneva (1985)
M. Gargallo Fernandez, J.B. Marset, I.B. Lesmes, J.Q. Izquierdo, X.F. Sala, J. Salas-Salvado, [FESNAD-SEEDO consensus summary: evidence-based nutritional recommendations for the prevention and treatment of overweight and obesity in adults]. Endocrinol. Nutr. 59, 429–437 (2011)
SCOOP-VLCD task 7.3 Reports on tasks for scientific cooperation. Collection of data on products intended for use in very-low-calorie-diets. Report Brussels European Comission, September (2002)
S. Kaul, M.P. Rothney, D.M. Peters, W.K. Wacker, C.E. Davis, M.D. Shapiro, D.L. Ergun, Dual-energy X-ray absorptiometry for quantification of visceral fat. Obesity 20, 1313–1318 (2012)
L.K. Micklesfield, J.H. Goedecke, M. Punyanitya, K.E. Wilson, T.L. Kelly, Dual-energy X-ray performs as well as clinical computed tomography for the measurement of visceral fat. Obesity 20, 1109–1114 (2012)
Kelly TL, Wilson KE, Ruth CR Estimating visceral fat by dual-energy X-ray absorptiometry. US patent application number US2010-0234719 Hologic, Inc. (2010).
Kelly TL, Wilson KE, Ruth CR Visceral fat measurement. US patent application number US2011-0235881 Hologic, Inc. (2011).
I. Shai, D. Schwarzfuchs, Y. Henkin, D.R. Shahar, S. Witkow, I. Greenberg, R. Golan, D. Fraser, A. Bolotin, H. Vardi, O. Tangi-Rozental, R. Zuk-Ramot, B. Sarusi, D. Brickner, Z. Schwartz, E. Sheiner, R. Marko, E. Katorza, J. Thiery, G.M. Fiedler, M. Bluher, M. Stumvoll, M.J. Stampfer, Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N. Engl. J. Med. 359, 229–241 (2008)
B. Cabia, S. Andrade, M.C. Carreira, F.F. Casanueva, A.B. Crujeiras, A role for novel adipose tissue-secreted factors in obesity-related carcinogenesis. Obes. Rev. 17, 361–376 (2016)
M.C. Amato, G. Pizzolanti, V. Torregrossa, G. Misiano, S. Milano, C. Giordano, Visceral adiposity index (VAI) is predictive of an altered adipokine profile in patients with type 2 diabetes. PLoS One 9, e91969 (2014)
A.B. Crujeiras, B. Cabia, M.C. Carreira, M. Amil, J. Cueva, S. Andrade, L.M. Seoane, M. Pardo, A. Sueiro, J. Baltar, T. Morais, M.P. Monteiro, R. Lopez-Lopez, F.F. Casanueva, Secreted factors derived from obese visceral adipose tissue regulate the expression of breast malignant transformation genes. Int. J. Obes. 40, 514–523 (2015)
I.J. Neeland, C.R. Ayers, A.K. Rohatgi, A.T. Turer, J.D. Berry, S.R. Das, G.L. Vega, A. Khera, D.K. McGuire, S.M. Grundy, J.A. de Lemos, Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. Obesity 21, E439–447 (2013)
D. Vissers, W. Hens, J. Taeymans, J.P. Baeyens, J. Poortmans, L. Van Gaal, The effect of exercise on visceral adipose tissue in overweight adults: a systematic review and meta-analysis. PLoS One 8, e56415 (2013)
I. Wolz, I. Hilker, R. Granero, S. Jimenez-Murcia, A.N. Gearhardt, C. Dieguez, F.F. Casanueva, A.B. Crujeiras, J.M. Menchon, F. Fernandez-Aranda, “Food Addiction” in Patients with Eating Disorders is Associated with Negative Urgency and Difficulties to Focus on Long-Term Goals. Front Psychol. 7, 61 (2016)
M.M. Ibrahim, Subcutaneous and visceral adipose tissue: structural and functional differences. Obes. Rev. 11, 11–18 (2010)
B.L. Wajchenberg, D. Giannella-Neto, M.E. da Silva, R.F. Santos, Depot-specific hormonal characteristics of subcutaneous and visceral adipose tissue and their relation to the metabolic syndrome. Horm. Metab. Res. 34, 616–621 (2002)
B. Bjorndal, L. Burri, V. Staalesen, J. Skorve, R.K. Berge, Different adipose depots: their role in the development of metabolic syndrome and mitochondrial response to hypolipidemic agents. J. Obes. 2011, 490650 (2011)
L. Webber, D. Divajeva, T. Marsh, K. McPherson, M. Brown, G. Galea, J. Breda, The future burden of obesity-related diseases in the 53 WHO European-Region countries and the impact of effective interventions: a modelling study. BMJ Open 4, e004787 (2014)
H.P. Booth, T.A. Prevost, A.J. Wright, M.C. Gulliford, Effectiveness of behavioural weight loss interventions delivered in a primary care setting: a systematic review and meta-analysis. Fam. Pract. 31, 643–653 (2014)
A. Abdullah, R. Wolfe, J.U. Stoelwinder, M. de Courten, C. Stevenson, H.L. Walls, A. Peeters, The number of years lived with obesity and the risk of all-cause and cause-specific mortality. Int. J. Epidemiol. 40, 985–996 (2011)
R.Z. Stolzenberg-Solomon, C. Schairer, S. Moore, A. Hollenbeck, D.T. Silverman, Lifetime adiposity and risk of pancreatic cancer in the NIH-AARP Diet and Health Study cohort. Am. J. Clin. Nutr. 98, 1057–1065 (2013)
J.P. Reis, C.M. Loria, C.E. Lewis, T.M. Powell-Wiley, G.S. Wei, J.J. Carr, J.G. Terry, K. Liu, Association between duration of overall and abdominal obesity beginning in young adulthood and coronary artery calcification in middle age. Jama. 310, 280–288 (2013)
M. Arnold, N. Pandeya, G. Byrnes, A.G. Renehan, G.A. Stevens, M. Ezzati, J. Ferlay, J.J. Miranda, I. Romieu, R. Dikshit, D. Forman, I. Soerjomataram, Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol. 16, 36–46 (2015)
J.A. Nazare, J. Smith, A.L. Borel, P. Aschner, P. Barter, L. Van Gaal, C.E. Tan, H.U. Wittchen, Y. Matsuzawa, T. Kadowaki, R. Ross, C. Brulle-Wohlhueter, N. Almeras, S.M. Haffner, B. Balkau, J.P. Despres, Usefulness of measuring both body mass index and waist circumference for the estimation of visceral adiposity and related cardiometabolic risk profile (from the INSPIRE ME IAA study). Am. J. Cardiol. 115, 307–315 (2015)
Acknowledgments
We acknowledge the Pronokal Division of Protein Supplies SL Spain for providing free of charge the diet of the ketosis group.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
BM, ABC, DB, AG, and FFC received advisory board fees and or research grants from Pronokal Protein Supplies Spain.
Additional information
Basilio Moreno and Ana B Crujeiras authors equally contributed to this work.
Rights and permissions
About this article
Cite this article
Moreno, B., Crujeiras, A.B., Bellido, D. et al. Obesity treatment by very low-calorie-ketogenic diet at two years: reduction in visceral fat and on the burden of disease. Endocrine 54, 681–690 (2016). https://doi.org/10.1007/s12020-016-1050-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-016-1050-2