Abstract
Prolactin is an anterior pituitary hormone necessary for fertility, pregnancy maintenance, lactation, and aspects of maternal behavior. In rodents, there is a surge of prolactin on the afternoon of proestrus, and a semi-circadian pattern of prolactin surges during early pregnancy, with a diurnal and nocturnal surge every day. Both of these patterns can be replicated in ovariectomized rats. A prior study demonstrated that central antagonism of κ-opioid receptors, the target of dynorphin, largely abolished the nocturnal prolactin surge in pregnant rats. We build on this to determine whether dynorphin, perhaps from the arcuate population that co-express kisspeptin, neurokinin B, and dynorphin (KNDy neurons), also contributes to the estradiol- or cervical stimulation-induced surges in ovariectomized rats. Ovariectomized rats were treated with either estradiol or cervical stimulation to induce prolactin surge(s). Blood samples were taken around the expected surge time to determine the effect of either acute κ-opioid receptor antagonism or previous chemical ablation of the KNDy population on prolactin levels. Dynorphin antagonism does significantly disrupt the nocturnal prolactin surge, but it does not contribute to the estradiol-induced surge. Chemical ablation of KNDy neurons had opposite effects; ablation of 40 % of the KNDy neurons had no impact on the nocturnal prolactin surge, while a somewhat larger ablation significantly reduced the size of the estradiol-induced surge. We conclude that dynorphin is likely a controlling factor for the nocturnal surge induced by cervical stimulation, and that other KNDy neuron products must play a role in the estradiol-induced surge.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The anterior pituitary hormone prolactin is necessary for fertility, reproduction, and aspects of maternal behavior [1]. Besides its role in lactation, prolactin is also elevated on the afternoon of proestrus during the rodent estrous cycle. The surge of prolactin, along with other reproductive hormones like luteinizing hormone (LH), help prime the female for ovulation, mating, and pregnancy [2]. This single surge of prolactin can be reproduced in ovariectomized (OVX) rats treated with estradiol (OVE). The continued exposure to estradiol (E2) produces a circadian prolactin surge in the afternoon (~1700 in our facilities). During the first half of pregnancy, prolactin exhibits a semi-circadian surge pattern: one nocturnal (~0300) and one diurnal surge (~1700, similar to the E2 surge). Induction of these surges does not require steroid hormone exposure, can occur in the absence of a fertile mating [3], and are important for pregnancy maintenance [2]. Levels of prolactin that are chronically too high or low have been linked to miscarriage, infertility, and sexual dysfunction [4, 5], as well as abnormal maternal behavior and post-partum depression [6]. The study of prolactin regulation is therefore of importance to women’s reproductive health and some evidence suggests that a long-term pattern of prolactin surges exist in women after intercourse with orgasm [7]. Cervical stimulation (CS), used to mimic mating, triggers this semi-circadian prolactin release pattern in OVX rats.
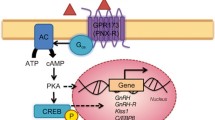
Prolactin is tonically inhibited by dopamine from the tuberoinfundibular dopamine (TIDA) neurons of the arcuate nucleus. TIDA terminals release dopamine at the median eminence, where the dopamine is carried via portal vessels to the pituitary gland to inhibit prolactin release. As a result, a prerequisite for prolactin surges is a decrease in dopamine neuron activity [8]. Additional factors that influence prolactin may act on TIDA neurons or directly on lactotrophs [2]. Heterogeneity within the arcuate and the complexity of the regulatory circuit have made it challenging to pinpoint how these other factors contribute to the generation of circadian and semi-circadian prolactin release patterns, despite decades of research.
A number of studies have investigated the role played by opioids in prolactin release, although these studies often involved peripheral drug administration or non-specific receptor blockers [9–12]. A much more definitive study used antagonists for different types of opioid receptors delivered directly into the lateral ventricle to show that dynorphin is a crucial factor controlling the nocturnal prolactin surge in pregnant rats [13]. In particular, the authors demonstrated that the nocturnal prolactin surge was greatly attenuated when κ-opioid receptors, the targets of dynorphin, were blocked with the antagonist norbinaltorphimine (nor-BNI). Recently, our lab has shown that dynorphin plays a role in regulating the LH surge in OVE rats [14]. This dynorphin originates from the arcuate population of neurons that co-secrete kisspeptin, neurokinin B, and dynorphin (KNDy neurons). These prior studies motivated the current study, which determines (1) whether nor-BNI attenuates the nocturnal prolactin surge in OVX-CS rats as it did in pregnant rats, (2) whether nor-BNI attenuates the prolactin surge in OVE rats, and (3) whether KNDy neurons are responsible for the contributions of dynorphin on either prolactin rhythm. The KNDy neurons have been studied almost exclusively for their role in controlling the activity of gonadotropin-releasing hormone neurons and LH release [15–18]. However, recent work has demonstrated that kisspeptin, likely released by KNDy neurons, stimulates prolactin secretion via inhibitory effects on the TIDA neurons in OVE rats [19].
Dynorphin preferentially binds to the κ-opioid receptor, which initiates an inhibitory signal transduction pathway [20]. In situ hybridization studies have demonstrated κ-opioid receptor gene expression within the hypothalamus [21], and opioid fibers are located near arcuate dopamine neurons [22]. Recently, a study in mice showed that dynorphin application inhibited the firing patterns of arcuate dopaminergic neurons [23]. In this report, we use immunohistochemistry to show the presence of κ-opioid receptors in the arcuate nucleus, providing a site of action of dynorphin at or near the TIDA neurons. Using the κ-opioid specific antagonist nor-BNI, we demonstrate that dynorphin plays a major role in the nocturnal prolactin surge induced by CS, but it does not contribute to the afternoon prolactin surge induced by E2. To determine the role played by KNDy neurons in the prolactin rhythms, we chemically ablated a large fraction of the neurons as described in Mittelman-Smith et al. [24] and as used recently in our studies on LH [14]. We found that ablation of ~40 % of the KNDy neurons has no effect on the nocturnal CS-induced prolactin surge. A somewhat larger ~50 % ablation significantly attenuates the afternoon E2-induced prolactin surge. We conclude that KNDy neurons play a role in the afternoon surge of prolactin induced by E2, but not through dynorphin. They appear to play little or no role in the CS-induced nocturnal surge, although such a role cannot be excluded given the partial ablation.
Materials and methods
Animals
Adult female Sprague–Dawley rats weighting 220–280 g (Charles River, Raleigh, NC) were housed in groups of three in a Laboratory Animal Resources facility under a 12-h light, 12-h dark cycle (lights on at 0600) and controlled temperature. Food and water were provided ad libitum. Animal procedures were approved by the Florida State University Animal Care and Use Committee. Upon arrival at our facilities, all rats were ovariectomized bilaterally through a single ventral midline incision under isoflurane anesthesia (Aerrane; Baxter, Deerfiled, IL) and allowed to recover for 1 week. Immediately after surgery, each rat was given a single dose (0.2 mg/100 g body weight) of metacam (Boehringer Ingelheim, St Joseph, MO). After further surgeries, rats were housed singly.
Jugular vein catheterization and blood sampling
Rats were anesthetized with isoflurane and a Micro-Renathane catheter tube (MRE-040, 0.04′ outer diameter × 0.025′ inner diameter, Braintree Scientific, Braintree, MA) filled with sterile saline (0.9 % NaCl, Teknova, Hollister, CA) was inserted through the external jugular vein into the right atrium. It was stitched in place subcutaneously and exteriorized at the back of the animal. After surgery, the tube was filled with gentamicin sulfate (Alexis, San Diego, CA) to prevent bacterial growth. Rats were given an injection of metacam (0.2 mg/100 g body weight). The evening before the first day of the experiment, a tube extension filled with saline was connected to the catheter to allow for continuous sampling without disturbing the animals. Blood samples of 300 μL were withdrawn into heparinized syringes. The same volume of saline was immediately replaced.
Cervical stimulation
At 1700 h on the afternoon following jugular cannulation, the CS group received the first bout of cervical stimulation. Stimulation was applied with an electrode rod with two platinum wires protruding from the tip. The second stimulation was performed at 0900 h the next morning. This protocol is known to produce rhythmic prolactin release [25]. Blood sampling began the following day.
Drug injections
Nor-BNI (Tocris Bioscience, Bristol, United Kingdom) was dissolved in sterile saline to a concentration of 5 µg/µL. An injection pump (KDS100; KD Scientific, Holliston, MA) controlled a Hamilton syringe (Hamilton, Reno, NV) connected with PE-10 polyethylene tubing to a stainless steel needle (0.2 mm diameter) inserted into the cannula of freely moving animals. A total dose of 25 μg of nor-BNI, or saline vehicle, was injected at a flow rate of 5 µL/min. The needle was left in place another 30–60 s to avoid reflux. Injections were performed 2–3 h before the expected peak surge time.
Stereotaxic surgeries
Rats were anesthetized with a 100 µL/100 g body weight dose of ketamine (Ketaset; Fort Dodge Animal Health, Fort Dodge, IA; 49 mg/ml) and xylazine (Anased; Lloyd Laboratories, Shenandoah, IA; 1.8 mg/ml). Animals were positioned in a stereotaxic apparatus with the incisor bar at −3.3 mm. For lateral ventricle cannulation, a 22-gauge guide cannula (C313G; Plastics One, Inc., Roanoke, VA) was implanted in the left lateral ventricle (coordinates: 1.0 mm posterior to bregma, 1.6 mm lateral to the midline, and 3.4–3.8 mm below the skull surface). Correct vertical placement of the cannula in the ventricle was verified by the displacement of the meniscus in a water manometer that detects pressure differences among compartments. The cannula was attached to the skull with two stainless steel screws and acrylic cement. A plastic capped mandril (C313DC, Plastics One, Inc.) kept the cannula clear of debris.
The neurotoxin saporin conjugated to the selective neurokinin B receptor agonist [MePhe7]neurokinin B (Nk3-SAP, #KIT-63; Advanced Targeting Systems, San Diego, CA) was used to chemically ablate KNDy neurons. A 26-gauge bilateral guide cannula (C235G-1.0/SP; Plastics One) was targeted at the arcuate nucleus (coordinates: 2.4–3.5 mm posterior to bregma, 0.5 mm lateral, and 9.75 below the skull). Each rat received two bilateral injections of 10 ng/100 nL of either Nk3-SAP or Blank-SAP (control) dissolved in PBS. Injections were performed at a rate of 0.05 μL solution/min with a 33-gauge needle connected to a Hamilton syringe (Hamilton) controlled by an injector pump (KDS100; KD Scientific Inc.). The needle was left in place for 5 min to prevent reflux. The scalp was closed with sutures. All rats were given an injection of metacam (0.2 mg/100 g body weight) and allowed to recover for 1 week.
Perfusions and immunohistochemistry
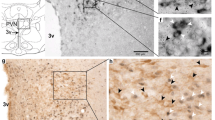
Rats were deeply anesthetized with ketamine and xylazine and transcardially perfused with saline, followed by cold 4 % paraformaldehyde. Brains were post-fixed an additional 4 h and allowed to sink in 30 % sucrose. 30 μm slices were cut on a freezing microtome (Microm HM 430, Thermo Scientific) and stored in cryoprotectant. During slicing, injection cannula tracts could be visualized and animals with crooked or misplaced injections were excluded. The arcuate nucleus was separated into an anterior “half” for TH and KOR analysis, and a posterior “half” for kisspeptin analysis. Briefly, slices were washed in PBS + 0.01 % Triton X, blocked in normal donkey serum, and then incubated for 24–48 h at 4 °C in primary antibody (rabbit anti-kisspeptin, AB9754, Millipore, Billerica, MA; mouse anti-TH, MAB318, Millipore; mouse anti-KOR, KOR-1 D-8, Santa Cruz, Dallas, TX). Tissue stained for KOR was first treated with a citric acid antigen retrieval solution for 30 min at 80 °C before blocking and primary antibody incubation. Following that, the free-floating slices were incubated for 2 h at room temperature with secondary antibody (Alexa Fluor donkey anti-rabbit 555, Life Technologies, Grand Island, NY; biotinylated goat anti-mouse IgG, BA-9200, Vector Labs, Burlingame, CA). Chromogenic staining included further incubation with an avidin–biotin complex solution (elite ABC kit, Vector Labs). Nickel sulfate (25 mg/ml), 3,3′-diaminobenzidine-HCL (DAB, 0.2 mg/ml), and 0.03 % H2O2 were used to develop the sections. Finally, all slices were mounted, dried, and cover slipped. No staining was observed when primary antibodies were excluded. Images were taken on a Leica microscope using a Nikon camera and software (NIS Elements AR 3.2). Ten slices were analyzed for each animal within each group. Cell counts were performed using ImageJ.
RIA
Blood was centrifuged at 1200×g for 15 min at 4 °C. The plasma was removed and frozen at −20 °C until the assay. Plasma prolactin was measured with a rat prolactin RIA kit provided by Dr. Albert F. Parlow through the National Hormone and Pituitary Program (Torrance, CA). 125I was purchased from PerkinElmer Life Sciences (Shelton, CT), and PRL-I-6 was radioiodinated by the chloramine-T method. The antiserum for prolactin was anti-rat PRL-S9 and the reference preparation was PRL-RP3. The lower limit of detection was 0.05 ng/ml, and the intra- and inter-assay coefficients of variation were 4.5 and 11.2 %, respectively.
HPLC with electrochemical detection
Median eminence samples were quickly dissected from decapitated animals sacrificed at peak surge times (0300 or 1700) and stored at −80 °C. Samples were later thawed and homogenized in 200 µL 0.2 M perchloric acid and 0.1 mM EDTA. Samples were centrifuged for 20 min at 8000×g at 4°C. The supernatant was collected and centrifuged through a 0.2-mm nylon microfiltration tube. Samples were then placed in autosampler vials for HPLC measurement. Briefly, 20 µL of sample was injected into the system by an autoinjector (model 524; ESA, Inc., Chelmsford, MA) connected to a Prominence degasser piston pump (LC-20AD; Shimadzu, Kyoto, Japan) with a flow of 0.4 mL/min. Separation occurred on a 15 mm × 3.2 mm C18 reverse phase column (3 μm particle size, MD-150, ESA). The conditioning cell (ESA 5021) was set at 300 mV, and the analytical cell (ESA 5011) was set to 100 mV on channel 1 and −225 mV on channel 2. Both channels were set with a gain of 10 nA, 5 s filter, 1 V output, and 0 % offset. Current changes were detected by a Coulochem II (ESA) and recorded using EZStart 7.3 SP1 software. Dopamine and DOPAC (3,4-dihydroxyphenylacetic acid, its metabolite) were identified based on peak retention times and concentration was quantified as area under the curve against a known standard. The ratio of DOPAC to DA in the median eminence is an indicator of TIDA neuronal activity [26].
Statistical analysis
Prolactin data were analyzed using SAS software (SAS Institute, Inc, Cary, NC), and statistical differences were determined by a generalized linear mixed model. Dopamine and cell count data were analyzed with GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA), and statistical differences were determined by a Student’s t test. All data passed tests for normality and equal variance. Data are expressed as mean ± SEM. p < 0.05 was considered statistically significant.
Experimental design
Experiment 1: Immunohistochemistry for κ-opioid receptor protein and tyrosine hydroxylase
Ovariectomized rats were perfused and brains were post-fixed and sunk in sucrose. Sequential sections were taken through the arcuate on a freezing microtome. Sections were stored in cryoprotectant until assayed. Immunohistochemistry was performed as detailed above.
Experiment 2: Effect of nor-BNI on the nocturnal CS surge
Animals were implanted with icv cannulas and allowed to recover for 1 week. After receiving jugular cannulas, rats received two bouts of electrical cervical stimulation. Blood sampling began the morning following the second bout. Samples were taken at 0100, 0300, 0500, 1200, 1500, 1700, and 1900 h. One full day of blood sampling would confirm the presence of prolactin surges and success of the CS protocol. The following day, the specific κ-opioid receptor antagonist norbinaltorphimine (nor-BNI) or saline was administered 2–3 h before the expected peak time of the nocturnal surge.
Experiment 3: Effect of nor-BNI on the OVE surge
Animals were implanted with icv cannulas and allowed to recover for 1 week. During the jugular cannulation surgery, OVX rats received one subcutaneous 5-mm Silastic implant (0.062 in. ID, 0.125 in. OD) containing approximately 500 µg 17β-estradiol (Sigma, St. Louis, MA). This dose of estradiol produces daily afternoon surges of prolactin [25]. On the second day after surgery, blood samples were taken every other hour from 0900 to 2100 h. Injections were performed on the second day 2–3 h before the expected peak time of the surge.
Experiment 4: Effect of KNDy neuron ablation on CS surges
OVX rats received bilateral Blank-SAP (control) or Nk3-SAP stereotaxic injections directly into the arcuate. After 7–10 days of recovery to allow the toxin to take effect, rats were cannulated and cervically stimulated. On the second day after cannulation, two full days of blood sampling began at the following times: 0100, 0300, 0500, 1200, 1500, 1700, and 1900 h. At the end of the experiment, animals were perfused and brains were immunohistochemically stained for kisspeptin to quantify neuronal loss. Kisspeptin staining was chosen because virtually all kisspeptin-positive cells in the arcuate express the Nk3 receptor necessary for saporin toxin up-take [16]. Additionally, the majority of dynorphin-expressing neurons in the arcuate express the Nk3 receptor, further suggesting that we have specifically targeted the KNDy population [27].
Experiment 5: Effect of KNDy neuron ablation on OVE surge
OVX rats received bilateral Blank-SAP (control) or Nk3-SAP stereotaxic injections directly into the arcuate. Estradiol pellets were implanted at this time. After 1 week of recovery to allow the toxin to take effect, rats underwent the jugular cannulation surgery. Blood sampling began the next day at the following times: 1000, 1200, 1400, 1600, 1800, and 2000 h. At the end of the experiment, animals were perfused and brains processed for kisspeptin immunohistochemistry as described above.
Results
The κ-opioid receptor is expressed in the arcuate nucleus
Immunohistochemistry demonstrates the presence of κ-opioid receptors in the arcuate nucleus of OVX rats (Fig. 1b). Staining for tyrosine hydroxylase produced a similar pattern and location (Fig. 1a), but technical difficulties precluded a double labeling analysis. The κ-opioid receptor staining is in agreement with previous literature in the sheep [28, 29] and in situ hybridization studies in mouse [30], rat [21], and guinea pig [31].
Nor-BNI attenuates the nocturnal prolactin surge in CS rats, but not the afternoon prolactin surge in OVE rats
One day of prolactin surges was first measured to confirm the effectiveness of CS; the nocturnal and diurnal surges were not statistically different between groups. A single injection of the specific κ-opioid receptor antagonist nor-BNI given prior to the next nocturnal surge greatly attenuated the surge at the time point immediately following injection (at 0100 h, p = 0.03, Fig. 2a). The surge remained reduced at the following time point, although this did not reach significance (at 0300 h, Fig. 2a). Measurements of the DOPAC/DA ratio, collected at the 0300 h time point, were nearly doubled in the drug-treated animals versus controls, although this change failed to reach significance (p = 0.07, Fig. 2b).
CS-induced prolactin surges measured at nocturnal, diurnal, and basal time points in OVX rats. Saline vehicle or nor-BNI (5 μg/μl) was injected around 0000 h, just before the nocturnal surge begins (n = 9–10). a Nor-BNI immediately and significantly decreases the nocturnal surge (gray triangle) at 0100 h (p = 0.03) versus controls (black square). N the nocturnal surge, D diurnal surge. b Nor-BNI tends to increase the DOPAC/DA ratio at 0300 h (p = 0.07) (n = 5–6)
In sharp contrast, when nor-BNI was injected prior to the afternoon prolactin surge in OVE rats there was no discernable effect on either the prolactin level (Fig. 3a) or the DOPAC/DA ratio (Fig. 3b). Administering two injections of nor-BNI prior to the surge (at 1300 and 1500) also failed to produce any significant effect on prolactin (Fig. 3a, inset). These results demonstrate that the mechanisms controlling these two prolactin surges are fundamentally different.
Prolactin measurements before and after injection of saline (black square) or nor-BNI (gray triangle) in OVE rats (n = 4). Saline vehicle or nor-BNI (5 μg/μl) was injected around 1400 h, when the prolactin levels begin to rise. a One injection of nor-BNI does not affect the afternoon prolactin surge. Two injections are also ineffectual (a inset, n = 5–6). b Nor-BNI also fails to affect dopamine activity measured by the DOPAC/DA ratio at 1700 (n = 4–5)
KNDy ablation fails to affect prolactin surges in CS rats, but attenuates the afternoon prolactin surge in OVE rats
Saporin toxin treatment decreased kisspeptin expression in the arcuate of OVX-CS rats by ~40 % (Fig. 4a). CS was nevertheless able to induce a normal semi-circadian pattern of prolactin release in these partially-ablated animals. Further, these surges were not statistically different from those of controls (Fig. 5). As expected, the saporin toxin had no effect on the number of TH-expressing cells in the arcuate (Fig. 4b), consistent with prior studies [14, 24].
Saporin toxin decreases kisspeptin neuron number without affecting TH neuron number. Ten sections were analyzed per animal, and n = 3 animals per group. a Kisspeptin cells are significantly (p < 0.05) decreased in ablated OVX-CS rats but b TH-positive cells are unaffected. Similarly, c kisspeptin cells are significantly decreased (p < 0.05) in ablated OVE rats, but d TH-positive cells numbers remain unchanged
CS-induced prolactin surges measured at nocturnal, diurnal, and basal time points in OVX rats treated with either Blank-SAP (black square) or Nk3-SAP (gray triangle) 1 week before (n = 4–13). Over two and a half days, the prolactin surge pattern is unaffected by previous neurotoxin treatment. N the nocturnal surge, D diurnal surge
In a separate experiment on OVE rats, saporin toxin treatment decreased the number of kisspeptin-expressing cells by ~50 % (Fig. 4c). It also led to a significant overall decrease in prolactin (significant main effect of time p = 0.003, Fig. 6). Post-hoc analysis detected that the 1400 time point was significantly lower in treated animals (p < 0.01). Because we did not measure multiple baseline time points before or after the surge, we could not establish whether ablation may have also decreased basal prolactin levels, but our single basal measurement at noon (before the surge) suggests this is not the case. A relatively similar level of ablation was sufficient to induce a significant increase in the afternoon LH surge in OVE rats [14]. This result stands in contrast to the lack of effect of partial KNDy neuron ablation on CS-induced prolactin surges and further shows the dissimilarity of the mechanisms underlying these rhythms. The number of TH-expressing cells in the arcuate was unaffected by the neurotoxin (Fig. 4d).
Prolactin measurements during the afternoon in OVE rats (n = 5–7) 1 week after Blank-SAP (black square) or Nk3-SAP injection (gray triangle). KNDy ablation significantly decreases the afternoon prolactin surge versus sham-treated animals. Post-hoc tests find the 1400 time point significantly lower than controls (p < 0.01)
Discussion
In this report, we show that central administration of a κ-opioid receptor antagonist attenuates the nocturnal prolactin surge induced by CS in OVX rats but has no effect on the afternoon surge of prolactin induced by E2. The attenuation of the nocturnal surge is consistent with a reported inhibitory effect on the nocturnal surge in pregnant rats [13]. Although a larger sample size may have detected a more subtle drug effect, the lack of effect on the afternoon surge of OVE rats suggests that the mechanisms driving these two surge patterns are different. Although we do not know the location of the κ-opioid receptors blocked by the antagonist, we present data indicating that κ-opioid receptors are expressed in the arcuate in a location similar to that of the TIDA neurons, which provide the primary prolactin control. We also do not know the origin of the dynorphin that contributes to the nocturnal prolactin surge. One potential source is the population of arcuate KNDy neurons. However, we found that partial chemical ablation of these neurons had no effect on the semi-circadian prolactin rhythm. Partial ablation of these neurons did, however, lead to a reduction in the afternoon prolactin surge in OVE rats. Since antagonism of κ-opioid receptors had no effect on this surge, the data suggest that the KNDy neurons exert their influence here through either neurokinin B or kisspeptin. The latter is consistent with a prior finding that kisspeptin stimulates prolactin secretion in OVE rats by inhibiting TIDA neurons [17].
Our demonstration of κ-opioid receptor protein in the arcuate builds on prior data in which κ-opioid receptor mRNA was detected in this region of the hypothalamus in mice, rats, and guinea pigs [21, 30, 31]. Additionally, dynorphin fibers are observed in close apposition to TIDA neurons in the rat [22] and dynorphin inhibits firing activity of dopaminergic arcuate neurons [23]. Dynorphin antagonism has been shown here and elsewhere [13] to influence prolactin by increasing dopamine levels, so it is likely that the TIDA neurons express the κ-opioid receptor and that binding of dynorphin to these receptors has an inhibitory effect on TIDA neuron activity (and thus a stimulatory effect on prolactin secretion). Although μ-opioid receptors have also been implicated in prolactin control [13], the nor-BNI used in our study is a κ-specific antagonist. The bulk of the data therefore suggest that the κ-opioid receptors that are important for control of the nocturnal prolactin surge are located on TIDA neurons.
The source of the dynorphin involved in the CS-induced nocturnal prolactin surge has not been resolved. We showed that ablation of ~40 % of the KNDy neurons had no significant effect on this surge. Given that a slightly larger (~50 %) ablation of KNDy neurons significantly reduced the E2-induced prolactin surge, it is likely that the ~40 % ablation would have been large enough to make a measurable impact on the nocturnal surge if the dynorphin came from KNDy neurons. Since no effect was observed, it is unlikely that KNDy neurons play a significant role in this surge. However, since the ablation was incomplete, we cannot completely rule out such a role. Alternatively, other dynorphin populations that would have been unaffected by the toxin do exist and may be primary sources of dynorphin for the nocturnal prolactin surge. For example, some hypothalamic POMC neurons produce dynorphin [32]. Their involvement in controlling food intake raises the possibility that they provide metabolic information to the reproductive axis.
The finding that partial KNDy neuron ablation affected the afternoon prolactin surge in OVE rats may be surprising, since kisspeptin expression in KNDy neurons is down-regulated by E2 [33]. However, prodynorphin mRNA levels do not decrease in the arcuate, even when E2 levels are sufficient to decrease kisspeptin immunoreactivity [34]. Indeed, findings from our lab (current report, [14]) show that these neurons are actively participating in the control of fertility even in the presence of E2. Zhang and Gallo [35, 36] have previously suggested a role for dynorphin in the proestrus LH surge and Helena et al. [14] show that dynorphin from the KNDy neurons participates in the E2-induced LH surge. However, our results do not support a role for dynorphin in the E2-induced prolactin surge or corresponding dopamine activity, in agreement with an earlier study showing no effect of nor-BNI administration on DOPAC levels in OVE animals [37]. These combined findings demonstrate independent circuits regulating LH and prolactin surges in the E2-primed animal.
Overall, this study shows several important findings. First, κ-opioid receptor protein is expressed in the arcuate nucleus near TIDA neurons. Second, dynorphin is a major contributor to the nocturnal prolactin surge in OVX-CS rats (and pregnant rats, [13]). Third, dynorphin appears to play little or no role in the afternoon surge of prolactin in OVE rats. Fourth, KNDy neurons do contribute to the afternoon surge in OVE rats but appear to play a minimal role in the nocturnal prolactin surge of OVX-CS rats. Taken together, they suggest that the neural mechanisms underlying these patterns of prolactin release are different.
Abbreviations
- CS:
-
Cervical stimulation
- DA:
-
Dopamine
- DOPAC:
-
3,4-Dihydroxyphenylacetic acid
- E2 :
-
Estradiol
- EDTA:
-
Ethylenediaminetetraacetic acid
- HPLC:
-
High performance liquid chromatography
- KNDy:
-
Kisspeptin, neurokinin B, & dynorphin
- KOR:
-
κ opioid receptor
- LH:
-
Luteinizing hormone
- nor-BNI:
-
Norbinaltorphimine
- OVE:
-
Ovariectomized with estrogen replacement
- OVX:
-
Ovariectomized
- PBS:
-
Phospho-buffered saline
- RIA:
-
Radioimmunoassay
- TH:
-
Tyrosine hydroxylase
References
M.E. Freeman, B. Kanicska, A. Lerant, G.M. Nagy, Prolactin: structure, function and regulation of secretion. Physiol. Rev. 80(4), 1523–1631 (2000)
M.E. Freeman, Neuroendocrine control of the ovarian cycle of the rat, in Knobil and Neill’s Physiology of Reproduction, ed. by J.D. Neill (Academic Press, San Diego, 2006), pp. 2327–2388
M.E. Freeman, M.S. Smith, S.J. Nazian, J.D. Neill, Ovarian and hypothalamic control of daily surges of prolactin secretion during pseudopregnancy in the rat. Endocrinology 94, 875–882 (1974)
P. Kadioglu, A.S. Yalin, O. Tiryakioglu, N. Gazioglu, G. Oral, O. Sanli, K. Onem, A. Kadioglu, Sexual dysfunction in women with hyperprolactinemia: a pilot study report. J. Urol. 174, 1921–1925 (2005)
M. Egli, B. Leeners, T.H.C. Kruger, Prolactin secretion patterns: basic mechanisms and clinical implications for reproduction. Reproduction 140, 643–645 (2010)
C.M. Larsen, D.R. Grattan, Prolactin-induced mitogenesis in the subventricular zone of the maternal brain during early pregnancy is essential for normal postpartum behavioral responses in the mother. Endocrinology 151(8), 3805–3814 (2010)
T.H. Kruger, B. Leeners, E. Naegeli, S. Schmidlin, M. Schedlowski, U. Hartmann, M. Egli, Prolactin secretory rhythm in women: immediate and long term alterations after sexual contact. Hum. Reprod. 27(4), 1139–1143 (2012)
D.R. Grattan, I.C. Kokay, Prolactin: a pleiotropic neuroendocrine hormone. J. Neuroendocrinol. 20, 752–763 (2008)
D.J. Sirinathsinghji, A.R. Audsley, Endogenous opioid peptides participate in the modulation of prolactin release in response to cervicovaginal stimulation in the female rat. Endocrinology 117(2), 549–556 (1985)
C.A. Sagrillo, J.L. Voogt, Endogenous opioids mediate the nocturnal prolactin surge in the pregnant rat. Endocrinology 129(2), 925–930 (1991)
Y. Hou, J.L. Voogt, Effects of naloxone infusion on nocturnal prolactin secretion and Fos/FRA expression in pregnant rats. Endocrine 10(2), 145–152 (1999)
B. Zhang, Y. Hou, J.L. Voogt, Effects of opioid antagonism on prolactin secretion and c-Fos/TH expression during lactation in rats. Endocrine 25(2), 131–136 (2004)
Z.B. Andrews, D.R. Grattan, Opioid receptor subtypes involved in the regulation of prolactin secretion during pregnancy and lactation. J. Neuroendocrinol. 15, 227–236 (2003)
C.V. Helena, N. Toporikova, B. Kalil, A.M. Stathopoulos, R.O. Carolino, J.A. Anselmo-Franci, R. Bertram, KNDy neurons modulate the steroid-induced luteinizing hormone surge in ovariectomized rats. Endocrinology 156(11), 4200–4213 (2015)
X.F. Li, J.S. Kinsey-Jones, Y. Cheng, A.M.I. Knox, Y. Lin, N.A. Petrou, A. Roseweir, S.L. Lightman, S.R. Milligan, R.P. Millar, K.T. O’Byrne, Kisspeptin signalling in the hypothalamic arcuate nucleus regulates GnRH pulse generator frequency in the rat. PLoS ONE 4(12), e8334 (2009)
V.M. Navarro, M.L. Gottsh, C. Chavkin, H. Okamura, D. Clifton, R.A. Steiner, Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J. Neurosci. 29(38), 11859–11866 (2009)
M. Kirilov, J. Clarkson, X. Liu, J. Roa, P. Campos, R. Porteous, G. Schutz, A.E. Herbison, Dependence of fertility on kisspeptin-Gpr54 signaling at the GnRH neuron. Nat. Commun. 4, 2492 (2013)
K.E. Beale, J.S. Kinsey-Jones, J.V. Gardiner, E.K. Harrison, El Thompson, M.H. Hu, M.L. Sleeth et al., The physiological role of arcuate kisspeptin neurons in the control of reproductive function in female rats. Endocrinology 155(3), 1091–1098 (2014)
R.E. Szawka, A.B. Rieiro, C.M. Leite, C.V.V. Helena, C.R. Franci, G.M. Anderson, G.E. Hoffman, J.A. Anselmo-Franci, Kisspeptin regulates prolactin release through hypothalamic dopaminergic neurons. Endocrinology 151(7), 3247–3257 (2014)
J.J. Wagner, R.M. Caudle, C. Chavkin, Kappa-opioids decrease excitatory transmission in the dentate gyrus of the guinea pig hypothalamus. J. Neurosci. 12(1), 132–141 (1992)
A. Mansour, C.A. Fox, S. Burke, F. Meng, R.C. Thompson, H. Akil, S.J. Watson, Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J. Comp. Neurol. 350(3), 412–438 (1994)
M.D. Fitzsimmons, J.A. Olschowka, S.J. Wiegand, G.E. Hoffman, Interaction of opioid peptide-containing terminals with dopaminergic perikarya in the rat hypothalamus. Brain Res. 581(1), 10–18 (1992)
X. Zhang, A.N. van den Pol, Dopamine/tyrosine hydroxylase neurons of the hypothalamic arcuate nucleus release GABA, communicate with dopaminergic and other arcuate neurons, and respond to dyrnorphin, met-enkephalin, and oxytocin. J. Neurosci. 35(45), 14966–14982 (2015)
M.A. Mittelman-Smith, H. Williams, S.J. Krajewski-Hall, J. Lai, P. Ciofi, N.T. McMullen, N.E. Rance, Arcuate kisspeptin/neurokinin B/dynorphin (KNDy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinology 153(6), 2800–2812 (2012)
M.F. Cordellini, G. Piazzetta, K.C. Pinto, A.M. Delattre, F. Matheussi, R.O.G. Carolino, R.E. Szawka, J.A. Anselmo-Franci, A.C. Ferraz, Effect of different doses of estrogen on the nigrostriatal dopaminergic system in two 6-hydroxydopamine-induced lesion models of Parkinson’s disease. Neurochem. Res. 36(6), 955–961 (2011)
K.J. Lookingland, H.D. Jarry, K.E. Moore, The metabolism of dopamine in the median eminence reflects the activity of tuberoinfundibular neurons. Brain Res. 419(1–2), 303–310 (1987)
M.C. Burke, P.A. Letts, S.J. Krajweski, N.E. Rance, Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: morphologic evidence of interrelated function within the arcuate nucleus. J. Comp. Neurol. 498, 712–726 (2006)
C.F. Witty, P.W. Weems, R.L. Goodman, L.M. Coolen, M.N. Lehman, Kappa opioid receptor is present within a majority of KNDy neurons in the ewe, in Neuroscience Meeting Planner, NN33, (Society for Neuroscience, Washington, DC, 2014) Online
P.W. Weems, C.F. Witty, M. Amstalden, L.M. Coolen, R.L. Goodman, M.N. Lehman, Kappa opioid receptor is co-localized in GnRH and KNDy cells in the female ovine and rodent brains. (submitted)
A.M. DePaoli, K.M. Hurley, K. Yasada, T. Reisine, G. Bell, Distribution of kappa opioid receptor mRNA in adult mouse brain: an in situ hybridization histochemistry study. Mol. Cell. Neurosci. 5(4), 327–335 (1994)
Y.J. Wang, K. Rasakham, P. Huang, D. Chudnovskaya, A. Cowan, L.Y. Liu-Chen, Sex difference in κ-opioid receptor (KOPR)-mediated behaviors, brain region KOPR level and KOPR-mediated guanosine 5′-O-(3-[35S] thiotriphosphate) binding in the guinea pig. J. Pharmacol. Exp. Ther. 339(2), 438–450 (2011)
N. Maolood, B. Meister, Dynorphin in pro-opiomelanocortin neurons of the hypothalamic arcuate nucleus. Neuroscience 154(3), 1121–1131 (2008)
J.T. Smith, M.J. Cunningham, E.F. Rissman, D.K. Clifton, R.A. Steiner, Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146(9), 3686–3692 (2005)
P. Mostari, N. Ieda, C. Deura, S. Minabe, S. Yamada, Y. Uenoyama, K. Maeda, H. Tsukamura, Dynorphin-kappa opioid receptor signaling partly mediates estrogen negative feedback effect on LH pulses in female rats. J. Reprod. Dev. 59(3), 266–272 (2013)
Q. Zhang, R.V. Gallo, Effect of prodynorphin-derived opioid peptides on the ovulatory luteinizing hormone surge in the proestrous rat. Endocrine 18(1), 27–32 (2002)
Q. Zhang, R.V. Gallo, Presence of κ-opioid tone at the onset of the ovulatory luteinizing hormone surge in the proestrous rat. Brain Res. 290, 135–139 (2003)
E.J. Wagner, J. Manzanares, K.E. Moore, K.L. Lookingland, Neurochemical evidence that estrogen-induced suppression of kappa-opioid-receptor-mediated regulation of tuberoinfundibular dopaminergic neurons is prolactin-independent. Neuroendocrinology 59, 197–201 (1994)
Acknowledgments
We thank Kim Hughes for helpful discussions, Frank Johnson for the use of his microscope, Charles Badland for assistance with figure preparation, and Lique Coolen for the antibody protocol. We also thank Frank Nenninger and Jose Arias-Cristancho, for generous assistance with surgeries, animal care, and blood sampling. This work was partially supported by the National Institutes of Health grant DK-43200.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None of the authors report any conflict of interest.
Human and Animal Rights
Animal procedures were approved by the Florida State University Animal Care and Use Committee.
Rights and permissions
About this article
Cite this article
Stathopoulos, A.M., Helena, C.V., Cristancho-Gordo, R. et al. Influence of dynorphin on estradiol- and cervical stimulation-induced prolactin surges in ovariectomized rats. Endocrine 53, 585–594 (2016). https://doi.org/10.1007/s12020-016-0938-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-016-0938-1