Abstract
Adipokines and inflammatory markers have been linked to kidney disease in animal models; however, evidence from prospective human studies is sparse. Recruited from Beijing and Shanghai in 2005, a total number of 2220 non-institutionalized Chinese individuals aged 50–70 years with baseline estimated glomerular filtration rate (eGFR) >60 mL/min/1.73 m2 were prospectively followed for 6 years. Plasma levels of resistin, retinol-binding protein 4 (RBP4), interleukin-6 (IL-6), C-reactive protein (CRP), and tumor necrosis factor-α receptor 2 (TNF-R2) were determined at baseline. Kidney function decrease was assessed by measurements of eGFR over 6 years. Incident-reduced eGFR was defined as the onset of eGFR <60 mL/min/1.73 m2, according to the Modification of Diet in Renal Disease Study Equation for Chinese. During the 6 years of follow-up, 333 (15.0 %) participants had incident-reduced eGFR. Each 1 standard deviation elevated concentration of resistin [relative risk (RR) 1.10; 95 % CI 1.00–1.24] and TNFR-2 (RR 1.30; 95 % CI 1.13–1.49) at baseline were significantly associated with a higher risk of incident-reduced eGFR. Comparing the highest with the lowest quartiles, the RR of incident-reduced eGFR was 1.43 (95 % CI 1.01–2.03) for resistin and 2.03 (95 % CI 1.41–2.93) for TNF-R2 (both P trend < 0.05) after adjustment for baseline demographic characteristics, lifestyle behaviors, BMI, plasma lipid profile, hypertension, and diabetes. These associations remained significant when further controlling for levels of RBP4, IL-6, and CRP, none of which was significantly associated with the risk of incident-reduced eGFR. In this prospective cohort study, elevated levels of resistin and TNF-R2, but not other adipokines and inflammatory markers, were independently associated with a greater risk of kidney function decline in middle-aged and elderly Chinese.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) is a condition that leads to numerous complications such as end-stage renal failure, cardiovascular disease, and premature death [1–3]. CKD has become one of the worldwide public health issues with substantial social and economic consequences [1–4]. In recent years, the prevalence of CKD and those of its major risk factors, including obesity, hypertension, and type 2 diabetes, have increased substantially in China [1, 5–7]. For instance, the prevalence was 10.8 % for CKD, 11.6 % for type 2 diabetes, and 12 % for obesity in adult Chinese in 2010 [1, 5, 6]. Thus, identifying major contributors for CKD is important to facilitate disease prevention and management.
The role of adipokines and inflammatory markers in pathogenesis of kidney dysfunction has attracted increased attention in recent years [8–16]. Pretreated rats with human recombinant tumor necrosis factor (TNF) and interleukin-1 beta enhanced the severity of glomerular injury [9]. Human recombinant TNF also induced glomerular endothelial cell damage in rabbits [10]. However, data from human studies, particularly from prospective studies, are sparse and inconsistent [8, 13, 17–19]. Previously, adipokines such as resistin and retinol-binding protein 4 (RBP4) and inflammatory markers such as C-reactive protein (CRP) or interleukin-6 (IL-6) were reported to be positively associated with the risk of CKD in some cross-sectional studies [11, 12, 20–22], but not in others [23]. To date, associations of CRP and IL-6 with CKD risk were investigated longitudinally in some cohort studies, and results were inconsistent [8, 13, 17, 18]. For example, increased CRP and IL-6 levels were associated with declined kidney function in the Multi-Ethnic Study of Atherosclerosis [17], whereas no significant associations were observed in the Cardiovascular Health Study [18]. In the present study, we aimed to investigate associations of multiple adipokines and inflammatory markers with the kidney function decline among the general Chinese population.
Materials and methods
Study population
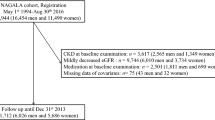
The Nutrition and Health of Aging Population in China study was initiated in 2005 when 3289 Chinese adults aged 50–70 years were selected and enrolled through multistage sampling from residents in one rural and two urban districts in each of two cities, Beijing and Shanghai, with comparable socioeconomic status [24]. This cohort study was designed to determine the effects of environmental and genetic factors and their interaction on the development of chronic diseases [24]. In 2011, all initial participants (n = 3289) were contacted, and 2529 participants (76.9 %) were successfully followed up. The remaining 760 participants (23.1 %) were considered as lost to follow-up due to either failure to be contacted (n = 554) or reluctance to participate (n = 206). Detailed information regarding the baseline and the follow-up surveys have been reported elsewhere [25]. After excluding adults with estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 at baseline (n = 50) or without blood samples at follow-up (n = 259), a total of 2220 men and women were included in the last analysis. All participants provided written informed consent, and the study was approved by the Institutional Review Board of the Institute for Nutritional Sciences.
Data collection
During a face-to-face interview conducted by trained health professionals, a standardized questionnaire was used at baseline and follow-up to collect information about social demographics, smoking (yes/no), alcohol consumption (yes/no), physical activity, and family history of disease. Educational levels were defined according to self-reported school years (0–6, 7–9, ≥10 years). Physical activity level was classified as low, moderate, or high according to the International Physical Activity Questionnaire scoring protocol with minor modification [24]. Participants were also invited to attend a physical examination. Body weight, height, waist circumference, and blood pressure were measured by trained health workers following a standardized protocol [24]. Body mass index (BMI) was calculated as mass in kilograms divided by height in meters squared. Following a 5-min rest, blood pressure was measured three times on the right arm using an electronic blood pressure monitor (Omron HEM-705CP, Vernon Hills, IL, USA), and the mean values of the last 2 measurements were used in the analysis. Hypertension was defined as systolic blood pressure ≥140 mmHg, or diastolic blood pressure ≥90 mmHg, or use of anti-hypertensive medications, or self-reported doctor-diagnosed hypertension. Type 2 diabetes was defined as fasting plasma glucose concentration ≥7.0 mmol/L, or taking anti-diabetic medications, or self-reported doctor-diagnosed diabetes.
Laboratory measurements
Fasting peripheral venous blood samples were collected in tubes with ethylene diamine tetraacetic acid and centrifuged at 3000 rpm for 15 min at both baseline and follow-up, and stored at −80 °C until analysis. Baseline and follow-up levels of glucose, total cholesterol, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol were measured on an automatic analyzer (Hitachi 7080, Tokyo, Japan) with commercial reagents (Wako Pure Chemical Industries, Osaka, Japan). Baseline plasma resistin was measured by Luminex xMAP Technology (Linco Research, St Charles, MO, USA) on a Bio-Rad Multiplex Suspension Array System. The intra- and inter-assay coefficients of variation (CVs) ranged from 1.4 to 7.9 % [26]. The basal level of RBP4 was measured in duplication by a sandwich enzyme-linked immunosorbent assay developed in-house [27]. The intra- and inter-assay CVs were 2–8 and 4–9 %, respectively. Plasma high-sensitive CRP was measured by a particle-enhanced immunoturbidimetric assay (Ultrasensitive CRP kit; Orion Diagnostica, Espoo, Finland), with intra- and inter-assay CVs all <12 % [24]. Plasma IL-6 was detected by means of ELISA (Quantikine HS IL-6 Immunoassay; R&D Systems, Minneapolis, MN). The intra- and inter-assay CVs for the IL-6 levels were all <8 % [28]. Soluble tumor necrosis factor-α receptor 2 (TNF-R2) was detected with Human Death Receptor 3-Plex kit (Biosource International Inc., Camarillo, CA, USA) in the same system used for measuring resistin. The average intra- and inter-assay CVs were 8 and 10 %, respectively.
Assessment of kidney function decline
For all of the participants, plasma creatinine (Pcr) was measured both at baseline and 6-year follow-up by means of an enzymatic method with intra- and inter-assay CVs <3 %. In addition, the Jaffe’s kinetic method was also used to measure Pcr for 50 participants randomly selected from the same population. Both methods were performed on a Hitachi 7080 automatic analyzer (Hitachi, Tokyo, Japan). A calibration equation was subsequently developed: Pcr (mg/dL) (Jaffe’s kinetic method) = 0.9246 × (Pcr [mg/dL] [enzymatic method]) + 0.3104 (r = 0.97; P < 0.0001) [29].
Based on the Modification of Diet in Renal Disease (MDRD) Study Equation for Chinese with minor modification, eGFR was calculated as eGFR (mL/min/1.73 m2) = 175 × Pcr (mg/dL)−1.234 (Jaffe’s kinetic method) × age−0.179 × 0.79 (if female) [30]. Kidney function decrease was assessed by measurements of eGFR over 6 years. Incident-reduced eGFR was defined as an eGFR <60 mL/min/1.73 m2 during follow-up [31].
In a sensitivity analysis, the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation was used to calculate eGFR [32, 33]. Moreover, to reduce misclassification due to slight changes of eGFR close to the low eGFR threshold [17], both eGFR decline >3 % per year and follow-up eGFR <60 mL/min/1.73 m2 were used to define incident-reduced eGFR [34]. In addition, the rapid decline of kidney function was defined as eGFR decline >3 mL/min/1.73 m2 per year [17].
Statistical analysis
Baseline characteristics were compared between participants with and without incident-reduced eGFR by means of the Student’s t test and χ 2 test whenever appropriate. Because person-years could not be accurately estimated, logistic regression models were applied to estimate the relative risks (RRs) of incident-reduced eGFR according to quartiles of baseline adipokines (resistin and RBP4) and inflammatory markers (CRP, IL-6, and TNF-R2), adjusting for age, sex, region (Beijing/Shanghai), residence (urban/rural), BMI, educational attainment, smoking, alcohol consumption, physical activity, HDL, LDL, diabetes, and hypertension [14, 17]. Due to the high cumulative incidence of reduced eGFR (15 %, 333/2220), the log-Poisson model provided consistent but not fully efficient estimations of RRs and confidence intervals (CIs); and it was applied to calculate RRs, 95 % CIs, and P-trend for incident-reduced eGFR [35]. In addition, each biomarker was modeled continuously per standard deviation (SD) to allow between comparisons [17]. The associations between individual cytokines and the risk of incident-reduced eGFR were also estimated in fully adjusted models stratified by age (<60, ≥60), sex, region, residence, smoking, hypertension, diabetes, and baseline eGFR (>90 and 60–90 mL/min/1.73 m2). Likelihood ratio tests were performed to examine potential interactions. Moreover, in the multivariable model, the adipokines and inflammatory markers were mutually adjusted in quartiles to examine whether the associations were independent of each other. In a secondary analysis, to further evaluate whether the inflammatory markers and adipokines could improve the ability of predicting CKD risk beyond traditional risk factors, areas under the receiver-operating characteristic curves (AUCs) were calculated as (1) Model 1—adjusting for age, sex, region (Beijing/Shanghai), residence (urban/rural), BMI, educational attainment, smoking, alcohol consumption, physical activity, HDL, LDL, diabetes, and hypertension; (2) Model 2—the Model 1 plus adjustment of the baseline eGFR; and (3) Model 3—the Model 2 plus adjustment of inflammatory markers and adipokines. The strengths of AUC statistics between the three models were subsequently compared. All analyses were performed with SAS (version 9.3; SAS Institute Inc., Cary, NC). Two-sided P values <0.05 were considered statistically significant.
Results
During the 6 years of follow-up, 333 (15.0 %) participants had incident-reduced eGFR. Baseline characteristics were compared between participants with and without incident-reduced eGFR (Table 1). Participants who had incident-reduced eGFR were older, more likely to than their counterparts have lower educational attainment, higher systolic/diastolic blood pressure, and higher prevalence of hypertension. Median levels of TNF-R2 were significantly higher in incident-reduced eGFR cases than non-cases (1.73 vs. 1.56 ng/mL, P < 0.001); while median resistin concentration was marginally higher in the cases than in the non-cases (9.6 vs. 8.5 ng/mL, P = 0.08). No significant differences were observed for other adipokines or inflammatory markers between incident cases of reduced eGFR and non-cases.
Table 2 shows the associations among adipokines, inflammatory markers, and eGFR. Plasma resistin and TNF-R2 were positively associated with the risk of incident-reduced eGFR (P trend <0.01). The associations were largely unchanged after further adjustment for BMI, educational attainment, smoking, alcohol consumption, physical activity, HDL, LDL, hypertension, and diabetes (Model 3; all P trend < 0.05). Compared with the individuals in the lowest quartile, subjects in the highest quartile had an RR of 1.43 (95 % CI 1.01–2.03) for resistin, and 2.03 (95 % CI 1.41–2.93) for TNF-R2. Similar results were also obtained when log-Poisson regression was used to model these associations: comparing the extreme quartiles, the RRs (95 % CIs) were 1.33 (1.01–1.77) for resistin and 1.77 (1.30–2.41) for TNF-R2 (Model 3; both P trend <0.05). In contrast, null associations were observed between RBP4, CRP, IL-6, and the risk of incident-reduced eGFR (Table 3). In addition, each 1-SD elevated concentration of resistin (RR 1.10; 95 % CI 1.00–1.24) and TNFR-2 (RR 1.30; 95 % CI 1.13–1.49) at baseline was significantly associated with a higher risk of incident-reduced eGFR. No association was observed between other biomarkers (RBP4, CRP, and IL-6) and incident-reduced eGFR (Table 3). Moreover, AUC statistics of 0.67 was estimated for the model consisting of age, sex, region, residence, BMI, educational attainment, smoking, alcohol consumption, physical activity, HDL, LDL, diabetes, and hypertension. Further inclusion of baseline eGFR significantly increased the AUC to 0.76 (P < 0.01). The AUC statistics was further strengthened to 0.78 (P = 0.06), when adding baseline resistin and TNFR-2 in the prediction model.
In the stratified analyses, the associations between resistin, TNF-R2 and the risk of incident-reduced eGFR remained positive in most subgroups defined by age, sex, region, residence, current smoking status, hypertension, diabetes, and baseline eGFR, although some of the associations were not significant possibly due to reduced power (all P for interaction >0.5) (Table 4). In sensitivity analyses, when additionally adjusting for RBP4, CRP, and IL-6 in the multivariable models, the associations remained significant (comparing the extreme quartile, RRs and 95 % CIs were 1.41 [1.00–2.02] for resistin and 2.02 [1.39–2.92] for TNF-R2, both P trend <0.05). When additionally adjusting for each other, the results for resistin and TNF-R2 were similar. When eGFR was calculated using the CKD-EPI equation [32, 33], the associations were attenuated but still significant [comparing the extreme quartiles, RRs and 95 % CIs were 1.36 [1.01–1.84] for resistin and 1.70 [1.25–2.32] for TNF-R2, both P trend <0.05) (Supplementary Table 1). Finally, when the alternative definitions (both eGFR decline >3 % per year and follow-up eGFR <60 mL/min/1.73 m2) for incident-reduced eGFR and rapid decline of kidney function (eGFR decline >3 mL/min/1.73 m2 per year) were utilized in our analysis, the results remained similar (Supplementary Tables 2 and 3).
Discussion
In this prospective investigation, we found that elevated plasma levels of resistin and TNF-R2 were significantly associated with an increased risk of kidney function decline in non-institutionalized middle-aged and elderly Chinese men and women. The associations were independent of established risk factors including other adipokines and inflammatory factors and robust to various definitions of kidney function decline.
In line with the findings of our cohort study, the positive association between plasma resistin levels and renal dysfunction was also reported in a few cross-sectional and prevalent case–control studies [11, 12, 20, 22], though no cohort data were available previously. For instance, in a case–control study, Mills et al. found a significant association between higher plasma resistin levels and an increased risk of kidney dysfunction; the multivariate-adjusted odds ratio (95 % CI) across the extreme resistin tertiles was 12.7 (6.5, 24.6) [11]. Resistin, a 12.5-kDa cysteine-rich protein, is mainly secreted in monocytes and macrophages in humans [36–38]. It remains unclear regarding the underlying mechanisms linking resistin levels with the pathogenesis of CKD. Existing studies suggested that resistin may promote endothelial dysfunction through enhancing oxidative stress [39], which might consequently induce glomeruli dysfunction [12]. Other studies indicated that the adverse effects of resistin could be attributed to its effects on stimulating the production of CRP and IL-6 [37, 40, 41]. Notably, plasma resistin was significantly correlated with CRP and IL-6 (Table 2), but its association with kidney function decline was apparently independent of these two inflammatory factors. Indeed, resistin was suggested to be a more sensitive marker reflecting pro-inflammatory status [42]. However, whether resistin could serve as an early predictor of kidney dysfunction remains to be elucidated in future studies.
In the present study, higher levels of TNF-R2 but not other inflammatory markers were significantly associated with a greater risk of kidney function decline. This observation was in accordance with the findings from a prospective cohort study conducted in 4926 Americans by Shankar et al. [13] (RR: 2.10; 95 % CI 1.55–2.84 across the extreme tertiles; P trend <0.001) and also a recent hospital-based study consisting of 262 South Korean patients by An et al. [19] (OR 3.24, 95 % CI 1.26–8.31, P = 0.015), as well as a few cross-sectional studies conducted also in western populations [21, 43–45]. As a soluble TNF-alpha receptor, TNF-R2 can mediate NF-κB activation, which has long been known as a prototypical pro-inflammatory signaling pathway which involves the enhanced expression of proinflammatory genes including adhesion molecules, chemokines, and cytokines [46, 47]. In the case of kidney dysfunction, evidence from animal studies showed that TNF receptors could mediate local inflammatory injury in the kidney [48]. In addition, TNF-R2 was also indicated to contribute to tubulointerstitial fibrosis and resulted in kidney damage [49]. Given its role as an upstream regulator of inflammatory pathway, TNF-R2 was suggested as an early predictor of progression to kidney dysfunction [13].
As two commonly studied inflammatory factors, the roles of CRP and IL-6 in the kidney disease remain controversial in cohort studies [8, 13, 17, 18]. For example, in the Multi-Ethnic Study of Atherosclerosis, significant associations of CRP and IL-6 with declined eGFR were only evidenced when using cystatin C-based equation to calculate eGFR, but not by creatinine-based equation [17]. In the Cardiovascular Health Study, higher levels of CRP were associated with higher levels of creatinine [8], but not associated with any decline of eGFR (cystatin C-based) in a subsequent analyses [18]. Shankar et al. also reported that IL-6, but not CRP, was associated with risk of CKD (calculated by creatinine-based eGFR) [13]. The discrepancies among these studies might be due to variations in the definitions of CKD or impaired renal function, various follow-up duration, characteristics of participants, and covariates adjusted. In addition, it was noticed that the CRP levels in our study population were much lower than those in previous studies (such as, comparing with the Multi-Ethnic Study of Atherosclerosis, median CRP was 0.70 vs. 2.35 mg/L for CKD cases; 0.64 vs. 1.78 mg/L for non-cases) [8, 17]. Thus, the different CRP levels might contribute to the heterogeneous findings between our study and other studies. As for RBP4, in contrast to the result reported in previous cross-sectional study [20], no significant association was observed between plasma RBP4 levels and the risk of kidney function decline in our prospective study. Nonetheless, further prospective studies are warranted to clarify the association between CRP, IL-6, RBP4, and the risk of kidney function decline among different populations.
To the best of our knowledge, our study is the first one that simultaneously investigated associations of multiple adipokines and inflammatory markers with risk of kidney function decline in a non-institutionalized Asia population. Moreover, comprehensive information about potential confounders was collected. A few limitations should also be mentioned. First, the follow-up rate of the study was 76.9 %, but it was acceptable comparable with other studies conducted in elderly participants [50]. Second, similar to other population-based studies, we were unable to measure renal function directly by a gold standard such as iothalamate clearance. Instead, our analyses included the modified MDRD study equation for Chinese, along with the CKD-EPI equation and the Bash’s CKD definition, and all of the approaches yielded consistent results. Third, the study did not have information regarding to intakes of medications like angiotensin receptor blockers or angiotensin-converting enzyme inhibitors, and therefore, the potential impacts of these medications on plasma creatinine were unable to be controlled for. In addition, our study was performed in middle-aged and elderly Chinese, and the findings of this study therefore may not be generalized to younger populations or other ethnicities. Finally, as in any epidemiology study, the study cannot demonstrate causality and exclude the role of residual confounding.
In conclusion, in this cohort study, elevated levels of plasma resistin and TNF-R2 were independently associated with a higher risk of kidney function decline after a 6-year follow-up in middle-aged and elderly Chinese men and women. More prospective studies are warranted to confirm our findings. Meanwhile, future experimental studies are also required to clarify the potential mechanisms underlying these associations.
References
L.X. Zhang, F. Wang, L. Wang et al., Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet 379, 815–822 (2012)
A.S. Levey, R. Atkins, J. Coresh et al., Chronic kidney disease as a global public health problem: approaches and initiatives—a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 72, 247–259 (2007)
A.K. Bello, E. Nwankwo, A.M. EL Nahas, Prevention of chronic kidney disease: a global challenge. Kidney Int. 68, S11–S17 (2005)
J. Coresh, E. Selvin, L.A. Stevens et al., Prevalence of chronic kidney disease in the United States. JAMA 298, 2038–2047 (2007)
Y. Xu, L.M. Wang, J. He et al., Prevalence and control of diabetes in Chinese adults. JAMA 310, 948–958 (2013)
Chinese Center for Disease Control and Prevention, Major findings in chronic diseases and risk factors of national DSPs in 2010 (2011), http://www.chinacdc.cn/gwswxx/mbsqc/201109/t20110906_52141.htm
D. Gu, K. Reynolds, X. Duan et al., Prevalence of diabetes and impaired fasting glucose in the Chinese adult population: International Collaborative Study of Cardiovascular Disease in Asia (InterASIA). Diabetologia 46, 1190–1198 (2003)
L. Fried, C. Solomon, M. Shlipak et al., Inflammatory and prothrombotic markers and the progression of renal disease in elderly individuals. J. Am. Soc. Nephrol. 15, 3184–3191 (2004)
N.I. Tomosugi, S.J. Cashman, H. Hay et al., Modulation of antibody-mediated glomerular injury in vivo by bacterial lipopolysaccharide, tumor necrosis factor, and IL-1. J. Immunol. 142, 3083–3090 (1989)
T. Bertani, M. Abbate, C. Zoja et al., Tumor necrosis factor induces glomerular damage in the rabbit. Am. J. Pathol. 134, 419–430 (1989)
K.T. Mills, L.L. Hamm, A.B. Alper et al., Circulating adipocytokines and chronic kidney disease. PLoS One 8, e76902 (2013)
C. Menzaghi, L. Salvemini, G. Fini et al., Serum resistin and kidney function: a family-based study in non-diabetic, untreated individuals. PLoS One 7, e38414 (2012)
A. Shankar, L.P. Sun, B.E.K. Klein et al., Markers of inflammation predict the long-term risk of developing chronic kidney disease: a population-based cohort study. Kidney Int. 80, 1231–1238 (2011)
L.D. Bash, T.P. Erlinger, J. Coresh, J. Marsh-Manzi, A.R. Folsom, B.C. Astor, Inflammation, hemostasis, and the risk of kidney function decline in the Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Kidney Dis. 53, 596–605 (2009)
J. Axelsson, P. Stenvinkel, Role of fat mass and adipokines in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 17, 25–31 (2008)
H.J. Kim, N.D. Vaziri, Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure. Am. J. Physiol. Renal Physiol. 298, F662–F671 (2010)
J.S. Hiramoto, R. Katz, C.A. Peralta et al., Inflammation and coagulation markers and kidney function decline: the Multi-Ethnic Study of Atherosclerosis (MESA). Am. J. Kidney Dis. 60, 225–232 (2012)
C. Keller, R. Katz, M.J. Sarnak et al., Inflammatory biomarkers and decline in kidney function in the elderly: the Cardiovascular Health Study. Nephrol. Dial. Transpl. 25, 119–124 (2010)
J.N. An, K.D. Yoo, J.H. Hwang et al., Circulating tumour necrosis factor receptors 1 and 2 predict contrast-induced nephropathy and progressive renal dysfunction: a prospective cohort study. Nephrology 20, 552–559 (2015)
A. Cabre, I. Lazaro, J. Girona et al., Retinol-binding protein 4 as a plasma biomarker of renal dysfunction and cardiovascular disease in type 2 diabetes. J. Intern. Med. 262, 496–503 (2007)
A. Upadhyay, M.G. Larson, C.Y. Guo et al., Inflammation, kidney function and albuminuria in the Framingham Offspring cohort. Nephrol. Dial. Transpl. 26, 920–926 (2011)
R. Kawamura, Y. Doi, H. Osawa et al., Circulating resistin is increased with decreasing renal function in a general Japanese population: the Hisayama Study. Nephrol Dial Transpl 25, 3236–3240 (2010)
M. Pruijm, B. Ponte, P. Vollenweider et al., Not all inflammatory markers are linked to kidney function: results from a population-based study. Am. J. Nephrol. 35, 288–294 (2012)
X.W. Ye, Z.J. Yu, H.X. Li, O.H. Franco, Y. Liu, X. Lin, Distributions of C-reactive protein and its association with metabolic syndrome in middle-aged and older Chinese people. J. Am. Coll. Cardiol. 49, 1798–1805 (2007)
G. Zong, J.W. Zhu, L. Sun et al., Associations of erythrocyte fatty acids in the de novo lipogenesis pathway with risk of metabolic syndrome in a cohort study of middle-aged and older Chinese. Am. J. Clin. Nutr. 98, 319–326 (2013)
Q.B. Qi, J.X. Wang, H.X. Li et al., Associations of resistin with inflammatory and fibrinolytic markers, insulin resistance, and metabolic syndrome in middle-aged and older Chinese. Eur. J. Endocrinol. 159, 585–593 (2008)
Q.B. Qi, Z.J. Yu, X.W. Ye et al., Elevated retinol-binding protein 4 levels are associated with metabolic syndrome in Chinese people. J. Clin. Endocr. Metab. 92, 4827–4834 (2007)
X.W. Ye, O.H. Franco, Z.J. Yu et al., Associations of inflammatory factors with glycaemic status among middle-aged and older Chinese people. Clin. Endocrinol. 70, 854–862 (2009)
F. Wang, P. Ye, L.M. Luo, W.K. Xiao, H.M. Wu, Association of risk factors for cardiovascular disease and glomerular filtration rate: a community-based study of 4925 adults in Beijing. Nephrol. Dial. Transpl. 25, 3924–3931 (2010)
Y.C. Ma, L. Zuo, J.H. Chen et al., Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J. Am. Soc. Nephrol. 17, 2937–2944 (2006)
A.S. Levey, K.U. Eckardt, Y. Tsukamoto et al., Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 67, 2089–2100 (2005)
X.L. Kong, Y.C. Ma, J.H. Chen et al., Evaluation of the Chronic Kidney Disease Epidemiology Collaboration equation for estimating glomerular filtration rate in the Chinese population. Nephrol. Dial. Transpl. 28, 641–651 (2013)
A.S. Levey, L.A. Stevens, C.H. Schmid et al., A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150, 604–607 (2009)
L.D. Bash, J. Coresh, A. Kottgen et al., Defining incident chronic kidney disease in the research setting. Am. J. Epidemiol. 170, 414–424 (2009)
T. Skov, J. Deddens, M.R. Petersen, L. Endahl, Prevalence proportion ratios: estimation and hypothesis testing. Int. J. Epidemiol. 27, 91–95 (1998)
M.P. Reilly, M. Lehrke, M.L. Wolfe, A. Rohatgi, M.A. Lazar, D.J. Rader, Resistin is an inflammatory marker of atherosclerosis in humans. Circulation 111, 932–939 (2005)
M. Bokarewa, I. Nagaev, L. Dahlberg, U. Smith, A. Tarkowski, Resistin, an adipokine with potent proinflammatory properties. J. Immunol. 174, 5789–5795 (2005)
C.M. Steppan, S.T. Bailey, S. Bhat et al., The hormone resistin links obesity to diabetes. Nature 409, 307–312 (2001)
C. Chen, J. Jiang, J.M. Lu et al., Resistin decreases expression of endothelial nitric oxide synthase through oxidative stress in human coronary artery endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 299, H193–H201 (2010)
Y. Cho, S.E. Lee, H.C. Lee et al., Adipokine resistin is a key player to modulate monocytes, endothelial cells, and smooth muscle cells, leading to progression of atherosclerosis in rabbit carotid artery. J. Am. Coll. Cardiol. 57, 99–109 (2010)
P. Calabro, D.W. Chang, J.T. Willerson, E.T. Yeh, Release of C-reactive protein in response to inflammatory cytokines by human adipocytes: linking obesity to vascular inflammation. J. Am. Coll. Cardiol. 46, 1112–1113 (2005)
E. Nehus, S. Furth, B. Warady, M. Mitsnefes, Correlates of resistin in children with chronic kidney disease: the chronic kidney disease in children cohort. J. Pediatr. 161, 276–280 (2012)
G. Hasegawa, K. Nakano, M. Sawada et al., Possible role of tumor-necrosis-factor and interleukin-1 in the development of diabetic nephropathy. Kidney Int. 40, 1007–1012 (1991)
M.A. Niewczas, T. Gohda, J. Skupien et al., Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J. Am. Soc. Nephrol. 23, 507–515 (2012)
A.C. Carlsson, T.E. Larsson, J. Helmersson-Karlqvist, A. Larsson, L. Lind, J. Arnlov, Soluble TNF receptors and kidney dysfunction in the elderly. J. Am. Soc. Nephrol. 25, 1313–1320 (2014)
J.R. Bradley, TNF-mediated inflammatory disease. J. Pathol. 214, 149–160 (2008)
T. Lawrence, The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 1, a001651 (2009)
V. Vielhauer, T.N. Mayadas, Functions of TNF and its receptors in renal disease: distinct roles in inflammatory tissue injury and immune regulation. Semin. Nephrol. 27, 286–308 (2007)
G. Guo, J. Morrissey, R. McCracken, T. Tolley, S. Klahr, Role of TNFR1 and TNFR2 receptors in tubulointerstitial fibrosis of obstructive nephropathy. Am. J. Physiol. 277, 766–772 (1999)
S. Vega, J. Benito-Leon, F. Bermejo-Pareja et al., Several factors influenced attrition in a population-based elderly cohort: neurological disorders in Central Spain Study. J. Clin. Epidemiol. 63, 215–222 (2010)
Acknowledgments
This study was supported by the Ministry of Science and Technology of China (2012CB524900 and 2013BAI04B03); the National Natural Science Foundation of China (81202272, 81321062, 30930081, and 81021002); ZY3-CCCX-3-2001, ZYSNXD-CC-HPGC-JD-003; the International Postdoctoral Exchange Fellowship Program 2015; the Knowledge Innovation Program of Shanghai Institutes for Biological Sciences; Chinese Academy of Sciences [2013KIP107]; and the SA-SIBS Scholarship Program.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The manuscript has neither been published previously, nor under consideration for publication elsewhere. There is no conflict of interest in this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, G., Deng, Y., Sun, L. et al. Elevated plasma tumor necrosis factor-α receptor 2 and resistin are associated with increased incidence of kidney function decline in Chinese adults. Endocrine 52, 541–549 (2016). https://doi.org/10.1007/s12020-015-0807-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-015-0807-3