Abstract
Centrally acting prolactin has been shown to have anti-stress effects by modulating the activity of the hypothalamic–pituitary–adrenal axis. We tested the hypothesis that prolactin directly targets hypothalamic corticotropin-releasing hormone (CRH) neurons. In situ hybridisation confirmed expression of mRNA encoding the long, but not the short, isoform of the prolactin receptor (PRLR) within the paraventricular nucleus (PVN) of the virgin rat; however, only 6% of CRH neurons expressed long-form Prlr mRNA. Examination of the functional response of CRH neurons to intracerebroventricular prolactin (500 ng) showed that these neurons did not respond with activation of phosphorylated signal transducer and activator of transcription 5 (pSTAT5), a marker of long-form PRLR activation. However, as only a subset of neurons expressing Crh mRNA could be detected using immunohistochemistry, we utilised a transgenic mouse model to label CRH neurons with a fluorescent reporter (CRH-Cre-tdTomato). In lactating animals, chronically elevated prolactin levels resulted in significantly increased pSTAT5 expression in the PVN. Overall, few tdTomato-labelled CRH neurons were double-labelled, although a small subset of CRH neurons in the caudal PVN were pSTAT5 positive (approximately 10% of tdTomato neurons at this level, compared to 1% in the rostral PVN). These data suggest that most CRH neurons do not respond directly to prolactin. To confirm that prolactin was not activating another signalling pathway, we used a transgenic mouse line to label PRLR-expressing neurons with Cre-dependent green fluorescent protein (GFP) expression (CRH-Cre-Prlrlox/lox). No GFP-expressing cells were evident in the PVN, indicating that in the mouse, as in the rat, the CRH neurons do not express either PRLR isoform. Together these data showed that the anti-stress effects of prolactin are not the result of prolactin directly regulating CRH neurons.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prolactin, primarily synthesised and secreted by the anterior pituitary lactotrophs, is a pleiotropic hormone with over 300 known functions (Bole-Feysot et al. 1998). Its most well-characterised role is the stimulation of mammary gland development and milk synthesis in pregnancy and lactation; however, prolactin is also known to act within the brain. Apart from the regulation of its own secretion (Grattan 2015), central actions of prolactin are predominantly associated with adaptations required for successful pregnancy and lactation and include facilitating changes to circuitries regulating body weight homeostasis to meet increased metabolic demands (Augustine and Grattan 2008; Augustine et al. 2008), stimulating the onset of maternal behaviour (Bridges et al. 1990; Bridges and Ronsheim 1990; Larsen and Grattan 2010) and suppressing anxiety and stress responses (Schlein et al. 1974; Torner et al. 2002).
The mechanism by which prolactin influences the stress response is not currently known, but may involve an action upon the corticotropin-releasing hormone neurons (CRH) in the hypothalamic paraventricular nucleus (PVN). These neurons integrate signals from stress-processing circuitries to control downstream activation at the pituitary and adrenal levels of the hypothalamic–pituitary–adrenal (HPA) axis. Chronic intracerebroventricular (ICV) prolactin significantly reduces stress-induced HPA axis activation in virgin rats, while the converse effect is observed if central prolactin receptors (PRLRs) are downregulated (Donner et al. 2007; Torner et al. 2001). During lactation, a characteristically hyperprolactinemic state, the reactivity of the HPA axis to a variety of stressors is suppressed (Brunton et al. 2005; da Costa et al. 1996; Lightman and Young 1989; Neumann et al. 1998). This effect is reversed if central PRLRs are blocked (Torner et al. 2002), indicating that the chronic elevation of prolactin signalling in lactation may play a significant role in regulating the reactivity of the HPA axis at this time. Centrally acting prolactin has also been shown to inhibit the formation of stress-induced gastric ulcers and prevent stress-induced hypocalcemia, although these actions have been associated with acute activation, rather than inhibition, of CRH neurons (Drago et al. 1985; Fujikawa et al. 2004, 2005).
The cellular effects of prolactin are transduced by membrane-bound PRLRs belonging to the class 1 cytokine receptor family (Forsyth and Wallis 2002). There are two major isoforms of the PRLR in rodents, long (PRLRL) and short (PRLRS), which differ only in the intracellular portion of the receptor peptide (Bole-Feysot et al. 1998). PRLRL is the dominant signalling form of the receptor within the brain (Freeman et al. 2000), and Prlr L mRNA is expressed within the PVN (Augustine et al. 2003; Bakowska and Morrell 1997; Brown et al. 2011; Kokay et al. 2006). Interestingly, restraint stress has been shown to upregulate Prlr L mRNA expression in this nucleus (Fujikawa et al. 1995). PRLR signal transduction occurs predominately through the Janus kinase 2-signal transducer and activator of transcription 5 (JAK2-STAT5) signalling pathway which is coupled exclusively to the long form of the receptor (Bole-Feysot et al. 1998). Phosphorylated STAT5 (pSTAT5) is markedly upregulated in the PVN during lactation (Augustine et al. 2016; Brown et al. 2011), coincident with the induction of maternal stress hyporesponsiveness; however, whether there is a causative relationship between prolactin-mediated upregulation of the JAK2-STAT5 pathway and suppression of CRH neuron activity is currently unknown.
In addition to signalling through the JAK2-STAT5 pathway, prolactin has been shown to activate the mitogen-activated protein kinase (MAPK) signalling cascade through binding to both the long and short isoforms of the PRLR (Das and Vonderhaar 1995). Prolactin-induced phosphorylation of extracellular-signal-regulated kinase (pERK), a marker of activation of the MAPK signalling pathway, has been shown in CRH neurons in the rat (Blume et al. 2009). However, it is unclear whether prolactin-induced pERK activation is the result of direct action on CRH neurons.
Based on the established regulation of the HPA axis by prolactin, we hypothesised that the CRH neurons would express the PRLR, allowing a direct regulation of these cells by prolactin. Initial experiments focussed upon measuring Prlr mRNA expression and PRLR-mediated signalling in CRH neurons in the virgin rat. To overcome technical limitations with immunohistochemical identification of CRH neurons, subsequent experiments were carried out using a reporter mouse model in which CRH neurons were labelled by expression of a marker protein, tdTomato (Taniguchi et al. 2011; Wamsteeker Cusulin et al. 2013). This was particularly important in lactating animals as tdTomato expression was maintained despite the marked reductions in endogenous Crh mRNA expression that have been shown in lactating mice (Gustafson P, Bunn S and Grattan D, unpublished data) and rats (Shanks et al. 1999; Walker et al. 2001; Windle et al. 1997). This enabled us to use prolactin-induced pSTAT5 expression [which is only detected in the PVN of mice during lactation (Brown et al. 2011, 2010)] to determine whether prolactin acts directly on CRH neurons through the long form of the PRLR. Finally, we used a mouse model in which Cre-mediated recombination of the Prlr gene resulted in expression of green fluorescent protein (GFP) under the control of the Prlr promoter (Brown et al. 2016). This model had the value of testing whether the short or long PRLR isoform was expressed by CRH neurons. Together the data showed that prolactin modulation of the stress response is not mediated by direct action upon the CRH neurons.
Materials and methods
Animals and housing
All animal work was conducted in accordance with approval from the University of Otago Animal Ethics Committee. Adult female Sprague-Dawley rats aged 10–14 weeks, CRH-Cre-tdTomato, CRH-Cre-Prlrlox/lox, and calcium-calmodulin kinase II α (CamK)-Cre-Prlrlox/lox mice aged 8–12 weeks were obtained from the Taieri Resource Unit at the University of Otago. All animals were group-housed, unless otherwise stated, under controlled temperature (22 ± 1 °C) and lighting conditions (12:12-h light:dark cycle, lights on at 0600 h). All animals were allowed ad libitum access to food and water throughout the duration of the experiments.
The present studies used both non-pregnant and lactating animals. Estrous cyclicity was recorded by daily monitoring of vaginal cytology and virgin animals were killed on the morning of diestrus. To generate lactating mice, females were individually housed with a C57BL/6J male. The presence of a copulatory plug was considered day 1 of pregnancy and the male removed. Animals were monitored until the day of birth which was considered day 1 of lactation. Litters were normalised to six pups per litter on day 3 of lactation, and lactating dams were killed on day 7 of lactation.
CRH-Cre-tdTomato mice were generated by crossing heterozygous CRH-IRES-Cre (Taniguchi et al. 2011) and homozygous Ai9 Cre-dependent tdTomato (Madisen et al. 2010) mouse lines. The presence or absence of Cre in genomic DNA samples was determined by reverse transcription polymerase chain reaction (RT-PCR). The following primers were used for genotyping: 5′-CTAGGCCACAGAATTGAAAGATCT-3′ (forward) and 5′-GTAGGTGGAAATTCTAGCATCATCC-3′ (reverse) for the wildtype allele and 5′-GCGGTCTGGCAGTAAAAACTAT-3′ (forward) and 5′-GTGAAACAGCATTGCTGTCACTT-3′ (reverse) for the mutant allele. Two products, 100 and 324 bp in size, were present in CRH-Cre-positive mice, while a single, 324 bp product, was present in CRH-Cre-negative (control) animals. To visualise Prlr-expressing CRH neurons, CRH-Cre-Prlrlox/lox mice were generated by crossing CRH-Cre mice with the Prlrlox/lox mouse line generated by our group in which the Prlr gene is modified to include loxP sites around exon 5 and an inverted GFP sequence (Brown et al. 2016). The following primers were used for Prlrlox/lox genotyping: 5′-TGTCCAGACTACAAAACCAGTGGC-3′ (forward) and 5′-CAGTGCTCTGGAGAGCTGGC-3′ (reverse wildtype) and 5′-ACCTCCCCCTGAACCTGAAACATAA-3′ (reverse mutant). In wildtype animals, a 546 bp product was present where as in Prlrlox/lox animals, a 400 bp product was present. As a positive control for Cre-mediated recombination of the Prlrlox/lox gene in CRH-Cre-Prlrlox/lox mice, CamK-Cre-Prlrlox/lox mice were used to show GFP expression driven by the Prlr promoter in CamK-Cre-expressing animals (Brown et al. 2016). These animals were generated by crossing Prlrlox/lox mice with CamK-Cre (Casanova et al. 2001). In these animals, the presence of Cre was detected using the following genotyping primer sequences: 5′-GGTTCTCCGTTTGCACTCAGG-3′ (forward), 5′-CTGCATGCACGGGACAGCTCT-3′ (reverse wildtype) and 5′-GCTTGCAGGTACAGGAGGTAGT-3′ (transgenic reverse). In wildtype animals, a 285 bp product was present, while in Cre-expressing animals, a 380 bp product was present.
Dual-label in situ hybridisation
Tissue collection and preparation
For tissue collection for in situ hybridisation, diestrous rats (n = 4) were anaesthetised with an overdose of sodium pentobarbitone (65 mg/kg) and transcardially perfused with 2% paraformaldehyde (PFA) in 0.1 M phosphate buffer (pH 7.4). The brains were dissected and post-fixed overnight in 2% PFA before cryoprotection in 30% sucrose in phosphate buffer. Coronal sections (16 µm thickness) through the PVN of the hypothalamus [−1.2 to −1.92 mm relative to Bregma, refer to (Paxinos and Watson 2006)] were cut in a cryostat at −20 °C and mounted onto aminopropyltriethoxy-silane-coated glass microscope slides. Sections were dried before they were stored at −20 °C until processing for in situ hybridisation.
Preparation of probes
Using the mRNA sequences from Genbank, primer pairs were designed for Prlr L (accession number M57668, nucleotides 1244–1644), Prlr S (accession number NM_012630, nucleotides 1041–1257) and Crh (accession number NM_031019.1, nucleotides 473–675). DNA templates were prepared by PCR with the sequences encoding T7 and SP6 polymerase promoter sites incorporated onto the ends of the primer sequences. DNA templates were characterised by Sanger sequencing. Antisense or sense 35S UTP-labelled RNA probes for the Prlr L and Prlr S were then transcribed from the cDNA templates using a Riboprobe® in vitro transcription kit (Promega) and the appropriate polymerase (T7 for the antisense probe, SP6 for the sense probe). A digoxigenin-11-UTP-labelled RNA probe specific for Crh was transcribed using a DIG RNA labelling kit (Roche). The RNA probes were purified using Mini Quick spin columns (Roche) to remove unincorporated nucleotides.
In situ hybridisation for PrlrS, PrlrL and Crh mRNA
Brain sections were thawed for 5 min at 55 °C before being post-fixed in 2% PFA [in 0.1 M phosphate buffer (pH 7.4)] for 5 min. Sections were then washed in 0.5× saline-sodium citrate buffer (SSC; 75 mM NaCl, 7.5 mM sodium citrate), digested in a proteinase K solution (2 µg/ml; 10 min) and acetylated in 0.1 M triethanolamine with 0.25% acetic anhydride for 10 min. Sections were washed in 2× SSC buffer before each section was covered with 100 µl of hybridisation buffer (100 mM dithiothreitol, 0.3 M NaCl, 20 mM Tris pH 8, 5 mM ethylenediaminetetraacetic acid (EDTA), 1× Denhardt’s solution, 10% dextran sulphate, 50% formamide) and incubated for 2–3 h at 42 °C. Probes were denatured at 95 °C for 3 min and 20 µl of the 35S-labelled Prlr S probe (1.2 × 106 cpm/section) or 20 µl of a mixture of the 35S-labelled Prlr L (1.2 × 106 cpm/section) and the digoxigenin-labelled Crh (50 ng/section) probes in hybridisation buffer was pipetted onto the sections and hybridised overnight at 55 °C. Sections were treated with Ribonuclease A (20 µg/L) and then washed in SSC buffer of increasing stringency (from 2× SSC at room temperature to 0.1 × SSC at 55 °C). As only the long form Prlr mRNA was detected in the PVN, dual-label in situ hybridisation with Crh mRNA was restricted to this isoform only. To detect the digoxigenin-labelled Crh probe, sections were incubated for 30 min with 2% digoxigenin blocking reagent before incubation with a sheep anti-digoxigenin antibody conjugated to alkaline phosphatase (1:2000; Roche) overnight at 4 °C. Sections were then incubated in a NBT/BCIP (nitroblue tetrazolium chloride/5-bromo-4chloro-3-indolyl-phosphate) solution overnight. The reaction was stopped by washing sections in saline-sodium phosphate-EDTA buffer (150 mM NaCl, 10 mM NaH2PO4, 1 mM EDTA). Sections were dried and exposed to scientific imaging film (7 days for Prlr L in the brain; 3 and 20 days for Prlr S in the ovary and brain, respectively) for autoradiographic screening of the 35S-hybridisation. The Prlr L /Crh mRNA dual-labelled slides were then dipped in LM-1 Hypercoat emulsion (Amersham Biosciences) and the sections exposed for 4–5 weeks at 4 °C before being developed in Kodak D19 and fixed in Ilford Hypan. Sections were cleared with xylene and coverslipped with VectaMount mounting medium.
Quantification and analysis
Digoxigenin-labelled Crh mRNA-expressing neurons in the PVN were identified by the presence of a blue/purple precipitate in the cytoplasm. Prlr L mRNA-expressing cells were identified by clusters of silver grains. Sections (n = 2 to 3 per animal) were photographed under a 40× objective using an Olympus BX51 microscope (Tokyo, Japan) fitted with a Spot RT digital camera (Diagnostic Instruments, MI, USA). ImageJ™ (National Institutes of Health, Bethesda, MD, USA) was used to determine the total number of digoxigenin-labelled cells and the number of cells dual-labelled with Prlr L mRNA. Cells expressing silver grains at 5× background were considered Prlr L -positive. Data are presented as the mean ± SEM.
pSTAT5/CRH double-label immunohistochemistry in the rat
ICV surgery, tissue collection and preparation
Rats (n = 12) were anaesthetised on the day of proestrus with 2% halothane, placed into a stereotaxic frame and a guide cannula (22 G; PlasticsOne, Roanoke, VA) inserted into the left lateral ventricle (1.3 mm lateral to the coronal suture at Bregma and 3 mm ventral to the surface of the brain). The cannula was fixed in place with stainless steel screws and dental cement and sealed. Correct cannula placement was confirmed 48 h following the surgery by administration of angiotensin II [10 ng diluted in 2 μl artificial cerebrospinal fluid (aCSF)]. Consumption of >5 ml water in the 30 min following angiotensin II administration indicated correct cannula placement. Animals were monitored daily until completion of one full estrous cycle. Beginning on the morning of metestrus, rats were treated with bromocriptine to block endogenous prolactin secretion (three doses of 500 μg/250 μl saline with 10% ethanol at 0900, 1700 and 0900 h, i.p.). Four hours following the last bromocriptine injection (1300 h), diestrus rats received either prolactin (500 ng diluted in 2 μl aCSF; n = 6) or vehicle (aCSF; n = 6) delivered into the lateral ventricle over 30 s. Injections were carried out with a Hamilton syringe (2 μl) fitted with an injection cannula (28 G). The injection cannula was left in the guide cannula for 30 s following the injection before being removed and the guide cannula sealed. Rats were anaesthetised 25 min later by lethal overdose of sodium pentobarbitone (65 mg/kg) and transcardially perfused as described above using 4% PFA. Brains were post-fixed overnight, transferred into 30% sucrose until they sank and then frozen on powered on dry ice before storage at −80 °C. Three sets of coronal sections [30-μm thick; −1.2 to −1.92 mm relative to Bregma, refer to (Paxinos and Watson 2006)] through the rat brain were cut using a freezing microtome.
Immunohistochemical staining procedure
Immunohistochemical labelling of pSTAT5 was performed on one set of sections as previously described (Sapsford et al. 2012). In summary, antigen retrieval was carried out by incubating sections in 0.01 M Tris-HCl (pH 10) at 90 °C for 10 min. Endogenous peroxidases were blocked using 0.9% hydrogen peroxide/40% methanol in Tris-buffered saline (TBS) before sections were incubated in rabbit anti-pSTAT5 antibody (Tyr694, 1:1000, Cell Signaling Technology, Beverly, MA) for 36 h at 4 °C followed by incubation with a biotin-conjugated goat anti-rabbit secondary antibody for 1.5 h (1:300; BA-1000, Vector Laboratories, Burlingame, CA). Sections were subsequently incubated with an avidin–biotin solution prepared using the Vectastain Elite ABC kit (1:100, Vector Laboratories) for 1 h before pSTAT5 immunolabelling was detected using nickel-enhanced diaminobenzidine tetrahydrochloride (DAB) (Vector DAB substrate kit), which generated a black nuclear stain. Sections were then washed in TBS, endogenous peroxidases blocked as described above and incubated with rabbit anti-CRH (T-4037, 1:1000, Peninsula Laboratories Inc., CA, USA) for 36 h at 4 °C. Sections were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (1:200) for 4 h before immunolabelling was detected using non-nickel-enhanced DAB to generate a brown cytoplasmic stain. Sections were mounted onto gelatin-coated slides, dried overnight before dehydration through graded ethanol and cleared using xylene. Sections were coverslipped using distyrene plasticiser xylene (DPX).
Quantification and analysis
Quantitative analysis of CRH immunolabelling in the PVN was made by counting the number of CRH-positive cells in two–three anatomically matched sections per animal using an Olympus BX51 microscope at high magnification (Tokyo, Japan). Prolactin-responsiveness of the CRH neurons was determined by counting the number CRH neurons expressing prolactin-induced pSTAT5. Data are presented as the mean ± SEM.
pSTAT5/tdTomato double-label immunohistochemistry in the mouse
Tissue collection and preparation
Diestrus Cre-negative (n = 3; control), diestrus and lactating Cre-positive (n = 4 per group) CRH-Cre-tdTomato mice were anaesthetised with sodium pentobarbitone (100 mg/kg) and transcardially perfused using 4% PFA in 0.1 M phosphate buffer (pH 7.4). Brains were post-fixed in 4% PFA for 1 h before being cryoprotected in 30% sucrose. Brains were frozen on dry ice and stored at −80 °C until used for immunohistochemistry. Three sets of coronal sections through the PVN [−0.46 to −1.34 mm relative to Bregma, refer to (Paxinos and Franklin 2004)] were cut using a freezing microtome.
Immunohistochemical staining procedure
One set of sections were immunolabelled for pSTAT5 as described previously (Brown et al. 2010, 2011). Antigen retrieval was carried out as described above for the rat. Sections were then incubated in a blocking solution containing 0.25% bovine serum albumin and 0.3% Triton-X in TBS before endogenous peroxidases were blocked using 3% hydrogen peroxide/40% methanol in TBS. Sections were incubated in rabbit anti-pSTAT5 antibody (Tyr694, 1:2000, Cell Signaling Technology, MA, USA) for 72 h at 4 °C followed by incubation with a biotin-conjugated goat anti-rabbit IgG secondary antibody for 2 h (1:500; Invitrogen, CA, USA). Sections were subsequently incubated with an avidin–biotin solution prepared using the Vectastain Elite ABC kit (1:100, Vector Laboratories, CA, USA) before pSTAT5 immunolabelling was detected using nickel-enhanced DAB and glucose oxidase, which generated a black nuclear stain. Sections were then immunolabelled for tdTomato by incubation with rabbit anti-DsRed primary antibody for 48 h (1:5000; Clontech Laboratories Inc., CA, USA). Following the primary antibody incubation, sections were incubated with goat anti-rabbit HRP-conjugated secondary antibody (1:500; Vector Laboratories, CA, USA) for 1.5 h before immunolabelling was detected using DAB to generate a brown cytoplasmic stain.
Quantification and analysis
Qualitative comparisons of tdTomato immunolabelling were made between diestrus CRH-Cre-positive and CRH-Cre-negative mice, using two well-characterised populations of CRH neurons [PVN and central nucleus of the amygdala (CeA)]. Quantitative comparisons of tdTomato and pSTAT5 immunolabelling were made between CRH-Cre-positive diestrous and lactating animals. The number of tdTomato-expressing cells, the number of pSTAT5-labelled nuclei and the number of double-labelled cells were counted from three rostral and two caudal PVN sections (90 µm apart), corresponding to the locations of the neuroendocrine and pre-autonomic neuron populations, respectively (Biag et al. 2012), using ImageJ™. Images were captured at high magnification (×40) using an Olympus BX61 light microscope (Tokyo, Japan). Data are presented as the mean ± SEM for each PVN subdivision and statistical comparisons were made using two-way analyses of variance (ANOVA) [factors: reproductive status (diestrous and lactation) × PVN subdivision (rostral and caudal)]. Pre-planned pairwise comparisons were made between the groups using post-hoc Tukey–Kramer tests, where p < 0.05 was considered to be a statistically significant difference.
Single-label GFP immunohistochemistry in the mouse
Tissue collection and preparation
Diestrus and lactating CRH-Cre-Prlrlox/lox animals (n = 2 per group), diestrus CamK-Cre-Prlrlox/lox (n = 4; positive control) and Cre-negative Prlrlox/lox animals (n = 3; negative control) were anaesthetised with sodium pentobarbitone (100 mg/kg) and transcardially perfused using 4% PFA as described above. Brains were post-fixed, cryoprotected in 30% sucrose and frozen as described above. Three sets of coronal sections through the hypothalamus [+0.62 to −2.30 mm relative to Bregma, refer to (Paxinos and Franklin 2004)] were cut using a sliding microtome.
Immunohistochemical staining procedure
One set of sections were labelled for GFP as previously described (Brown et al. 2016). Endogenous peroxidases were blocked by incubating sections in a 3% hydrogen peroxide solution in TBS. Sections were then incubated in blocking solution with 2% normal goat serum for 1 h before sections were incubated in rabbit anti-GFP antibody (1: 40 000, Life Technologies, CA, USA) for 72 h at 4 °C. Sections were incubated with biotin-conjugated goat anti-rabbit IgG secondary antibody for 1.5 h (1:500; Invitrogen, CA, USA) prior to a 1 h incubation in an avidin–biotin solution prepared using the Vectastain Elite ABC kit (1:100; Vector Laboratories, CA, USA). GFP immunolabelling was detected using nickel-enhanced DAB.
Data presentation
Qualitative comparisons were made between GFP immunolabelling in CRH-Cre-Prlrlox/lox and CamK-Cre-Prlrlox/lox mice. Representative images from the PVN have been presented, as well as the medial nucleus of the amygdala (MeA) as a control region where GFP is known to be expressed following Cre-mediated Prlrlox/lox gene recombination (Brown et al. 2016).
Results
Prlr L mRNA does not colocalise with Crh mRNA in the diestrous rat PVN
Expression of Prlr mRNA encoding the long and short isoforms of the PRLR was visualised by exposing autoradiographic film to sections labelled with 35S-labelled probes specific for Prlr L or Prlr S mRNA. Consistent with previous reports (Augustine et al. 2003; Bakowska and Morrell 1997; Kokay et al. 2006), Prlr L mRNA was expressed within the PVN (Fig. 1a). In contrast, no Prlr S mRNA was detected within this nucleus despite visualisation of mRNA expression in the choroid plexus and ovary using the same probe (Fig. 1b). Dual-label in situ hybridisation was used to determine if Prlr L mRNA was expressed by CRH neurons (Fig. 1c-f). Crh mRNA-expressing neurons were identified by digoxigenin immunoreactivity, while Prlr L -expressing cells were identified by discrete clusters of silver grains. Prlr L -expressing cells were clearly detected in the PVN (Fig. 1a, e, f); however, there was limited colocalisation with Crh mRNA expression (approximately 6% of CRH neurons co-expressed Prlr L mRNA) (Fig. 1e, f, i). No labelling was present in control sections labelled with sense probes (data not shown).
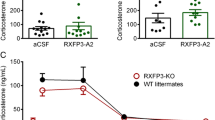
CRH neurons in the diestrous rat do not express Prlr L mRNA and are not responsive to ICV prolactin. Representative autoradiograms showing a Prlr L and b Prlr S mRNA in the PVN of the rat. Prlr L mRNA is detected in the PVN and choroid plexus (black arrow in a). Despite detecting Prlr S in control tissues (choroid plexus, black arrow in b) and the ovary (inset in b), no labelling was detected in the PVN. c Schematic representation of a coronal section through the rat brain at the level of the PVN [−1.72 mm relative to Bregma, refer to (Paxinos and Watson 2006)]. d Dual-label in situ hybridisation showing Crh mRNA-expressing neurons (cytoplasmic labelling) and Prlr L mRNA in the PVN at low power. High power images (e, f) show Crh mRNA positive/Prlr L mRNA negative neurons (white arrow heads) and Crh mRNA negative/Prlr L mRNA positive neurons (black arrow heads), where Prlr L mRNA is identifiable as silver grains (black dots). g, h) Representative images showing CRH (brown cytoplasmic staining; black arrow heads) and pSTAT5 (black nuclear staining; white arrow heads) immunoreactive neurons in the rat PVN. Box in g indicates the position of the high power image. i Quantification of the number of Crh mRNA-expressing neurons and the number of neurons dual-labelled with Prlr L mRNA (n = 4). j Quantification of the number of CRH neurons and the number of neurons double-labelled with pSTAT5 (n = 6). Data represents the mean ± SEM. 3v = third ventricle
Prolactin-induced pSTAT5 does not colocalise with CRH neurons in the rat
In the absence of any detectable Prlr L mRNA expression by CRH neurons, we confirmed that CRH neurons were not functionally responsive to prolactin in the diestrous rat using immunohistochemistry to identify ICV prolactin-induced pSTAT5 and colocalising this with CRH immunolabelling. Phosphorylated-STAT5 immunoreactive cells were present in the PVN of prolactin-treated animals (Fig. 1g, h), but not vehicle-treated controls (data not shown). Little colocalisation of pSTAT5 with CRH immunoreactive cells was observed (0.5 ± 0.2 neurons per section) (Fig. 1h, j). Note that the number of CRH neurons per section detected by immunohistochemistry (27.4 ± 4.8; Fig. 1j) was much less than the number detected by in situ hybridisation (139.6 ± 33.9; Fig. 1i), suggesting that we were only detecting a subset of CRH neurons with this method.
pSTAT5 does not colocalise with tdTomato-labelled CRH neurons in the mouse
To overcome the difficulty in identifying CRH neurons by immunohistochemistry, we moved to a reporter mouse model to label CRH neurons. The specificity of tdTomato expression in the CRH-Cre-tdTomato mouse model was confirmed by immunohistochemistry (Fig. 2). In animals which expressed Cre, tdTomato immunolabelling was present in the PVN (Fig. 2a, c) and CeA (Fig. 2a, e), regions containing well-defined populations of CRH neurons. In animals which did not have Cre, no immunolabelling was present in either the PVN (Fig. 2b, d) or CeA (Fig. 2b, f). tdTomato immunoreactivity in the rostral subdivision of the PVN was greater than in the caudal division [F(1, 12) = 211.3, p < 0.0001; two-way ANOVA], although this was unaffected by reproductive status [F(1, 12) = 0.4594, p = 0.5108; two-way ANOVA], and thus there was no interaction between the factors [F(1, 12) = 0.8177, p = 0.3837; two-way ANOVA] (Figs. 3a–d, 4a).
Validation of the CRH-Cre-tdTomato mouse line. Representative images showing immunolabelling of tdTomato-expressing neurons in CRH-Cre-tdTomato reporter mice. Low power images showing the presence of tdTomato immunolabelling (brown staining) in the PVN and central nucleus of the amgydala (CeA) of CRH-Cre-positive mice (a) and the absence of labelling in CRH-Cre-negative mice (b). High power images showing tdTomato immunolabelling in the PVN (c) and CeA (e) of CRH-Cre-positive mice. No labelling is evident in either the PVN (d) or CeA (f) of CRH-Cre-negative mice. Images are representative of n = 3 to 4 animals per group. 3v = third ventricle
pSTAT5 does not colocalise with tdTomato-labelled CRH neurons. Representative images showing pSTAT5 and tdTomato immunolabelling in the PVN of diestrous and lactating CRH-Cre-tdTomato mice. a–d Low and high power images showing tdTomato immunolabelling (brown cytoplasmic staining) in diestrus (a, b) and lactating (c, d) CRH-Cre-tdTomato mice. Immunolabelling for pSTAT5 (black nuclear stain) is evident only in lactation and does not colocalise with pSTAT5 (c, d). e–h tdTomato-labelled CRH neurons at the caudal margin of the PVN (white arrows). Neurons double-labelled with pSTAT5 and tdTomato (black arrows) are evident at two levels of the caudal PVN (f, h). Boxes indicate the position of the high power images. Images are representative of n = 4 animals per group. 3v = third ventricle. Refer to (Paxinos and Franklin 2004) for Bregma coordinates
Quantification of the number of tdTomato and pSTAT5 immunoreactive neurons in the PVN of CRH-Cre-tdTomato mice. a The number of tdTomato neurons is greater in the rostral subdivision of the PVN, but this is unchanged between diestrous and lactating CRH-Cre-tdTomato mice. b The number of pSTAT5 immunoreactive cells increases significantly in lactation in both subdivisions of the PVN. c Limited colocalisation of pSTAT5 and tdTomato immunoreactive cells in lactating mice, which is restricted to the caudal subdivision of the PVN. Note the difference in scale on the Y-axis in (c). Data represent the mean ± SEM (n = 4 animals per group) and were analysed using a two-way ANOVA followed by a Tukey–Kramer test for multiple comparisons (****Represents p < 0.0001)
Conversely, the number of pSTAT5 labelled nuclei was significantly affected by reproductive status (F(1, 12) = 348.5, p < 0.0001; two-way ANOVA), with the number of nuclei per section greater in lactating animals as the result of the chronic elevation of circulating prolactin (Brown et al. 2011) (Figs. 3, 4b). This was observed in both PVN subdivisions (F(1, 12) = 1.899, p = 0.1933; two-way ANOVA), and thus there was no interaction between the factors (F(1, 12) = 3.801, p = 0.0750; two-way ANOVA).
Despite the increase in pSTAT5 in lactation, few tdTomato neurons expressed this marker of PRLRL activation (Fig. 4c). The distribution of pSTAT5 labelled cells in the PVN relative to the tdTomato-expressing population showed marked rostral to caudal variation. Rostrally, there was little overlap in the distribution of pSTAT5-labelled and tdTomato-labelled cells (approximately − 0.82 mm relative to Bregma; Fig. 3c). Caudally, the distribution of the two populations overlapped with pSTAT5-labelled cells interspersed among tdTomato-labelled cells (approximately − 0.94 to − 1.06 mm relative to Bregma; Fig. 3e, g). Interestingly, those few tdTomato neurons which were double-labelled in lactation were almost exclusively located at the caudal margin of the PVN (0.9 ± 0.3 and 7.3 ± 0.7 double-labelled neurons per section in the rostral and caudal divisions, respectively; F(1, 12) = 74.69, p < 0.0001; two-way ANOVA; approximately Bregma level − 1.06 mm) (Fig. 3e, g). Given that little pSTAT5 was detected in the PVN of diestrous animals, and thus no double-labelled neurons were identified, this effect of PVN subdivision on the number of double-labelled neurons was specific to the lactation group (F (1, 12) = 124.2, p < 0.0001).
CRH neurons in the mouse do not express either PRLR isoform
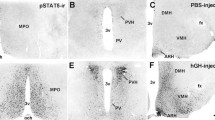
As pSTAT5 is not the only pathway activated by prolactin, we next used another transgenic line (Prlrlox/lox) in which Cre-mediated recombination would result in expression of GFP under control of the Prlr promoter, thus labelling Prlr-expressing cells (Brown et al. 2016). We looked in both diestrous and lactating mice to determine whether PRLR expression in CRH neurons differs depending on reproductive status. In positive control CamK-Cre-Prlrlox/lox animals, where deletion of PRLRs was targeted to CamK-expressing forebrain neurons (Brown et al. 2016), GFP immunolabelling was identified in a variety of hypothalamic nuclei, including the PVN and MeA (Fig. 5b, e). No GFP expression was detected in Cre-negative control animals (Fig. 5a, d). Surprisingly, no GFP expression was present in CRH-Cre-Prlrlox/lox mice in the PVN, MeA (Fig. 5c, f) nor any other brain region, in either diestrous or lactating animals. This suggested that while possible recombination occurred, the Prlr promoter was not activated in these cells to drive GFP expression and thus showed that neither isoform of the PRLR is expressed by CRH neurons.
Absence of GFP immunolabelling in CRH-Cre-Prlrlox/lox mice shows CRH neurons do not express PRLR. Representative images showing immunolabelling for GFP in the PVN and MeA in transgenic mice where Cre-mediated recombination is specific to all forebrain neurons (CamK-Cre-Prlrlox/lox; positive control) or CRH neurons (CRH-Cre-Prlrlox/lox). The presence of GFP immunolabelled cells in the CamK-Cre-Prlrlox/lox line (b, e) indicates Cre-mediated inversion of the Prlrlox/lox gene, resulting in the expression of GFP under the control of the Prlr promoter. GFP-labelled neurons are evident in the PVN and the MeA but no GFP cells are present in animals without Cre (a, d; negative control). In the CRH-Cre-Prlrlox/lox line (c, f), no GFP-labelled cells are present, indicating that PRLRs are not expressed by PVN CRH neurons. 3v = third ventricle. Refer to (Paxinos and Franklin 2004) for Bregma coordinates
Discussion
Studies of prolactin effects upon the stress axis have primarily been conducted in non-pregnant female and male rats when circulating prolactin levels are typically low and the HPA axis is responsive to a variety of physical and psychological stressors. Under these conditions, acute elevation in prolactin secretion in response to stress has been reported to protect against stress-induced gastric ulcer formation and hypocalcemia (Fujikawa et al. 2004, 2005). Conversely, chronic elevation of prolactin ICV has been shown to suppress HPA axis reactivity to stress (Donner et al. 2007; Torner et al. 2002, 2001). Both the acute and chronic anti-stress effects of prolactin are inhibited if the long form of the PRLR is blocked centrally (Fujikawa et al. 2005; Torner et al. 2002, 2001), indicating that these actions of prolactin are mediated within the brain. Evidence of Prlr mRNA expression within the PVN (Bakowska and Morrell 1997; Brown et al. 2011; Kokay et al. 2006) led us to hypothesise that prolactin acts directly upon the CRH neurons to regulate these stress responses. Using data from both rat and mouse models, we show here that CRH neurons do not express PRLRs and thus any actions of prolactin upon the HPA axis are likely to be mediated indirectly.
In the present study, consistent with previous reports (Bakowska and Morrell 1997; Kokay et al. 2006), Prlr L mRNA was detected within the rat PVN; however, there was little evidence of colocalisation with Crh mRNA expression. Furthermore, prolactin-induced pSTAT5 did not colocalise with CRH immunoreactive neurons in the PVN of the rat. Together these data showed that prolactin action upon the HPA axis is not likely to result from activation of PRLRL on CRH neurons. One caveat to this interpretation, however, was the limited visualisation of the CRH neurons by immunohistochemistry in the present study. Colchicine pre-treatment is typically used to block trafficking of the CRH protein to the axonal terminals at the median eminence and thus allow the cell bodies to be visualised by immunohistochemistry (Bloom et al. 1982). However, as this approach was not compatible with pSTAT5 immunolabelling, presumably because colchicine interferes with trafficking of this signalling molecule to the nucleus (Yip S., personal communication), it was likely that only a subset of CRH neuron cell bodies were detected in the present study. As direct prolactin action upon those CRH neurons not detected by immunohistochemistry could not be excluded, a transgenic CRH-Cre-tdTomato reporter mouse line was utilised in subsequent experiments to ensure visualisation of the CRH neuron population irrespective of CRH protein trafficking and levels of Crh mRNA expression.
In the CRH-Cre-tdTomato reporter mouse line, tdTomato immunoreactivity was specific to those animals which expressed Cre recombinase and the pattern of tdTomato expression corresponded with regions known to contain populations of CRH neurons (PVN and CeA), consistent with previous characterisation of this mouse model (Wamsteeker Cusulin et al. 2013). Assessment of prolactin-responsiveness of tdTomato-labelled CRH neurons was carried out in lactating mice because prolactin-induced pSTAT5 is not readily detected within the PVN of diestrous animals (Brown et al. 2011). The number of CRH neurons detected using tdTomato was preserved in lactating animals despite basal Crh mRNA expression being markedly suppressed at this time (Gustafson P, Bunn S and Grattan D, unpublished data). Consistent with the rat data, the majority of tdTomato-labelled CRH neurons in the CRH-Cre reporter mouse line did not express pSTAT5. However, a sub-population of dual-labelled cells was identified at the caudal margin of the PVN. Although the increase in pSTAT5 labelling within the brain during lactation has been attributed to the hyperprolactinemic state (Brown et al. 2011), we sought to confirm that these presumptive prolactin-responsive CRH neurons expressed pSTAT5 as a result of PRLRL activation, rather than another hormone or cytokine signalling through the JAK2-STAT5 pathway. To achieve this, we crossed a novel Prlrlox/lox mouse line to the CRH-Cre line to identify PRLR-expressing neurons (Brown et al. 2016). The Prlrlox/lox construct included an inverted GFP sequence which when recombined into the correct orientation, marked cells which express PRLRs by GFP expression driven by the Prlr promoter (Brown et al. 2016). In positive controls, where Cre recombinase was expressed by forebrain neurons, GFP expression was present in the PVN and other regions such as the MeA. However, no GFP expression was detected in the PVN, nor in any brain region, when Cre recombinase expression was driven by the CRH promoter. Thus, these data are consistent with the rat data showing an absence of Prlr mRNA in CRH neurons and suggest that CRH neurons are not directly prolactin responsive. This was consistent for both diestrous and lactating animals, showing that there was no upregulation of PRLR expression by CRH neurons in lactation. From these data, it can be concluded that the pSTAT5 expression by those tdTomato-labelled neurons in the caudal PVN during lactation was not the result of PRLRL activation. Given that the JAK2-STAT5 pathway is not exclusively coupled to the PRLRL, it is possible that another cytokine or hormone receptor coupled to the STAT5 pathway was activated in these cells. For example, growth hormone receptor mRNA and growth hormone-induced pSTAT5 have recently been characterised in the PVN of the mouse (Furigo et al. 2016). The significance of pSTAT5 signalling in these neurons is not known; however, as this caudal region corresponds to the pre-autonomic division of the PVN (Biag et al. 2012), this suggests a possible role in autonomic nervous system regulation.
The PVN CRH neurons identified in the rat and mouse showed little pSTAT5 expression, indicating that neither the acute stimulatory (Fujikawa et al. 2004; Weber and Calogero 1991) or chronic suppressive (Donner et al. 2007) actions of prolactin upon CRH neurons are mediated by activation of the JAK2-STAT5 pathway in these cells. In rats, ICV prolactin has been shown to stimulate the MAPK pathway in CRH neurons (Blume et al. 2009). Both PRLRL and PRLRS can mediate prolactin activation of the MAPK signalling cascade (Das and Vonderhaar 1995); however, we have shown here that Prlr L mRNA is not expressed by CRH neurons in rats. Reports of PRLRS expression within the PVN are inconsistent. One in situ hybridisation study has detected Prlr S mRNA expression within this nucleus (Bakowska and Morrell 2003), but our group could not confirm this result with RT-qPCR (Augustine et al. 2003). In the present study, we found no evidence of Prlr S mRNA expression within the PVN of the diestrous rat by in situ hybridisation, despite the presence of labelling in positive control tissues. Nevertheless, it is possible that low levels of Prlr S mRNA expression are present in the PVN but were unable to be detected by the methodological approach in our study. The absence of GFP immunolabelling in the PVN of CRH-Cre-Prlrlox/lox mice was consistent with a lack of Prlr S mRNA expression detected in the rat, as the Prlrlox/lox construct was designed such that Cre-mediated recombination would result in GFP marking the expression of either PRLR isoform (Brown et al. 2016). Interestingly, in cultured hypothalamic neurons prolactin has been shown to act through the short PRLR isoform to have a stimulatory effect upon Crh promoter activity (Blume et al. 2009). While this effect is consistent with the acute prolactin-induced stimulation of Crh mRNA expression reported in vivo (Fujikawa et al. 2004), the inhibitory effect of prolactin upon stress-induced gastric ulcer formation and hypocalcemia has been attributed to long-form PRLR activation (Fujikawa et al. 2005). Additionally, prolactin acting through the long form of the PRLR has been shown to mediate the chronic prolactin-induced suppression of the HPA axis (Donner et al. 2007; Torner et al. 2002, 2001). Our data show that any prolactin action upon CRH neurons in vivo is not likely to be mediated by either PRLR isoform directly on these cells. It must be acknowledged, however, that experiments in the present study were undertaken in basal non-stressed conditions. It is possible that under stress conditions, PRLRs could be upregulated in CRH neurons and thus allow direct prolactin regulation of these neurons. Restraint stress has been shown to increase Prlr mRNA expression in the PVN of male rats (Fujikawa et al. 1995); however, the phenotype of the Prlr-expressing cells has not been determined. Thus, additional research under stress conditions would be required to exclude this possibility.
The target of prolactin which mediates this apparent indirect action upon the CRH neurons is not known. The present study has shown that the PVN contains many non-CRH, prolactin-responsive neurons. Thus, prolactin may target a neuronal population within, or adjacent to, this nucleus to regulate CRH neuron activity. Of the defined neuronal populations within the PVN, currently only the oxytocin neurons are known to be targeted directly by prolactin acting through the PRLRL (Augustine et al. 2016; Kokay et al. 2006; Sapsford et al. 2012). Oxytocin has anti-stress effects (Neumann et al. 2000a, b), making these neurons a potential candidate for mediating the actions of prolactin upon CRH neurons. However, as the anti-stress effects of oxytocin are limited to male and non-pregnant/non-lactating female rats (Neumann et al. 2000a, b), these neurons are not likely to mediate the suppression of HPA axis activity in lactation. Alternatively, prolactin may act externally to the PVN to influence CRH neuron activity. Prlr L mRNA and prolactin-induced pSTAT5 is detected in a variety of stress-responsive brain regions including parts of the limbic system and brainstem nuclei (Brown et al. 2011, 2010; Ulrich-Lai and Herman 2009) which project to the CRH neurons and thus regulate the activity of the HPA axis. It is also possible that rather than targeting the CRH neurons, prolactin regulates pituitary adrenocorticotropic hormone (ACTH) secretion (and thus downstream corticosterone secretion) through its modulation of the neuroendocrine dopaminergic neurons in the arcuate nucleus (Olah et al. 2009). During lactation, the suckling stimulus increases ACTH secretion (Olah et al. 2009; Walker et al. 1992). This is associated with dephosphorylation/deactivation of tyrosine hydroxylase, the rate-limiting enzyme in the dopamine synthesis pathway, in the median eminence (Feher et al. 2010). Furthermore, treatment with bromocriptine (D2 dopamine receptor agonist) in lactation prevents the suckling-associated increase in ACTH secretion (Olah et al. 2009). These data indicate that the regulation of ACTH secretion is not limited to changes at the level of the CRH neurons and suggest that prolactin actions on the stress response might be mediated by additional neuronal populations.
Prolactin has been shown to have an important physiological role in the regulation of the stress response. Our data show that prolactin regulation of HPA axis activity is not mediated by prolactin directly targeting the CRH neurons. Using data from the rat and mouse models, we showed that the CRH neurons do not express either the long or short PRLR isoform and are not responsive to prolactin-induced activation of the JAK2-STAT5 signalling pathway. While it is difficult to absolutely prove a negative observation, through the use of several complementary approaches in two different models the present study strongly indicates that prolactin acts indirectly to regulate CRH neurons and thus the downstream activation of the HPA axis.
References
Augustine RA, Grattan DR (2008) Induction of central leptin resistance in hyperphagic pseudopregnant rats by chronic prolactin infusion. Endocrinology 149:1049–1055
Augustine RA, Kokay IC, Andrews ZB, Ladyman SR, Grattan DR (2003) Quantitation of prolactin receptor mRNA in the maternal rat brain during pregnancy and lactation. J Mol Endocrinol 31:221–232
Augustine RA, Ladyman SR, Grattan DR (2008) From feeding one to feeding many: hormone-induced changes in bodyweight homeostasis during pregnancy. J Physiol 586:387–397. doi:10.1113/jphysiol.2007.146316
Augustine RA, Bouwer GT, Seymour AJ, Grattan DR, Brown CH (2016) Reproductive regulation of gene expression in the hypothalamic supraoptic and paraventricular nuclei. J Neuroendocrinol 28. doi:10.1111/jne.12350
Bakowska JC, Morrell JI (1997) Atlas of the neurons that express mRNA for the long form of the prolactin receptor in the forebrain of the female rat. J Comp Neurol 386:161–177. doi:10.1002/(Sici)1096-9861
Bakowska JC, Morrell JI (2003) The distribution of mRNA for the short form of the prolactin receptor in the forebrain of the female rat. Mol. Brain Res 116:50–58. doi:10.1016/S0169-328X(03)00213-4
Biag J, Huang Y, Gou L et al (2012) Cyto- and chemoarchitecture of the hypothalamic paraventricular nucleus in the C57BL/6 J male mouse: a study of immunostaining and multiple fluorescent tract tracing. J Comp Neurol 520:6–33. doi:10.1002/cne.22698
Bloom FE, Battenberg EL, Rivier J, Vale W (1982) Corticotropin releasing factor (CRF): immunoreactive neurones and fibers in rat hypothalamus. Regul Pept 4:43–48
Blume A, Torner L, Liu Y, Subburaju S, Aguilera G, Neumann ID (2009) Prolactin activates mitogen-activated protein kinase signaling and corticotropin releasing hormone transcription in rat hypothalamic neurons. Endocrinology 150:1841–1849. doi:10.1210/en.2008-1023
Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA (1998) Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Rev 19:225–268
Bridges RS, Ronsheim PM (1990) Prolactin (PRL) regulation of maternal behavior in rats: bromocriptine treatment delays and PRL promotes the rapid onset of behavior. Endocrinology 126:837–848. doi:10.1210/endo-126-2-837
Bridges RS, Numan M, Ronsheim PM, Mann PE, Lupini CE (1990) Central prolactin infusions stimulate maternal behavior in steroid-treated, nulliparous female rats. Proc Natl Acad Sci USA 87:8003–8007
Brown RS, Kokay IC, Herbison AE, Grattan DR (2010) Distribution of prolactin-responsive neurons in the mouse forebrain. J Comp Neurol 518:92–102. doi:10.1002/cne.22208
Brown RS, Herbison AE, Grattan DR (2011) Differential changes in responses of hypothalamic and brainstem neuronal populations to prolactin during lactation in the mouse. Biol Reprod 84:826–836. doi:10.1095/biolreprod.110.089185
Brown RSE, Kokay IC, Phillipps HR et al (2016) Conditional deletion of the prolactin receptor reveals functional subpopulations of dopamine neurons in the arcuate nucleus of the hypothalamus. J Neurosci 36:9173–9185. doi:10.1523/jneurosci.1471-16.2016
Brunton PJ, Meddle SL, Ma S, Ochedalski T, Douglas AJ, Russell JA (2005) Endogenous opioids and attenuated hypothalamic-pituitary-adrenal axis responses to immune challenge in pregnant rats. J Neurosci 25:5117–5126. doi:10.1523/JNEUROSCI.0866-05.2005
Casanova E, Fehsenfeld S, Mantamadiotis T, Lemberger T, Greiner E, Stewart AF, Schutz G (2001) A CamKIIalpha iCre BAC allows brain-specific gene inactivation. Genesis 31:37–42
da Costa AP, Wood S, Ingram CD, Lightman SL (1996) Region-specific reduction in stress-induced c-fos mRNA expression during pregnancy and lactation. Brain Res 742:177–184
Das R, Vonderhaar BK (1995) Transduction of prolactin’s (PRL) growth signal through both long and short forms of the PRL receptor. Mol Endocrinol 9:1750–1759. doi:10.1210/mend.9.12.8614411
Donner N, Bredewold R, Maloumby R, Neumann ID (2007) Chronic intracerebral prolactin attenuates neuronal stress circuitries in virgin rats. Eur J Neurosci 25:1804–1814. doi:10.1111/j.1460-9568.2007.05416.x
Drago F, Continella G, Conforto G, Scapagnini U (1985) Prolactin inhibits the development of stress-induced ulcers in the rat. Life Sci 36:191–197
Feher P, Olah M, Bodnar I et al (2010) Dephosphorylation/inactivation of tyrosine hydroxylase at the median eminence of the hypothalamus is required for suckling-induced prolactin and adrenocorticotrop hormone responses. Brain Res Bull 82:141–145. doi:10.1016/j.brainresbull.2010.02.006
Forsyth IA, Wallis M (2002) Growth hormone and prolactin–molecular and functional evolution. J Mammary Gland Biol Neoplasia 7:291–312
Freeman ME, Kanyicska B, Lerant A, Nagy G (2000) Prolactin: structure, function, and regulation of secretion. Physiol Rev 80:1523–1631
Fujikawa T, Soya H, Yoshizato H, Sakaguchi K, Doh-Ura K, Tanaka M, Nakashima K (1995) Restraint stress enhances the gene expression of prolactin receptor long form at the choroid plexus. Endocrinology 136:5608–5613. doi:10.1210/endo.136.12.7588315
Fujikawa T, Soya H, Tamashiro KL et al (2004) Prolactin prevents acute stress-induced hypocalcemia and ulcerogenesis by acting in the brain of rat. Endocrinology 145:2006–2013. doi:10.1210/en.2003-1446
Fujikawa T, Tamura K, Kawase T et al (2005) Prolactin receptor knockdown in the rat paraventricular nucleus by a morpholino-antisense oligonucleotide causes hypocalcemia and stress gastric erosion. Endocrinology 146:3471–3480. doi:10.1210/en.2004-1528
Furigo IC, Metzger M, Teixeira PD, Soares CR, Donato J Jr (2016) Distribution of growth hormone-responsive cells in the mouse brain. Brain Struct Funct. doi:10.1007/s00429-016-1221-1
Grattan DR (2015) 60 Years of neuroendocrinology: the hypothalamo-prolactin axis. J Endocrinol 226:T101–122. doi:10.1530/JOE-15-0213
Kokay IC, Bull PM, Davis RL, Ludwig M, Grattan DR (2006) Expression of the long form of the prolactin receptor in magnocellular oxytocin neurons is associated with specific prolactin regulation of oxytocin neurons. Am J Physiol Regul Integr Comp Physiol 290:R1216–R1225. doi:10.1152/ajpregu.00730.2005
Larsen CM, Grattan DR (2010) Prolactin-induced mitogenesis in the subventricular zone of the maternal brain during early pregnancy is essential for normal postpartum behavioral responses in the mother. Endocrinology 151:3805–3814. doi:10.1210/en.2009-1385
Lightman SL, Young WS 3rd (1989) Lactation inhibits stress-mediated secretion of corticosterone and oxytocin and hypothalamic accumulation of corticotropin-releasing factor and enkephalin messenger ribonucleic acids. Endocrinology 124:2358–2364
Madisen L, Zwingman TA, Sunkin SM et al (2010) A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13:133–140. doi:10.1038/nn.2467
Neumann ID, Johnstone HA, Hatzinger M et al (1998) Attenuated neuroendocrine responses to emotional and physical stressors in pregnant rats involve adenohypophysial changes. J Physiol 508(Pt 1):289–300
Neumann ID, Kromer SA, Toschi N, Ebner K (2000a) Brain oxytocin inhibits the (re)activity of the hypothalamo-pituitary-adrenal axis in male rats: involvement of hypothalamic and limbic brain regions. Regul Pept 96:31–38
Neumann ID, Torner L, Wigger A (2000b) Brain oxytocin: differential inhibition of neuroendocrine stress responses and anxiety-related behaviour in virgin, pregnant and lactating rats. Neuroscience 95:567–575
Olah M, Feher P, Ihm Z et al (2009) Dopamine-regulated adrenocorticotropic hormone secretion in lactating rats: functional plasticity of melanotropes. Neuroendocrinology 90:391–401. doi:10.1159/000232313
Paxinos G, Franklin KB (2004) The mouse brain in stereotaxic coordinates. 4th edn, Gulf Professional Publishing, Houston
Paxinos G, Watson C (2006) The rat brain in stereotaxic coordinates. 6th edn, Academic Press, Cambridge
Sapsford TJ, Kokay IC, Ostberg L, Bridges RS, Grattan DR (2012) Differential sensitivity of specific neuronal populations of the rat hypothalamus to prolactin action. J Comp Neurol 520:1062–1077. doi:10.1002/cne.22775
Schlein P, Zarrow M, Denenberg V (1974) The role of prolactin in the depressed or ‘buffered’ adrenocorticosteroid response of the rat. J Endocrinol 62:93–99
Shanks N, Windle RJ, Perks P, Wood S, Ingram CD, Lightman SL (1999) The hypothalamic-pituitary-adrenal axis response to endotoxin is attenuated during lactation. J Neuroendocrinol 11:857–865
Taniguchi H, He M, Wu P et al (2011) A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron 71:995–1013. doi:10.1016/j.neuron.2011.07.026
Torner L, Toschi N, Pohlinger A, Landgraf R, Neumann ID (2001) Anxiolytic and anti-stress effects of brain prolactin: improved efficacy of antisense targeting of the prolactin receptor by molecular modeling. J Neurosci 21:3207–3214
Torner L, Toschi N, Nava G, Clapp C, Neumann ID (2002) Increased hypothalamic expression of prolactin in lactation: involvement in behavioural and neuroendocrine stress responses. Eur J Neurosci 15:1381–1389
Ulrich-Lai YM, Herman JP (2009) Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 10:397–409. doi:10.1038/nrn2647
Walker CD, Lightman SL, Steele MK, Dallman MF (1992) Suckling is a persistent stimulus to the adrenocortical system of the rat. Endocrinology 130:115–125. doi:10.1210/endo.130.1.1309321
Walker CD, Tilders FJ, Burlet A (2001) Increased colocalization of corticotropin-releasing factor and arginine vasopressin in paraventricular neurones of the hypothalamus in lactating rats: evidence from immunotargeted lesions and immunohistochemistry. J Neuroendocrinol 13:74–85
Wamsteeker Cusulin JI, Fuzesi T, Watts AG, Bains JS (2013) Characterization of corticotropin-releasing hormone neurons in the paraventricular nucleus of the hypothalamus of Crh-IRES-Cre mutant mice. PLoS One 8:e64943
Weber RF, Calogero AE (1991) Prolactin stimulates rat hypothalamic corticotropin-releasing hormone and pituitary adrenocorticotropin secretion in vitro. Neuroendocrinology 54:248–253
Windle RJ, Wood S, Shanks N et al (1997) Endocrine and behavioural responses to noise stress: comparison of virgin and lactating female rats during non-disrupted maternal activity. J Neuroendocrinol 9:407–414
Acknowledgements
This work was supported by a Programme Grant from the Health Research Council of New Zealand (14–568). PG was supported by a University of Otago PhD Scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Papillon Gustafson and Ilona Kokay have contributed equally to this study.
Rights and permissions
About this article
Cite this article
Gustafson, P., Kokay, I., Sapsford, T. et al. Prolactin regulation of the HPA axis is not mediated by a direct action upon CRH neurons: evidence from the rat and mouse. Brain Struct Funct 222, 3191–3204 (2017). https://doi.org/10.1007/s00429-017-1395-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-017-1395-1