Abstract
Diabetes mellitus is a strong risk factor for chronic kidney disease and end-stage renal disease. Whether sex differences in chronic kidney disease and end-stage renal disease incidence exist among diabetic patients remains unclear. This systematic review and meta-analysis was conducted to evaluate the relative effect of diabetes on chronic kidney disease and end-stage renal disease risk in women compared with men. We systematically searched Embase, PubMed, and the Cochrane Library for both cohort and case–control studies until October 2015. Studies were selected if they reported a sex-specific relationship between diabetes mellitus and chronic kidney disease or end-stage renal disease. We generated pooled estimates across studies using random-effects meta-analysis after log transformation with inverse variance weighting. Ten studies with data from more than 5 million participants were included. The pooled adjusted risk ratio of chronic kidney disease associated with diabetes mellitus was 3.34 (95 % CI 2.27, 4.93) in women and 2.84 (95 % CI 1.73, 4.68) in men. The data showed no difference in diabetes-related chronic kidney disease risk between the sexes (pooled adjusted women-to-men relative risk ratio was 1.14 [95 % CI 0.97, 1.34]) except for end-stage renal disease—the pooled adjusted women-to men relative risk ratio was 1.38 (95 % CI 1.22, 1.55; p = 0.114, I² = 38.1 %). The study found no evidence of a sex difference in the association between diabetes mellitus and chronic kidney disease. However, the excess risk for end-stage renal disease was higher in women with diabetes than in men with the same condition, from which we assume that the female gender could accelerate the disease progression. Further studies are needed to support this notion and elucidate the underlying mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is a serious noncommunicable disease burden worldwide. Approximately 382 million people suffered from diabetes in 2013, and the prevalence is projected to increase substantially in the next decades. The number of cases is expected to rise to 592 million by 2035 [1]. Chronic kidney disease (CKD) is a global health concern as similar to diabetes. Prevalence is estimated to be 8–16 % worldwide, and DM is one of the most common risk factors [2]. Compared to non-diabetic patients, patients with diabetes have greater rate of the development of end-stage renal disease (ESRD) [3]. Furthermore, concurrent DM and CKD will lead to a higher risk of cardiovascular morbidity [4] and all-cause mortality [5].
A meta-analysis in 2000 suggested that the male gender is more prone to chronic renal disease in non-diabetic patients and is associated with a more rapid rate of progression [6]. Women are generally accepted to hold a reduced risk for non-diabetic kidney disease, indicating the female gender seems to be a protective factor, while the influence of sex differences on the incidence of diabetes-related kidney disease is still not well understood [7]. Several studies have reported higher CKD risk for men [8–10] or for women with diabetes [11–13], whereas some other reports have not found any difference between the sexes [14–16]. The data are inconsistent may be due to the different study designs, uncontrolled definition of outcomes, and variation in patient populations, especially for the fact that no study was primarily designed to examine this issue. Thus, a pooled analysis would be very helpful.
Therefore, we conducted this systemic review and meta-analysis in order to evaluate the sex-specific association between DM and risk of CKD and ESRD.
Methods
Search strategy
A computer-assisted search was systematically performed in Embase, PubMed, and the Cochrane Library until October 2015 for studies that have explored the association between DM and CKD or ESRD. We used a combined text word and Medical Subject Headings (MeSH) search strategy with the terms “diabetes mellitus”, “diabetes”, “chronic kidney disease”, “chronic renal disease”, “chronic renal insufficiency”, “chronic kidney insufficiency”, “chronic kidney failure”, “chronic renal failure”, “end stage renal disease”, “end-stage kidney disease”, “ESRD”, “sex”, “gender”, “men”, and “ women”. No restriction in publication date or country was imposed. References were scanned to identify other potentially relevant studies.
Selection criteria
Two independent reviewers (Yanjue Shen and Rongrong Cai) accessed the reports with the following inclusion criteria: (1) the study design was based on case–control or cohort study; (2) the study evaluated the association between DM and CKD or ESRD; (3) the study provided relative risks (RR) or odds ratios (OR), and their 95% confidence intervals (CIs) for the incidence of CKD or ESRD, or the data to calculate the parameters. Studies that adopted a cross-sectional design, did not adjust at least for age, merely reported the RRs or ORs for the prevalence of CKD or ESRD, or did not provide information about the variability around the point estimates were excluded. Besides, studies conducted in populations with a history of relevant outcomes were also excluded. The quality of the selected studies was evaluated according to the Newcastle-Ottawa Scale (NOS) [17], which ranged from zero star to nine stars, and the nine stars represent the highest methodological quality.
Data extraction
Baseline data were extracted from the included studies if possible as follows: first author of each study, study design (cohort or case–control study; prospective or retrospective study), study size (including the proportion of women), study location, study period, mean age at baseline in the enrolled studies, methods of diabetes assessment (registration, self-reported or medical assessment), variables adjusted in the analysis, and the risk estimates with corresponding 95 % CIs in men and women respectively. Any discrepancies were settled by discussion with the third author (Shaohua Wang).
Statistical analysis
The primary outcome was the incidence of all stages of CKD, and the secondary outcome was the incidence of ESRD only. CKD was defined as the abnormality of kidney structure or function for more than 3 months, and ESRD was the final common pathway for CKD [18]. CKD incidence was determined by incident albuminuria alone or through methods of determining the estimated glomerular filtration rate (eGFR), such as the simplified Modification of Diet in Renal Disease (MDRD) equation [19], the Cockcroft–Gault equation [20, 21], and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations [22, 23]. For each included report, the adjusted sex-specific RRs or ORs with 95 % CIs for individuals with diabetes versus those without diabetes were obtained to estimate the women-to-men relative risk ratio of RRs (RRR) and the corresponding 95 % CIs [24]. Considering the heterogeneity between studies, we have generated pooled estimates across studies using random-effects meta-analysis after log transformation with inverse variance weighting (both for log RR and log RRR).
The Cochran Q tests and I² statistics were used to assess the heterogeneity between studies. For the Q statistic, a p value below 0.10 meant statistically significant for heterogeneity. For I², a value greater than 50 % indicated the presence of substantial heterogeneity. Sensitivity analyses and meta-regression were employed to explore potential sources of heterogeneity [25]. Publication bias was assessed by using Egger’s regression test and funnel plot [26]. We also conducted a “trim-and-fill” analysis [27], which yielded an effect adjusted for funnel plot asymmetry. Finally, we used Stata version 11.0 to analyze the data, and a two-tailed p value of less than 0.05 was considered to be statistically significant.
Results
Search results
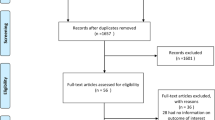
The search strategy identified 8637 reports after excluding duplicate articles (Fig. 1). Of these, 151 were retrieved for more detailed evaluation, and 10 studies that satisfied the inclusion and exclusion criteria were included in our meta-analysis, including 9 cohort studies [28–36] and 1 case–control study [37]. The baseline characteristics of the 5,542,757 participants are shown in Table 1. Of the ten included articles, three studies were conducted in Europe [28, 29, 31], four in North America [30, 34, 35, 37], and the other three in Asia [32, 33, 36]. The year of the baseline survey ranged from 1974 to 2011. The mean age of the individuals in the included studies ranged from 41 to 76 years. DM was determined by registration, self-reported or medical assessment. The quality rating of the involved studies ranged from 6 stars to 8 stars (supplementary Table 1). Potential confounders (at least for age) were controlled in all the studies.
Association between DM and risk of CKD and ESRD
The pooled summary RR for individuals with diabetes versus those without diabetes based on available data was 3.34 (95 % CI 2.27, 4.93) for women and 2.84 (95 % CI 1.73, 4.68) for men (Fig. 2). Significant heterogeneity was found among these studies (p < 0.001, I² = 99.3 % for women and p < 0.001, I² = 99.4 % for men). Exclusion of the studies that were not adjusted for hypertension [28, 29, 35, 37], the heterogeneity between the studies did not change significantly, with the value p < 0.001 and I² = 98.8 % both for women and men. The maximum-adjusted pooled RR was attenuated slightly both in women (RR 3.31 [95 % CI 2.06, 5.33]) and in men (RR 2.66 [95 % CI 1.41, 5.04]) (supplementary Fig. 1). Some factors may have accounted for the high heterogeneity between the studies, such as uncontrolled methods of selecting diabetes groups, potential confounding factors, and the large number of participants.

Note: Box sizes are in proportion to study weights. The study by Julia et al. provided separate estimates for patients with type 1 and type 2 diabetes. The study by Jay et al. provided separate estimates for cohorts from black race and the other race. Jan et al.1 and Yamagata et al.1 provided relative risk for incident CKD stage 1–2 in men and women; Jan et al.2 and Yamagata et al.2 provided relative risk for incident CKD stage 3–5 in men and women
Pooled adjusted relative risk for incident chronic kidney disease, comparing individuals with diabetes versus those without diabetes
Sex-specific association between DM and CKD
No evidence of a sex difference was found in the association between DM and all stages of CKD; the pooled adjusted women-to-men summary RRR for incident CKD was 1.14 (95 % CI 0.97, 1.34) with significant heterogeneity (p < 0.001, I² = 85.3 %) (Fig. 3). When we excluded the studies with only a CKD Stage 5 outcome, the heterogeneity between the studies was modified slightly, with the value p = 0.01 and I² = 62.2 %. In addition, the pooled adjusted RRR (RRR 0.97 [95 % CI 0.83, 1.12]) showed no significant association between diabetes and risk of CKD (supplementary Fig. 2). Meta-influence analysis showed a possible higher influence on the effect estimate attributable to the study by Jan et al. [29] in the analysis for specific association between DM and CKD without the studies with ESRD as outcome only (supplementary Fig. 3). Removal of this study did not substantially alter the pooled results (RRR 1.12 [95 % CI 0.97, 1.29]) and the heterogeneity decreased dramatically (p = 0.792, I² = 0.0 %). Moreover, sensitivity analyses omitting one study at a time did not alter the effect estimate, the summary RRRs ranged from 0.92 (95 % CI 0.79, 1.07) to 1.02 (95 % CI 0.89, 1.16) (supplementary Table 2). Meta-regression indicated that the pooled RRR for CKD was not affected by the mean age of the participants (p = 0.220), by baseline prevalence of diabetes (p = 0.861), nor by the baseline year of each study (p = 0.333) (supplementary Fig. 4).

Note: Box sizes are in proportion to study weights. The study by Julia et al. provided separate estimates for patients with type 1 and type 2 diabetes. The study by Jay et al. provided separate estimates for cohorts from black race and the other race. Jan et al.1 and Yamagata et al.1 provided adjusted women-to-men relative risk ratio for incident CKD stage 1–2; Jan et al.2 and Yamagata et al.2 provided adjusted women-to-men relative risk ratio for incident CKD stage 3–5
Pooled adjusted women-to-men relative risk ratio for incident chronic kidney disease, comparing individuals with diabetes versus those without diabetes
Sex-specific association between DM and ESRD
For the incidence of ESRD, the pooled women-to-men RRR was 1.38 (95 % CI 1.22, 1.55) (Fig. 4), indicating a significant sex difference between DM and ESRD. The p value was 0.114 and the I² statistic was 38.1 %, which denoted no significant heterogeneity between the studies. When we excluded the studies that were not adjusted for hypertension [28, 29, 35, 37], the maximum-adjusted pooled RRR was increased slightly (RR 1.39 [95 % CI 1.16, 1.67]) without heterogeneity (p = 0.123, I² = 44.9 %) (supplementary Fig. 5). Meta-influence analysis showed a possible higher influence on the effect estimate attributable to the study by Jay et al. (Black) [30] (supplementary Fig. 6). Removal of this study improved the strength of the association between DM and ESRD (RRR 1.43 [95 % CI 1.29, 1.59]), and the heterogeneity decreased dramatically (p = 0.366, I² = 8.3 %). In a sensitivity analysis in which studies were omitted one at a time with the remaining studies pooled, the summary RRRs ranged from 1.35 (95 % CI 1.20, 1.52) when the study by Falk et al. [28] was excluded to 1.43 (95 % CI 1.29, 1.59) when the study by Jay et al. (Black) [30] was removed (supplementary Table 3). In addition, we performed subgroup analysis to further elicit the association between diabetes and ESRD (Table 2). Results suggested that the pooled RRRs did not differ significantly in subgroup analyses. Furthermore, no statistical significance was found between prospective and retrospective studies (p = 0.093), between the European and the North American regions (p = 0.176), between the mean age below and above 60 years old (p = 0.151), nor between registration and other methods of DM assessment (p = 0.153).

Note: Box sizes are in proportion to study weights. The study by Julia et al. provided separate estimates for patients with type 1 and type 2 diabetes. The study by Jay et al. provided separate estimates for cohorts from black race and the other race
Pooled adjusted women-to-men relative risk ratio for incident end stage renal disease, comparing individuals with diabetes versus those without diabetes
Publication bias
Egger’s regression test provided no evidence of substantial publication bias ( p = 0.255) for the incidence of CKD. However, the visual inspection of funnel plot showed some extent of asymmetry (Fig. 5). Thus, we conducted the “trim-and-fill” analysis (supplementary Fig. 7). No funnel plot asymmetry was observed after including one study, and the adjustment did not alter the results (RRR 1.13 [95 % CI 0.96, 1.32]), thereby indicating the absence of significant publication bias. For the incidence of ESRD, the Egger’s regression test provided no evidence of substantial publication bias (p = 0.159).
Discussion
In this systematic review and meta-analysis with data for more than 5 million individuals from previous observational studies, we found that DM was a strong risk factor for CKD and ESRD in both men and women. However, no evidence on sex difference was noted in the association between DM and incident CKD except for ESRD after adjustment for several important variables. The pooled adjusted RRR for ESRD was 38 % higher in women with diabetes than in men with diabetes, thereby indicating that the female sex may exacerbate the disease progression. The results did not vary substantially by study design, region, mean age of individuals, and methods of diabetes assessment.
Several authors have explained the differences between DM and CKD or ESRD in sex distribution, but no uncontestable statement has been established to date. The United Kingdom Prospective Diabetes Study 74 [38] found that the male sex was at greater risk of incident albuminuria in diabetes. One possible reason for the discrepancy between their views and ours may be that their recruited individuals were aged between 25 and 65 years old. Although women exhibited a more positive CKD risk profile than men at younger ages, the trends seemed to reserve with aging, such as in hypertension [39]. A previous study noted that a higher percentage of women would suffer from hypertension than men after 65 years old. Similarly, the data from Luk [40] showed that the male gender was a predictive factor of incident eGFR < 60 mL/min per 1.73 m² by the MDRD equation adjusted for the Chinese population. Additionally, a recent study carried out by Margaret and his colleagues [12] reported a higher incidence of CKD in diabetic women as measured by CKD-EPI equations. However, comparisons were limited because different eGFR equations may contribute to different values from the actual GFR, and the exclusive of the first stage of CKD may also lead to different results. The inconsistent data may be alternatively explained by the lack of adjustment for potential confounding factors.
In earlier reports, diabetes has generally been found to cause a greater adverse effect on major CKD risk factors in women than in men, requiring more consideration in clarifying the association between the sexes. From such studies, we noticed that women with diabetes were more likely to have hypertension [41–44], dyslipidemia [41, 42, 44], and obesity [42], and were less likely to achieve the target value for glycated hemoglobin [41–43]. In other words, women with diabetes had a higher chance of failing treatment targets than men. Likewise, the greater change in central adiposity and insulin resistance in women than in men led to endothelial dysfunction and inflammation [45], which were recognized as novel risk factors in CKD and ESRD patients. A recent meta-analysis published in Lancet showed that the excess risk of stroke associated with diabetes was significantly higher in women compared with men [24]. Furthermore, females exhibited a second end-organ complication to a much greater extent than did men [13]. That is to say, diabetic women experience more serious outcomes, and the estimates should be revised upward to accommodate the greater excess risk observed in women. In our meta-analysis, adjustment for major CKD risk factors showed no significant difference from the age-adjusted estimates. This result implies that the greater excess risk of incident CKD observed in diabetic women couldn’t wholly account for the differences between the sexes.
The other potential mechanisms for this sex-specific difference may contribute to the diversity of sex hormones. The effect of sex hormones on diabetic CKD progression remains uncertain, while it is generally accepted that estrogens slow down the progression of CKD not related to diabetes [46–48], and testosterone seems to exacerbate such progression [49, 50]. Recent literature has speculated that estrogens are also renoprotective in diabetic women as they could reduce albuminuria, glomerulosclerosis, and tubulointerstitial fibrosis via regulating extracellular matrix synthesis and degradation [51–54]. Estrogens decrease the synthesis of collagen types I and IV, the expression of fibronectin and laminin proteins. Furthermore, estrogens augment the activity of matrix metalloproteinases [51, 53], regulate the expression of transforming growth factor-β [51, 54], and control podocyte signaling pathways [52] to protect the kidney. However, female protection does not appear to work in a diabetic environment possibly because of the imbalance in sex hormones induced by hyperglycemia [55]. During clamped hyperglycemia, only women exhibited reductions in renal vascular resistance and filtration fraction [56]. In our meta-analysis, we couldn’t find a difference between the sexes in incidence of CKD. On the contrary, female sex appeared to be a risk factor for incident ESRD. The inconsistent result between the CKD and ESRD may be attributed to the persistent effect of hyperglycemia and sharp decrease in estrogens in post-menopause women as most ESRD cases occur in patients were 65–74 years old [57]. Additionally, older women exhibit a more negative CKD risk profile and it will deteriorate to a greater extent than those of age-adjusted men before regressing to ESRD, which has been implied earlier to involve a cooperative effect. Thus, we may hypothesize that the female sex accelerates the disease progression. However, the precise mechanisms are poorly understood and require further investigation.
Strengths and limitations
To our knowledge, this study is the first systematic review and meta-analysis that investigated the possible sex differences in the incidence of diabetes-related CKD and ESRD. We believe that the main advantage of our study was the use of general keywords for extensive literature search and the skimming reading of more than 8000 relevant articles. In addition, it is preferable that the majority of the ten included studies were cohort studies because of evidently causal hypothesis verification. Furthermore, the mean NOS score of 7.2 suggested the high quality of the included studies. This meta-analysis involved several limitations. First, we did not take mortality into account as a competing risk factor. As a result, the high mortality among diabetic patients may have contributed to the inverse relationship between DM and CKD or ESRD. However, the most powerful risk factor for death is age, which is different from the factors for CKD, and is unlikely to differentially affect women more than men. Second, some heterogeneity across studies existed, but the subgroup and meta-regression analyses did not find substantial differences in the results. Third, the definition of the outcomes and the methods of diabetes assessment differed among studies. Thus, a variety of surrogate outcomes were used to calculate the effect sizes. For example, the CKD-EPI equation was regarded to be more accurate than the MDRD equation at the GFR of 60 mL/min/1.73 m² or more. However, determining the CKD incidence by using CKD-EPI equations is impossible to accomplish for all included studies. Fortunately, no difference within studies was noted. Hence, the use of RRR remains valid. Finally, we were unable to explore the effect of onset age of diabetes and the duration of diabetes because of insufficient data for these variables, which may contribute to the data inconsistency.
Conclusion
In conclusion, diabetes is a strong risk factor for CKD and ESRD on the basis of an appropriate assessment of known risk factors. Our systematic review and meta-analysis of ten observational studies could not find any difference in incident CKD between men and women with diabetes compared with those without diabetes, but the female sex appeared to accelerate the disease progression. Therefore, physicians should pay closer attention to female patients and provide early intervention prior to ESRD occurrence to retard disease progression. Besides, the measurement of testosterone and estradiol in both men and women may be highly beneficial. Awareness of early symptoms of CKD is favorable for improving clinical outcomes especially in women. Further large-scale prospective cohort studies are warranted to detect the underlying mechanisms and support our finding of the association between diabetes and risk of CKD and ESRD.
References
L. Guariguata, D.R. Whiting, I. Hambleton, J. Beagley, U. Linnenkamp, J.E. Shaw, Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 103(2), 137–149 (2014). doi:10.1016/j.diabres.2013.11.002
V. Jha, G. Garcia-Garcia, K. Iseki, Z. Li, S. Naicker, B. Plattner, R. Saran, A.Y.-M. Wang, C.-W. Yang, Chronic kidney disease: global dimension and perspectives. Lancet 382(9888), 260–272 (2013). doi:10.1016/s0140-6736(13)60687-x
M. Kastarinen, A. Juutilainen, H. Kastarinen, V. Salomaa, P. Karhapaa, J. Tuomilehto, C. Gronhagen-Riska, P. Jousilahti, P. Finne, Risk factors for end-stage renal disease in a community-based population: 26-year follow-up of 25,821 men and women in eastern Finland. J. Intern. Med. 267(6), 612–620 (2010). doi:10.1111/j.1365-2796.2009.02197.x
Y.T. Chang, J.L. Wu, C.C. Hsu, J.D. Wang, J.M. Sung, Diabetes and end-stage renal disease synergistically contribute to increased incidence of cardiovascular events: a nationwide follow-up study during 1998-2009. Diabetes Care 37(1), 277–285 (2014). doi:10.2337/dc13-0781
S. Nag, R. Bilous, W. Kelly, S. Jones, N. Roper, V. Connolly, All-cause and cardiovascular mortality in diabetic subjects increases significantly with reduced estimated glomerular filtration rate (eGFR): 10 years’ data from the South Tees Diabetes Mortality study. Diabetic Med. 24(1), 10–17 (2007). doi:10.1111/j.1464-5491.2007.02023.x
J. Neugarten, A. Acharya, S.R. Silbiger, Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. J. Am. Soc. Nephrol. 11(2), 319–329 (2000)
C. Maric, Sex, diabetes and the kidney. Am. J. Physiol. Renal Physiol. 296(4), F680–F688 (2009). doi:10.1152/ajprenal.90505.2008
A. Mollsten, M. Svensson, I. Waernbaum, Y. Berhan, S. Schon, L. Nystrom, H.J. Arnqvist, G. Dahlquist, Swedish Childhood Diabetes Study, G., Diabetes Incidence Study in, S., Swedish Renal, R. Cumulative risk, age at onset, and sex-specific differences for developing end-stage renal disease in young patients with type 1 diabetes: a nationwide population-based cohort study. Diabetes 59(7), 1803–1808 (2010). doi:10.2337/db09-1744
K. Raile, A. Galler, S. Hofer, A. Herbst, D. Dunstheimer, P. Busch, R.W. Holl, Diabetic nephropathy in 27,805 children, adolescents, and adults with type 1 diabetes: effect of diabetes duration, A1C, hypertension, dyslipidemia, diabetes onset, and sex. Diabetes Care 30(10), 2523–2528 (2007). doi:10.2337/dc07-0282
P. Hovind, L. Tarnow, P. Rossing, B.R. Jensen, M. Graae, I. Torp, C. Binder, H.H. Parving, Predictors for the development of microalbuminuria and macroalbuminuria in patients with type 1 diabetes: inception cohort study. Br. Med. J. 328(7448), 1105 (2004). doi:10.1136/bmj.38070.450891.FE
M.K. Yu, C.R. Lyles, L.A. Bent-Shaw, B.A. Young, A. Pathways, Risk factor, age and sex differences in chronic kidney disease prevalence in a diabetic cohort: the pathways study. Am. J. Nephrol. 36(3), 245–251 (2012). doi:10.1159/000342210
M.K. Yu, W. Katon, B.A. Young, Associations between sex and incident chronic kidney disease in a prospective diabetic cohort. Nephrology 20(7), 451–458 (2015). doi:10.1111/nep.12468
M.C. Monti, J.T. Lonsdale, C. Montomoli, R. Montross, E. Schlag, D.A. Greenberg, Familial risk factors for microvascular complications and differential male-female risk in a large cohort of American families with type 1 diabetes. J. Clin. Endocrinol. Metab. 92(12), 4650–4655 (2007). doi:10.1210/jc.2007-1185
P. Rossing, P. Hougaard, H.H. Parving, Risk factors for development of incipient and overt diabetic nephropathy in type 1 diabetic patients: a 10-year prospective observational study. Diabetes Care 25(5), 859–864 (2002)
J.A. Breyer, R.P. Bain, J.K. Evans, N.S. Nahman Jr., E.J. Lewis, M. Cooper, J. McGill, T. Berl, Predictors of the progression of renal insufficiency in patients with insulin-dependent diabetes and overt diabetic nephropathy. The Collaborative Study Group. Kidney Int. 50(5), 1651–1658 (1996)
K. Okada, M. Yanai, K. Takeuchi, K. Matsuyama, K. Nitta, K. Hayashi, S. Takahashi, Sex differences in the prevalence, progression, and improvement of chronic kidney disease. Kidney Blood Press. Res. 39(4), 279–288 (2014). doi:10.1159/000355805
A. Stang, Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25(9), 603–605 (2010). doi:10.1007/s10654-010-9491-z
P.E. Stevens, A. Levin, Kidney disease: improving global outcomes chronic kidney disease guideline development work group, M., Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 158(11), 825–830 (2013). doi:10.7326/0003-4819-158-11-201306040-00007
A.S. Levey, J. Coresh, T. Greene, J. Marsh, L.A. Stevens, J.W. Kusek, F. Van Lente, Chronic Kidney Disease Epidemiology, C.: Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin. Chem. 53(4), 766–772 (2007). doi:10.1373/clinchem.2006.077180
J.L. Teruel, J. Sabater, C. Galeano, M. Rivera, J.L. Merino, M. Fernandez Lucas, R. Marcen, J. Ortuno, [The Cockcroft-Gault equation is better than MDRD equation to estimate the glomerular filtration rate in patients with advanced chronic renal failure]. Nefrologia 27(3), 313–319 (2007)
A.A. Sanusi, A. Akinsola, A.A. Ajayi, Creatinine clearance estimation from serum creatinine values: evaluation and comparison of five prediction formulae in Nigerian patients. Afr. J. Med. Med. Sci. 29(1), 7–11 (2000)
A.S. Levey, L.A. Stevens, C.H. Schmid, Y.L. Zhang, A.F. Castro 3rd, H.I. Feldman, J.W. Kusek, P. Eggers, F. Van Lente, T. Greene, J. Coresh, E.P.I. Ckd, A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150(9), 604–612 (2009)
L. Hoste, L. Dubourg, L. Selistre, V.C. De Souza, B. Ranchin, A. Hadj-Aissa, P. Cochat, F. Martens, H. Pottel, A new equation to estimate the glomerular filtration rate in children, adolescents and young adults. Nephrol. Dial. Transpl. 29(5), 1082–1091 (2014). doi:10.1093/ndt/gft277
S.A.E. Peters, R.R. Huxley, M. Woodward, Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775 385 individuals and 12 539 strokes. Lancet 383(9933), 1973–1980 (2014). doi:10.1016/S0140-6736(14)60040-4
J.P. Higgins, S.G. Thompson, Quantifying heterogeneity in a meta-analysis. Stat. Med. 21(11), 1539–1558 (2002). doi:10.1002/sim.1186
M. Egger, G. Davey Smith, M. Schneider, C. Minder, Bias in meta-analysis detected by a simple, graphical test. Br. Med. J. 315(7109), 629–634 (1997)
S. Duval, E. Weinhandl, Correcting for Publication Bias in the Presence of Covariates. Methods Research Report (Prepared by the Minnesota Evidence-based Practice Center under Contract No. 290-02-0009.) AHRQ Publication No. 11-EHC041-EF. Rockville, MD: Agency for Healthcare Research and Quality. September 2011. Available at: www.effectivehealthcare.ahrq.gov/reports/final.cfm
F. Hoffmann, B. Haastert, M. Koch, G. Giani, G. Glaeske, A. Icks, The effect of diabetes on incidence and mortality in end-stage renal disease in Germany. Nephrol. Dial. Transpl. 26(5), 1634–1640 (2011). doi:10.1093/ndt/gfq609
J.C. van Blijderveen, S.M. Straus, R. Zietse, B.H. Stricker, M.C. Sturkenboom, K.M. Verhamme, A population-based study on the prevalence and incidence of chronic kidney disease in the Netherlands. Int. Urol. Nephrol. 46(3), 583–592 (2014). doi:10.1007/s11255-013-0563-3
J.L. Xue, P.W. Eggers, L.Y. Agodoa, R.N. Foley, A.J. Collins, Longitudinal study of racial and ethnic differences in developing end-stage renal disease among aged medicare beneficiaries. J. Am. Soc. Nephrol. 18(4), 1299–1306 (2007). doi:10.1681/ASN.2006050524
J. Hippisley-Cox, C. Coupland, Predicting the risk of chronic kidney disease in men and women in England and Wales: prospective derivation and external validation of the QKidney Scores. BMC Fam. Pract. 11, 49 (2010). doi:10.1186/1471-2296-11-49
K. Nagai, C. Saito, F. Watanabe, R. Ohkubo, C. Sato, T. Kawamura, K. Uchida, A. Hiwatashi, H. Kai, K. Ishida, T. Sairenchi, K. Yamagata, Annual incidence of persistent proteinuria in the general population from Ibaraki annual urinalysis study. Clin. Exp. Nephrol. 17(2), 255–260 (2013). doi:10.1007/s10157-012-0692-5
M. Tohidi, M. Hasheminia, R. Mohebi, D. Khalili, F. Hosseinpanah, B. Yazdani, A.A. Nasiri, F. Azizi, F. Hadaegh, Incidence of chronic kidney disease and its risk factors, results of over 10 year follow up in an Iranian cohort. PloS One 7(9), e45304 (2012). doi:10.1371/journal.pone.0045304
M.K. Haroun, Risk factors for chronic kidney disease: a prospective study of 23,534 men and women in Washington County, Maryland. J. Am. Soc. Nephrol. 14(11), 2934–2941 (2003). doi:10.1097/01.asn.0000095249.99803.85
R.F. Dyck, L. Tan, Rates and outcomes of diabetic end-stage renal disease among registered native people in Saskatchewan. Can. Med. Assoc. J. 150(2), 203–208 (1994)
K. Yamagata, K. Ishida, T. Sairenchi, H. Takahashi, S. Ohba, T. Shiigai, M. Narita, A. Koyama, Risk factors for chronic kidney disease in a community-based population: a 10-year follow-up study. Kidney Int. 71(2), 159–166 (2007). doi:10.1038/sj.ki.5002017
E.S. Johnson, D.H. Smith, M.L. Thorp, X. Yang, J. Juhaeri, Predicting the risk of end-stage renal disease in the population-based setting: a retrospective case-control study. BMC Nephrol. 12, 17 (2011). doi:10.1186/1471-2369-12-17
R. Retnakaran, C.A. Cull, K.I. Thorne, A.I. Adler, R.R. Holman, U.S. Group, Risk factors for renal dysfunction in type 2 diabetes: U.K. prospective diabetes study 74. Diabetes 55(6), 1832–1839 (2006). doi:10.2337/db05-1620
V.L. Roger, A.S. Go, D.M. Lloyd-Jones, E.J. Benjamin, J.D. Berry, W.B. Borden, D.M. Bravata, S. Dai, E.S. Ford, C.S. Fox, H.J. Fullerton, C. Gillespie, S.M. Hailpern, J.A. Heit, V.J. Howard, B.M. Kissela, S.J. Kittner, D.T. Lackland, J.H. Lichtman, L.D. Lisabeth, D.M. Makuc, G.M. Marcus, A. Marelli, D.B. Matchar, C.S. Moy, D. Mozaffarian, M.E. Mussolino, G. Nichol, N.P. Paynter, E.Z. Soliman, P.D. Sorlie, N. Sotoodehnia, T.N. Turan, S.S. Virani, N.D. Wong, D. Woo, M.B. Turner; American Heart Association Statistics, C., Stroke Statistics, S.: Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation 125(1), e2–e220 (2012). doi:10.1161/CIR.0b013e31823ac046
A.O. Luk, W.Y. So, R.C. Ma, A.P. Kong, R. Ozaki, V.S. Ng, L.W. Yu, W.W. Lau, X. Yang, F.C. Chow, J.C. Chan, P. C. Tong, Hong Kong Diabetes, R.: Metabolic syndrome predicts new onset of chronic kidney disease in 5,829 patients with type 2 diabetes: a 5-year prospective analysis of the Hong Kong Diabetes Registry. Diabetes Care 31(12), 2357–2361 (2008). doi:10.2337/dc08-0971
I. Gouni-Berthold, H.K. Berthold, C.S. Mantzoros, M. Bohm, W. Krone, Sex disparities in the treatment and control of cardiovascular risk factors in type 2 diabetes. Diabetes Care 31(7), 1389–1391 (2008). doi:10.2337/dc08-0194
G. Penno, A. Solini, E. Bonora, C. Fondelli, E. Orsi, G. Zerbini, R. Trevisan, M. Vedovato, G. Gruden, L. Laviola, A. Nicolucci, G. Pugliese, I. Renal; Cardiovascular Events Study, G.: Gender differences in cardiovascular disease risk factors, treatments and complications in patients with type 2 diabetes: the RIACE Italian multicentre study. J. Int. Med. 274(2), 176–191 (2013). doi:10.1111/joim.12073
D.J. Wexler, R.W. Grant, J.B. Meigs, D.M. Nathan, E. Cagliero, Sex disparities in treatment of cardiac risk factors in patients with type 2 diabetes. Diabetes Care 28(3), 514–520 (2005)
A. Ferrara, C.M. Mangione, C. Kim, D.G. Marrero, D. Curb, M. Stevens, J.V. Selby, Translating Research Into Action for Diabetes Study, G.: Sex disparities in control and treatment of modifiable cardiovascular disease risk factors among patients with diabetes: translating research into action for diabetes (TRIAD) study. Diabetes Care 31(1), 69–74 (2008). doi:10.2337/dc07-1244
S.G. Wannamethee, O. Papacosta, D.A. Lawlor, P.H. Whincup, G.D. Lowe, S. Ebrahim, N. Sattar, Do women exhibit greater differences in established and novel risk factors between diabetes and non-diabetes than men? The British Regional Heart Study and British Women’s Heart Health Study. Diabetologia 55(1), 80–87 (2012). doi:10.1007/s00125-011-2284-4
S. Xiao, D.G. Gillespie, C. Baylis, E.K. Jackson, R.K. Dubey, Effects of estradiol and its metabolites on glomerular endothelial nitric oxide synthesis and mesangial cell growth. Hypertension 37(2 Pt 2), 645–650 (2001)
L.L. Yanes, J.C. Sartori-Valinotti, J.F. Reckelhoff, Sex steroids and renal disease: lessons from animal studies. Hypertension 51(4), 976–981 (2008). doi:10.1161/HYPERTENSIONAHA.107.105767
S. Doublier, E. Lupia, P. Catanuto, S.J. Elliot, Estrogens and progression of diabetic kidney damage. Curr. Diabetes. Rev. 7(1), 28–34 (2011)
J.F. Reckelhoff, L.L. Yanes, R. Iliescu, L.A. Fortepiani, J.P. Granger, Testosterone supplementation in aging men and women: possible impact on cardiovascular-renal disease. Am. J. Physiol. Renal. Physiol. 289(5), F941–948 (2005). doi:10.1152/ajprenal.00034.2005
L.A. Fortepiani, L. Yanes, H. Zhang, L.C. Racusen, J.F. Reckelhoff, Role of androgens in mediating renal injury in aging SHR. Hypertension 42(5), 952–955 (2003). doi:10.1161/01.HYP.0000099241.53121.7F
A. Dixon, C. Maric, 17beta-Estradiol attenuates diabetic kidney disease by regulating extracellular matrix and transforming growth factor-beta protein expression and signaling. Am. J. Physiol. Renal Physiol. 293(5), F1678–F1690 (2007). doi:10.1152/ajprenal.00079.2007
P. Catanuto, S. Doublier, E. Lupia, A. Fornoni, M. Berho, M. Karl, G.E. Striker, X. Xia, S. Elliot, 17 beta-estradiol and tamoxifen upregulate estrogen receptor beta expression and control podocyte signaling pathways in a model of type 2 diabetes. Kidney Int. 75(11), 1194–1201 (2009). doi:10.1038/ki.2009.69
R.W. Mankhey, C.C. Wells, F. Bhatti, C. Maric, 17beta-Estradiol supplementation reduces tubulointerstitial fibrosis by increasing MMP activity in the diabetic kidney. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292(2), R769–R777 (2007). doi:10.1152/ajpregu.00375.2006
R.W. Mankhey, F. Bhatti, C. Maric, 17beta-Estradiol replacement improves renal function and pathology associated with diabetic nephropathy. Am. J. Physiol. Renal Physiol. 288(2), F399–F405 (2005). doi:10.1152/ajprenal.00195.2004
Q. Xu, C.C. Wells, J.H. Garman, L. Asico, C.S. Escano, C. Maric, Imbalance in sex hormone levels exacerbates diabetic renal disease. Hypertension 51(4), 1218–1224 (2008). doi:10.1161/HYPERTENSIONAHA.107.100594
D.Z. Cherney, E.B. Sochett, J.A. Miller, Gender differences in renal responses to hyperglycemia and angiotensin-converting enzyme inhibition in diabetes. Kidney Int. 68(4), 1722–1728 (2005). doi:10.1111/j.1523-1755.2005.00588.x
C.A. Jones, A.S. Krolewski, J. Rogus, J.L. Xue, A. Collins, J.H. Warram, Epidemic of end-stage renal disease in people with diabetes in the United States population: do we know the cause?. Kidney Int. 67(5), 1684–1691 (2005). doi:10.1111/j.1523-1755.2005.00265.x
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic Supplementary material
Rights and permissions
About this article
Cite this article
Shen, Y., Cai, R., Sun, J. et al. Diabetes mellitus as a risk factor for incident chronic kidney disease and end-stage renal disease in women compared with men: a systematic review and meta-analysis. Endocrine 55, 66–76 (2017). https://doi.org/10.1007/s12020-016-1014-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-016-1014-6