Abstract
As a multi-functional cellular organelle, mitochondrial metabolic reprogramming is well recognized as a hallmark of cancer. The center of mitochondrial metabolism is oxidative phosphorylation (OXPHOS), in which cells use enzymes to oxidize nutrients, thereby converting the chemical energy to the biological energy currency ATPs. OXPHOS also creates the mitochondrial membrane potential and serve as the driving force of other mitochondrial metabolic pathways and experiences significant reshape in the different stages of tumor progression. In this minireview, we reviewed the major mitochondrial pathways that are connected to OXPHOS and are affected in cancer cells. In addition, we summarized the function of novel bio-active molecules targeting mitochondrial metabolic processes such as OXPHOS, mitochondrial membrane potential and mitochondrial dynamics. These molecules exhibit intriguing preclinical and clinical results and have been proven to be promising antitumor candidates in recent studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Mitochondrial OXPHOS and Its Reprogramming in Glioblastoma Cells
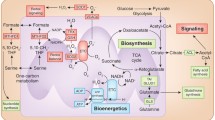
Mitochondria are dynamic organelles critical to a diverse array of essential metabolic functions, yet their most well characterized role is being the cellular “powerhouse”. In the presence of O2, the ETC drives the synthesis of ATP from ADP through ATP synthase (Complex V) by the mechanism of chemiosmotic coupling (Cooper, 2000; Voet et al., 2006) (Fig. 1). OXPHOS is integral in a vast number of biochemical processes including the tricarboxylic acid cycle (TCA, or Kreb cycle), generation of reactive oxygen species (ROS), maintenance of membrane potential, and regulation of mitochondrial morphology (Legros et al., 2002). Mitochondria in cells are connected by a dynamic network that can undergo structural alterations in a process termed mitochondrial dynamics. It is balanced by two highly regulated processes: mitochondrial fission and fusion (Mishra & Chan, 2016). Three dynamin-related proteins regulate mitochondrial fusion: mitofusin-1 (MFN1), mitofusin-2 (MFN2), and optic atrophy 1 (OPA1); whereas, two proteins regulate mitochondrial fission: dynamin-1-like protein (DRP1) and mitochondrial fission 1 protein (FIS1) (Mishra & Chan, 2016). Mitochondrial dynamics tightly correlate with OXPHOS activity and mitochondrial membrane potential: fusion requires high potential and active OXPHOS, while fission is induced by uneven mitochondrial membrane potential and damaged OXPHOS (Liesa & Shirihai, 2013).
The original description of the Warburg effect (Warburg et al., 1927) first illustrated the seeming paradoxical observation that unlike normal tissues, cancer cells tend to undergo glycolysis to produce lactate even in the presence of O2 in a process termed aerobic glycolysis. Compared to oxidative phosphorylation in which pyruvate gets completely oxidized into CO2 in the presence of O2, aerobic glycolysis produces significantly less ATP per glucose molecule. It is paradoxical because in the setting of increased cellular proliferation, one would expect cancer cells to adopt a more efficient metabolic pathway. In glioblastoma (GBM), the most aggressive and prevalent form of primary brain tumors (Kanderi & Gupta, 2020), it has been shown that as much as 90% of glucose is converted into lactate (DeBerardinis et al., 2007). It is now recognized that this reprogramming of metabolic pathway provide the advantage of satisfying the substantial requirement of biomass production, such as nucleotide, amino acids and lipids synthesis, in order to sustain the rapid proliferation of malignant cells (Vander Heiden et al., 2009). Glioma stem cells (GSCs), a subpopulation of GBM cells is thought to be responsible for tumor initiation, recurrence, metastasis and therapeutic resistance; thus, novel therapies targeting GSCs are appealing for treatment of this currently incurable tumor. Hypoxic condition in GBM leads to mitochondrial reprogramming in tumor cells to adapt to a hostile microenvironment (Muz et al., 2015). The major changes in mitochondrial metabolism of glioma cells consist of alterations in OXPHOS and secondary metabolic changes related to TCA cycle, mitochondrial membrane potential and mitochondrial dynamics (Guntuku et al., 2016; Strickland & Stoll, 2017). In this mini review, we will focus on the role of oxidative phosphorylation (OXPHOS) and related mitochondrial metabolisms in pathogenesis and potential therapy for GBM.
Targeting Mitochondrial OXPHOS in Treatment for GBM
In GBM cells, not only is the number of mitochondria abnormal, but also the mitochondrial morphology is significantly altered. In addition, biochemical evidence indicates that energy production via OXPHOS is defective in these cells (Chinopoulos & Seyfried, 2018; Seyfried & Mukherjee, 2005). However, other studies raise the possibility that some GBM cells rely on elevated OXPHOS to promote survival (Janiszewska et al., 2012). Recently, it has been shown that GSCs have higher mitochondrial activities and express higher level of OXPHOS proteins compared to isogenic differentiated glioma cells (Kuramoto et al., 2020). The rationale to inhibit OXPHOS in GBM is therefore twofold: first, in cells like GSCs with higher-than-normal OXPHOS requirement, inhibition would selectively target these cells and deprive them of their metabolic needs; second, in non-GSC glioma cells, the existing OXPHOS defects render them more vulnerable to further OXPHOS inhibition.
By pharmacologically inhibiting ETC (Complex I–IV) or F1F0 ATP synthase using small molecules inhibitors, OXPHOS is reduced leading to decreased ATP production. In addition, production of superoxide anion (O2−) is enhanced and mitochondrial permeability is altered leading to programmed cell death. Various natural and synthetic compounds can inhibit OXPHOS by targeting different complexes in the respiratory chain. Many are approved by Food and Drug Administration (FDA) for nontumor indications and are now being evaluated for application in oncology.
Complex I (NADH-ubiquinone oxidoreductase) inhibitors have different biochemical structures but similar activities: rotenoids, vanilloids and piericidins contain hydroquinone/quinone motifs, while metformin and biguanides do not; they all noncompetitively bind with different sites of the complex and exhibit significant ability to inhibit glioma growth (Degli Esposti, 1998). Complex II does not pump protons across the inner mitochondrial membrane and contribute to the creation of mitochondrial membrane potential (Ralph et al., 2011). Complex II (succinate dehydrogenase) inhibitors (e.g., α-tocopheryl succinate, gracillin and atpenins) have been reported to increase mitochondrial ROS production and sensitize cells to apoptosis. Intriguingly, Complex III (cytochrome c reductase) and Complex IV (cytochrome c oxidase) appears to be significantly more active than other components of ETC (Kuramoto et al., 2020) in GBM. Complex III inhibitors, Licochalcone A and antimycin A, have been shown to induce apoptosis and reduce viability of GSCs (Kuramoto et al., 2017; Mi-Ichi et al., 2005). Verteporfin, an FDA-approved photoactivating drug for macular degeneration, has been characterized as an OXPHOS inhibitor specifically in GSCs but not in differentiated cells through high throughput screening (Kuramoto et al., 2020). In addition, Atovaquone, an FDA-approved drug used for pneumocystis pneumonia and malaria infections, is a competitive inhibitor of Complex III and is reported to be effective against cancer stem cells (Fiorillo et al., 2016b). There are two FDA approved Complex IV inhibitors for cancer treatment: mitotane for adrenocortical cancer and arsenic trioxide for acute promyelocytic leukemia, the latter showed promising preclinical effect in GBM (Fang & Zhang, 2020).
Complex V (F1F0-ATP synthase) plays an important role in cancer metabolism including GBM, although its exact function remains controversial (Esparza-Moltó & Cuezva, 2018). Complex V is able to operate in reverse by hydrolyzing ATP and pump proton in the opposite direction to maintain mitochondrial membrane potential in the absence of ETC functionality. This has been demonstrated to be is necessary for GBM survival (Chinopoulos & Seyfried, 2018). Multiple Complex V inhibitors has shown promising anticancer potentials in vitro and in vivo. Bedaquiline (a.k.a., Sirturo), an FDA-approved antibiotic drug for multidrug-resistant pulmonary tuberculosis treatment, effectively targets Complex V, leading to mitochondrial dysfunction and decreased proliferation selectively in stem-like cancer cells (Fiorillo et al., 2016a). In addition to Bedaquiline, some natural and synthetic products such as resveratrol (related polyphenols and flavones), aurovertin B, quercetin, bicarbonate anion, tenoxin, lecucinostatin, fluro-aluminate, dicyclohexyl-carbodimide and azide also have the ability to inhibit ATP synthase (Neupane et al., 2019). The potential of these molecules in GBM treatment need to be further examined. Most recently, Gboxin, a compound identified from high throughput chemical screening using primary glioma stem cells, has been shown to effectively inhibit Complex V activity. Even though Gboxin binds nonspecifically to all cellular mitochondria, it specifically inhibit proliferation of GBM cells but not normal mouse embryonic fibroblasts and astrocytes (Shi et al., 2019).
Two other related mitochondrial processes are highly regulated by OXPHOS: mitochondrial membrane potential and mitochondrial dynamics. Despite the impaired OXPHOS in most GBM cells, their mitochondrial membrane potential is surprisingly hyperpolarized (∆Ψm ≈ − 220 mV vs. normal cells ≈ − 140 mV) cells (Shi et al., 2019). The elevated mitochondrial membrane potential allows the cancer cell to escape apoptosis (Guièze et al., 2019) and cell cycle arrest (Ramamoorthy et al., 2018). The mechanisms of how cancer cells maintain higher mitochondrial membrane potential despite impaired OXPHOS is still a mystery. Nevertheless, inhibiting OXPHOS has the additional effect of depolarizing the mitochondrial membrane, which can secondarily promote programmed cell death. Mitochondrial dynamics is emerging as another valuable target for GBM therapy, however our current understanding of the process is incomplete and controversial. While mitochondrial fusion has been shown to require ATP hydrolysis and high mitochondrial membrane potential (Bonnay et al., 2020), others have shown that mitochondrial fission is frequently observed in many cancers including GBM (Wan et al., 2014). Consistent with the latter finding, Mdivi-1, a selective inhibitor of DRP1, a dynamin family of large GTPases that control the final part of mitochondrial fission, blocks mitochondrial fission and can significantly slow proliferation of human GBM cells (Lee et al., 2013; Xie et al., 2015). The mechanism of Drp1 regulation appears to be mediated through a series of protein phosphorylation events. Drp1 is selectively activated in brain tumor initiating stem cells making it an attractive target for GSC specific therapy (Xie et al., 2015).
Conclusions and Prospects
Inhibitors of mitochondrial OXPHOS alone or in combination with other cancer drugs, have shown significant potential against malignant tumors including GBMs in both in vitro and in vivo studies. One of the main challenges of using OXPHOS inhibitors in cancer treatment is whether the degree of mitochondrial dysregulation in tumors is sufficient to confer selectively against tumor cells while sparing normal cells. The existing approved OXPHOS inhibitors have acceptable side effect profile suggesting that it is a viable approach. Emerging preclinical data also suggest that OXPHOS inhibition may have the benefit of specifically targeting GSC, a particularly elusive subpopulation that resist current therapy.
References
Bonnay, F., Veloso, A., Steinmann, V., Köcher, T., Deniz Abdusselamoglu, M., Bajaj, S., Rivelles, E., Landskron, L., Esterbauer, H., Zinzen, R. P., & Knoblich, J. A. (2020). Oxidative metabolism drives immortalization of neural stem cells during tumorigenesis. Cell, 182(6), 1490. https://doi.org/10.1016/j.cell.2020.07.039
Chinopoulos, C., & Seyfried, T. N. (2018). Mitochondrial substrate-level phosphorylation as energy source for glioblastoma: Review and hypothesis. The American Society of Neurochemistry. https://doi.org/10.1177/1759091418818261
Cooper, M. G. (2000). The mechanism of oxidative phosphorylation. Sinauer Associates.
DeBerardinis, R. J., Mancuso, A., Daikhin, E., Nissim, I., Yudkoff, M., Wehrli, S., & Thompson, C. B. (2007). Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proceedings of the National Academy of Sciences of the United States of America, 104(49), 19345. https://doi.org/10.1073/pnas.0709747104
Degli Esposti, M. (1998). Inhibitors of NADH-ubiquinone reductase: An overview. Biochimica Et Biophysica Acta (BBA) - Bioenergetics, 1364(2), 222. https://doi.org/10.1016/s0005-2728(98)00029-2
Esparza-Moltó, P. B., & Cuezva, J. M. (2018). The role of mitochondrial H +-ATP synthase in cancer. Frontiers in Oncology. https://doi.org/10.3389/fonc.2018.00053
Fang, Y., & Zhang, Z. (2020). Arsenic trioxide as a novel anti-glioma drug: A review. Cellular & Molecular Biology Letters. https://doi.org/10.1186/s11658-020-00236-7
Fiorillo, M., Lamb, R., Tanowitz, H. B., Cappello, A. R., Martinez-Outschoorn, U. E., Sotgia, F., & Lisanti, M. P. (2016a). Bedaquiline, an FDA-approved antibiotic, inhibits mitochondrial function and potently blocks the proliferative expansion of stem-like cancer cells (CSCs). Aging, 8(8), 1593. https://doi.org/10.18632/aging.100983
Fiorillo, M., Lamb, R., Tanowitz, H. B., Mutti, L., Krstic-Demonacos, M., Rita Cappello, A., Martinez-Outschoorn, U. E., Sotgia, F., & Lisanti, M. P. (2016b). Repurposing atovaquone: Targeting mitochondrial complex III and OXPHOS to eradicate cancer stem cells. Oncotarget, 7(23), 34084. https://doi.org/10.18632/oncotarget.9122
Guièze, R., Liu, V. M., Rosebrock, D., Jourdain, A. A., Hernández-Sánchez, M., Martinez Zurita, A., Sun, J., Ten Hacken, E., Baranowski, K., Thompson, P. A., Heo, J.-M., Cartun, Z., Aygün, O., BryanIorgulescu, J., Zhang, W., Notarangelo, G., Livitz, D., Li, S., Davids, M. S., … Wu, C. J. (2019). Mitochondrial reprogramming underlies resistance to BCL-2 inhibition in lymphoid malignancies. Cancer Cell, 36(4), 369. https://doi.org/10.1016/j.ccell.2019.08.005
Guntuku, L., Naidu, V. G., & Yerra, V. G. (2016). Mitochondrial dysfunction in gliomas: Pharmacotherapeutic potential of natural compounds. Current Neuropharmacology, 14(6), 567. https://doi.org/10.2174/1570159x14666160121115641
Janiszewska, M., Suvà, M. L., Riggi, N., Houtkooper, R. H., Auwerx, J., Clément-Schatlo, V., Radovanovic, I., Rheinbay, E., Provero, P., & Stamenkovic, I. (2012). Imp2 controls oxidative phosphorylation and is crucial for preserving glioblastoma cancer stem cells. Genes & Development, 26(17), 1926. https://doi.org/10.1101/gad.188292.112
Kanderi, T., & Gupta, V. (2020). Glioblastoma multiforme. Statpearls Publishing.
Kuramoto, K., Suzuki, S., Sakaki, H., Takeda, H., Sanomachi, T., Seino, S., Narita, Y., Kayama, T., Kitanaka, C., & Okada, M. (2017). Licochalcone A specifically induces cell death in glioma stem cells via mitochondrial dysfunction. FEBS Open Bio, 7(6), 835. https://doi.org/10.1002/2211-5463.12226
Kuramoto, K., Yamamoto, M., Suzuki, S., Sanomachi, T., Togashi, K., Seino, S., Kitanaka, C., & Okada, M. (2020). Verteporfin inhibits oxidative phosphorylation and induces cell death specifically in glioma stem cells. The FEBS Journal, 287(10), 2023. https://doi.org/10.1111/febs.15187
Lee, K. S., Wu, Z., Song, Y., Mitra, S. S., Feroze, A. H., Cheshier, S. H., & Lu, B. (2013). Roles of PINK1, mTORC2, and mitochondria in preserving brain tumor-forming stem cells in a noncanonical notch signaling pathway. Genes & Development, 27(24), 2642. https://doi.org/10.1101/gad.225169.113
Legros, F., Lombès, A., Frachon, P., & Rojo, M. (2002). Mitochondrial fusion in human cells is efficient, requires the inner membrane potential, and is mediated by mitofusins. Molecular Biology of the Cell, 13(12), 4343. https://doi.org/10.1091/mbc.e02-06-0330
Liesa, M., & Shirihai, O. S. (2013). Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metabolism, 17(4), 491. https://doi.org/10.1016/j.cmet.2013.03.002
Mi-Ichi, F., Miyadera, H., Kobayashi, T., Takamiya, S., Waki, S., Iwata, S., Shibata, S., & Kita, K. (2005). Parasite mitochondria as a target of chemotherapy: Inhibitory effect of licochalcone A on the Plasmodium falciparum respiratory chain. Annals of the New York Academy of Sciences. https://doi.org/10.1196/annals.1352.037
Mishra, P., & Chan, D. C. (2016). Metabolic regulation of mitochondrial dynamics. The Journal of Cell Biology, 212(4), 379. https://doi.org/10.1083/jcb.201511036
Muz, B., de la Puente, P., Azab, F., & Azab, A. K. (2015). The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia (auckland). https://doi.org/10.2147/HP.S93413
Neupane, P., Bhuju, S., Thapa, N., & Bhattarai, H. K. (2019). ATP synthase: Structure function and inhibition. Biomolecular Concepts, 10(1), 1. https://doi.org/10.1515/bmc-2019-0001
Ralph, S. J., Moreno-Sánchez, R., Neuzil, J., & Rodríguez-Enríquez, S. (2011). Inhibitors of succinate: Quinone reductase/complex II regulate production of mitochondrial reactive oxygen species and protect normal cells from ischemic damage but induce specific cancer cell death. Pharmaceutical Research, 28(11), 2695. https://doi.org/10.1007/s11095-011-0566-7
Ramamoorthy, M. D., Kumar, A., Ayyavu, M., & Dhiraviam, K. N. (2018). Reserpine induces apoptosis and cell cycle arrest in hormone independent prostate cancer cells through mitochondrial membrane potential failure. Anti-Cancer Agents in Medicinal Chemistry, 18(9), 1313. https://doi.org/10.2174/1871520618666180209152215
Seyfried, T. N., & Mukherjee, P. (2005). Targeting energy metabolism in brain cancer: Review and hypothesis. Nutrition & Metabolism. https://doi.org/10.1186/1743-7075-2-30
Shi, Y., Lim, S. K., Liang, Q., Iyer, S. V., Wang, H. Y., Wang, Z., Xie, X., Sun, D., Chen, Y. J., Tabar, V., Gutin, P., Williams, N., De Brabander, J. K., & Parada, L. F. (2019). Gboxin is an oxidative phosphorylation inhibitor that targets glioblastoma. Nature, 567(7748), 341. https://doi.org/10.1038/s41586-019-0993-x
Strickland, M., & Stoll, E. A. (2017). Metabolic reprogramming in glioma. Frontiers in Cell and Developmental Biology. https://doi.org/10.3389/fcell.2017.00043
Vander Heiden, M. G., Cantley, L. C., & Thompson, C. B. (2009). Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science (new York), 324(5930), 1029. https://doi.org/10.1126/science.1160809
Voet, D., Voet, J. G., & Pratt, C. W. (2006). Fundamentals of biochemistry (2nd ed.). Wiley.
Wan, Y. Y., Zhang, J. F., Yang, Z. J., Jiang, L. P., Wei, Y. F., Lai, Q. N., Wang, J. B., Xin, H. B., & Han, X. J. (2014). Involvement of Drp1 in hypoxia-induced migration of human glioblastoma U251 cells. Oncology Reports, 32(2), 619. https://doi.org/10.3892/or.2014.3235
Warburg, O., Wind, F., & Negelein, E. (1927). The metabolism of tumors in the body. The Journal of General Physiology, 8(6), 519. https://doi.org/10.1085/jgp.8.6.519
Xie, Q., Wu, Q., Horbinski, C. M., Flavahan, W. A., Yang, K., Zhou, W., Dombrowski, S. M., Huang, Z., Fang, X., Shi, Y., Ferguson, A. N., Kashatus, D. F., Bao, S., & Rich, J. N. (2015). Mitochondrial control by DRP1 in brain tumor initiating cells. Nature Neuroscience, 18(4), 501. https://doi.org/10.1038/nn.3960
Author information
Authors and Affiliations
Contributions
ZW and RL: Conception and design, Literature search, ZW, WH, RL: Manuscript preparation and Final approval.
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, Z., Ho, W.S. & Lu, R. Targeting Mitochondrial Oxidative Phosphorylation in Glioblastoma Therapy. Neuromol Med 24, 18–22 (2022). https://doi.org/10.1007/s12017-021-08678-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-021-08678-8