Abstract
The 20% ethanol extract of Polygala tenuifolia, Angelica tenuissima, and Dimocarpus longan (WIN-1001X) was derived from a modified version of Korean traditional herbal formula ‘Chungsimyeolda-tang’ which has been used for the treatment of cerebrovascular disorders. The Parkinson’s disease presents with impaired motor functions and loss of dopaminergic neurons. However, the treatment for Parkinson’s disease is not established until now. This study aims to elucidate the therapeutic advantages of WIN-1001X on animal models of Parkinson’s disease. WIN-1001X administration successfully relieved the Parkinsonism symptoms in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced Parkinson’s disease mice tested by rota-rod and pole tests. The loss of tyrosine hydroxylase activities in substantia nigra and striatum was also attenuated by administration of WIN-1001X. In mice with sub-chronical MPTP injections, autophagy-related proteins, such as LC3, beclin-1, mTOR, and p62, were measured using the immunoblot assay. The results were favorable to induction of autophagy after the WIN-1001X administration. WIN-1001X treatment on 6-hydroxydopamine-injected rats also exhibited protective effects against striatal neuronal damage and loss of dopaminergic cells. Such protection is expected to be due to the positive regulation of autophagy by administration of WIN-1001X with confirmation both in vivo and in vitro. In addition, an active compound, onjisaponin B was isolated and identified from WIN-1001X. Onjisaponin B also showed significant autophagosome-inducing effect in human neuroblastoma cell line. Our study suggests that relief of Parkinsonism symptoms and rescue of tyrosine hydroxylase activity in dopaminergic neurons are affected by autophagy enhancing effect of WIN-1001X which the onjisaponin B is one of the major components of activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) primarily presents with motor symptoms such as tremor, bradykinesia, rigidity, and postural instability. It is one of the most common neurodegenerative diseases. Substantial loss of dopaminergic neurons in substantia nigra pars compacta (SNpc) of the brain is considered the primary cause of PD. The depletion of dopamine production is mainly resulted from the degeneration of dopaminergic neurons and manifests clinically as motor dysfunctions.
In the field of PD research, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is a generally used chemical agent promoting PD in various animals including rodents. Being a lipophilic compound, injected MPTP penetrates brain through the blood–brain-barrier (BBB). Then, it is metabolized into 1-methyl-4-phenylpyridinium (MPP+), the toxic cation, by monoamine oxidase B (MAO-B) followed by accumulation in dopaminergic neurons through dopamine transporter causing neurotoxicity in the substantia nigra pars compacta (SNpc) and striatum (Kopin 1987). The behavioral and biological deficits, which occur following substantial lesion due to MPTP injection in vivo, closely resemble the symptoms of PD, and such symptoms are observed after loss of striatal dopamine more than 80% (Kopin and Markey 1988).
The important character of MPTP-injected animal PD model is that it yields large variations in the extent of striatal terminal degeneration, SNpc cell body loss, and dopamine depletion as well as behavioral deficits based on the dose and injection schedule of MPTP (Meredith and Rademacher 2011). Acute protocol implementing repeated MPTP injection in a single day induces rapid and greater loss of dopamine and dopaminergic neurons compared to the sub-chronic model where animals get single daily MPTP injection for several days period. The behavioral deficits of acute protocol are also expected to be greater in correlation with the dopaminergic loss. On the other hand, the pattern of neuronal death is known to be different between the two MPTP-injection models where sub-chronic MPTP injection tends to induce more of delayed apoptotic cell death and continuous loss of striatal dopamine compared to the acute animal model (Tatton and Kish 1997).
A selective catecholaminergic neurotoxin, 6-hydroxydopamine (6-OHDA), is another popular neurotoxic chemical agent traditionally used to induce the similar pathogenesis and progression of PD on catecholaminergic neurons (Ungerstedt 1968; Agid et al. 1973). Different degrees of dopaminergic neuronal degeneration were found in rats in accordance with 6-OHDA injected at different sites of the nigrostriatal tract (Kirik et al. 1998). Injection of 6-OHDA into the striatum induced gradual SNpc neuronal loss (Sauer and Oertel 1994), which makes it more useful to be utilized in the study of functional recovery where a partial dopamine depletion model is needed.
As degeneration of dopaminergic neurons become one of the major pathologies of PD, interest grew on the protective mechanisms that could save or delay such degeneration. Autophagy is one of such pathways that has been enormously studied in various disease models to exhibit protective roles by delaying the detrimental consequences of pathologies. As well covered by a number of reviews, increasing evidences are being continuously reported providing close correlation between autophagy mechanism and neurodegeneration including PD (Cheung and Ip 2009; Lynch-Day et al. 2012).
WIN-1001X is a code name for the 20% ethanol extract of Polygala tenuifolia, Angelica tenuissima, and Dimocarpus longan 1:1:1 combination which is a modified Korean traditional herbal formula ‘Chungsimyeolda-tang’ that has well been described in a historic literature ‘Dongui Sasang Shinpyun’ (Shim et al. 2008). Polygala tenuifolia, which has been consumed as a regional food material, is well reported of its beneficial effects on memory deficits, depression state, axonal degeneration, and neuroinflammation (Guo et al. 2016; H. Li et al. 2017; Kuboyama et al. 2017; Fan et al. 2017). The other constituent Angelica tenuissima shows its anti-inflammatory and immunomodulating effects as well as protection against neurodegenerative disease model (Chung et al. 2012; Weeratunga et al. 2016; Choi et al. 2018). Dimocarpus longan, a popular tropical fruit, is also reported to exhibit neuroprotective and anti-microbial/inflammatory functions (Lin et al. 2012; Lee et al. 2014; Tseng et al. 2014; Kunworarath et al. 2016).
In our previous reports based on modified ‘Chungsimyeolda-tang’, water extract from Polygala tenuifolia, Angelica tenuissima, and Dimocarpus longan exhibited a strong anti-apoptotic property in saving dopaminergic neurons from chemically induced PD mouse brains (Li et al. 2015). Furthermore, our group also reported that this water extract of traditional plants combination showing significant modulation of autophagy in cells with MPP+-induced neurotoxicity (Bae et al. 2015). Along with our reports, other groups of researchers found that onjisaponin B, one of the active components of WIN-1001X, and its metabolites, tenuifolins, are responsible for the autophagy induction in cell lines (Wu et al. 2013, 2015). Based on their findings, we have enriched our natural products extract to be optimized using 20% of ethanol solvent to increase such non-polar components while maintaining the polar components to previously reported level. In current study, we used the animal PD models induced by two different neurotoxins (MPTP and 6-OHDA) to estimate the effects of WIN-1001X focused on enhancement of behavioral deficits and autophagy for the first time in vivo.
Materials and Methods
Chemicals and Reagents
Dulbecco’s modified Eagle’s medium (DMEM) was obtained from Hyclone (Logan, UT, USA). Fetal bovine serum (FBS), 0.25% trypsin–EDTA and a penicillin/streptomycin mixture were obtained from Gibco BRL (Grand Island, NY, USA). MPTP, 6-OHDA, and ropinirole hydrochloride were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Rabbit anti-tyrosine hydroxylase (TH), rabbit anti-α-synuclein, rabbit anti-neighbor of BRCA1 gene 1 protein (NBR1), rabbit anti-microtubule-associated proteins 1A/1B light chain 3B (LC3), rabbit anti-beclin-1, rabbit anti-mammalian target of rapamycin (mTOR), rabbit anti-p62, rabbit anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibodies, and anti-rabbit horseradish peroxidase-linked immunoglobulin (Ig) G secondary antibodies were purchased from Cell Signaling Technology (Boston, MA, USA).
Preparation of WIN-1001X and Ropinirole
WIN-1001X was constituted with three herbs; Polygala tenuifolia Willd., Angelica tenuissima Nakai, and Dimocarpus longan Lour., which were supported by the Oriental Hospital at Daejeon University (Daejeon, Republic of Korea). The identification and authentication of these herbs were examined by the botany and drug department of Oriental Hospital. The voucher specimens (voucher No.: Polygala tenuifolia Willd., KN00847A; Angelica tenuissima Nakai, KN00735A; Dimocarpus longan Lour, KN00804A) are deposited at the herbarium of Korea Institute of Science & Technology (Gangneung, Republic of Korea). Hyunjin pharmaceutical Co. (Seoul, Republic of Korea) supported another batch of the herbs. Prof. Dae Sik Jang authenticated the origins of the herbs and their voucher specimens (voucher numbers: Polygala tenuifolia Willd., POTE1-2016; Angelica tenuissima Nakai, ANTE1-2016; Dimocarpus longan Lour, DILO1-2016) have been deposited in the Laboratory of Natural Product Medicine, College of Pharmacy, Kyung Hee University (Seoul, Republic of Korea). The even mixture of three herbs (15 g of each air-dried herb) was extracted with 1 L of ethanol (20% v/v), boiled twice and evaporated to 100 mL, then labeled as WIN-1001X, and freeze dried to obtain lyophilized WIN-1001X. The WIN-1001X stock solution was made from the lyophilized extract with saline and right before the treatment, the stock solution was diluted to the desired concentrations.
For the positive control of the study, ropinirole was selected to be administered orally dissolved in saline as well. Ropinirole is a non-ergoline dopamine receptor agonist approved for Parkinsonism by FDA in 1997 to relieve symptoms such as muscle spasms, poor muscle control, tremors, and stiffness. Other than symptomatic relief, there are several reports on the neuroprotective effects of ropinirole via anti-oxidation and anti-apoptotic pathway modulation as well (Iida et al. 1999; Parvez et al. 2010).
LC–MS Analysis of WIN-1001X
The LC–MS analysis of WIN-1001X was executed utilizing similar method in our previous publication by Bae et al. (2015). Using YMC-Triart C18 ExRS plus HPLC column (250 × 4.6 mm, S-5 μm, 8 nm) for LC separation with mobile phase (from 10 to 55% aqueous acetonitrile containing 0.05% formic acid for 60 min at 1 mL/min), the analysis was performed using Agilent 1200 series HPLC system combined with an Agilent 6120 quadrupole MSD. The ionization mode was API-ES and used a positive polarity. For mass ionization, the gas temperature, drying gas, and capillary voltage were set to 350 °C, 12 L/min, and 4000 V, respectively. The mass range was 200–1500. DAD detection was performed at 254 and 320 nm wavelengths and the column temperature was set at 30 °C.
Animals
Male C57BL/6J mice (7- and 10-week-old) and adult male Sprague-Dawley (SD) rats (7-week-old) were, respectively, purchased from Japan SLC, Inc. (Shizuoka, Japan) and Orient Bio, Inc. (Sungnam, Republic of Korea). All the animals were housed in the temperature and humidity-controlled facility with access to water and food ad libitum under a 12 h light/dark cycle. All the animal protocols were approved and in accordance with the Korea Institute of Science and Technology Animal Care Committee guidelines.
MPTP-Induced Acute Parkinson’s Disease Mouse Model
At the age of 8 weeks old, mice were randomly divided into 5 groups (n = 13/group, except n = 10 for control group): Group 1 and 2, mice were administered with normal saline as control; Group 3, mice were administered with ropinirole 5 mg/kg/day as a positive control; Group 4 and 5, mice were administered with WIN-1001X 100 and 200 mg/kg/day. On the third day of daily oral drug administration, intraperitoneal MPTP (20 mg/kg) injection was conducted. After 1 h from the daily drug administration, group 2–5 mice received 4 times of repeated MPTP injections at 2 h of intervals, while group 1 received saline injections as control. Daily drug administration continued for 3 more days and the motor performance tests were performed on the 6th day of drug administration. After the motor performance tests, the whole brains were perfused with and kept in 4% paraformaldehyde solution for immunohistochemistry (IHC) analysis.
MPTP-Induced Sub-chronic Parkinson’s Disease Mouse Model
C57BL/6J mice (12-week-old, n = 5–6/group) were randomly divided into 5 groups. The groups and drug dosages were same as the MPTP-induced acute PD mouse model. From the third day of daily oral drug administration, group 2–5 mice received daily single intraperitoneal injection of MPTP (30 mg/kg/day), 1 h after the drug treatment for 5 consecutive days, while group 1 mice received equivalent volume of saline injection as control. Drug and MPTP administrations were discontinued from 8th day and on the 14th day from the first administration of WIN-1001X and ropinirole, all the mice were sacrificed by cervical dislocation. SNpc and striatum parts of each mouse were dissected from the whole brain and stored at − 80 °C until they were further analyzed.
The 6-OHDA-Induced Experimental Parkinsonism in Rat
At the age of 8 weeks old, the SD rats were processed to induce Parkinsonism using 6-OHDA as previously described (Ogawa et al. 1985). Briefly, the surgery was carried out under anesthesia by intraperitoneal pentobarbital sodium (50 mg/kg) injection, and the stereotaxic coordinates of the striatum (AP: 1.0 mm, ML: + 3.0 mm and DV: − 6.0 mm) were determined according to the atlas of rat brain. After anesthesia, the rats were set on the stereotaxic apparatus, and then unilateral injection of 6-OHDA (dose 20 μg dissolved in 4 μL of 0.01% ascorbic acid saline solution) was performed into the right striatum using the Hamilton syringe (rate of 0.5 μL/min). For the sham-operated rats, 4 μL of vehicle solution was infused. After 2 weeks from the 6-OHDA injection, apomorphine-induced rotation behavior was observed to select the rats with proper lesion in the striatum (rats with over 90 rotations/h only). Then based on the observation, the rats were evenly distributed into four 6-OHDA-injected experimental groups treated with vehicle (n = 24); l-dopa 10 mg/kg (n = 24); WIN-1001X 50 and 100 mg/kg (n = 24 each), and additional sham-operated control group (n = 20). After the grouping, drugs were administered every day orally for 21 consecutive days. After the final apomorphine-induced rotation behavior test, ipsilateral striatum was dissected from the brain of anesthetized rat and stored at − 80 °C until they were further analyzed.
Motor Performance Test: Rota-Rod Test
The rota-rod test is being used to test motor coordination in rodent models of PD and could offer a useful quantitative tool to assess the efficacy of PD therapeutics. Seven days before the acute MPTP treated, all mice were placed on a rotating rod of the automated 4-lane rota-rod apparatus (3.6 cm diameter for rotating spindle; Jeung Do Bio & Plant Co., Seoul, Republic of Korea) and subjected to the adaptive sessions which run 10, 15, and 20 rpm for 2 min each. After 2 days of adaptive sessions, rota-rod training session was continued for 5 consecutive days until the MPTP injection. Training session was conducted as the same condition as the actual test session which runs at accelerated speed of 2–40 rpm for maximum 5 min in triplicated sessions per day. On the actual test day, the average time that each mouse spent on the rotating rod was recorded.
Motor Performance Test: Pole Test
The Pole test is a classic and useful method to assess the agility of the animals and possibly used for measurement of motor inhibition such as bradykinesia (Ogawa et al. 1985). Each mouse was placed head-upward on the upper region of a vertical pole with rough surface (diameter 8 mm, height 50 cm). Time taken for which the mouse turned its body so the forefeet to be lower than the hind limbs and hips (T-turn), and the time taken for the mouse to climb down the pole until the forefeet touched the marked area of the pole end (T-LA) were measured.
Immunohistochemistry Analysis
Briefly, the mouse brains from the MPTP-induced acute PD model were transferred to 4% paraformaldehyde, embedded in paraffin and sectioned (10 μm) near cerebrum and midbrain regions to expose the SNpc and striatum area. Sections were processed for IHC staining with antibody against TH (1:1000) (Li et al. 2015). Quantification of the effect of WIN-1001X in brain tissues was performed by counting the TH-positive cell numbers in the SNpc at × 100 magnification under a microscope and by measuring the optical density of TH-positive fibers in the Striatum at × 40 magnification using Image software (Bethesda, MD, USA). Data were expressed as a percentage of the value in the vehicle-treated control group.
Brain Tissue Western Blot Analysis
The harvested SNpc and striatum tissues were homogenized using a PRO-PREP™ protein extraction solution (iNtRON Biotechnology, Seoul, Republic of Korea). And then the lysate was centrifuged at 13,000 rpm for 30 min at 4 °C, after that the supernatant was collected in a new tube. The protein concentration was measured using the Bradford method. The protein samples of SNpc and striatum were separated by 8–15% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis, and then transferred to poly-vinylidene difluoride (PVDF) transfer membrane (0.45 μM; Merck & Co., Kenilworth, NJ, USA). The membranes were incubated with 5% skim milk dissolved in Tris-buffered saline containing 0.1% Tween-20 (TBST) (0.5 mM Tris–HCl pH 7.5, 150 mM NaCl, and 0.1% Tween-20) for 1 h and then continued to incubate with the primary antibodies at a dilution of 1:1000 overnight at 4 °C. After being washed three times with TBST, these membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody in TBST with 5% skim milk at a ratio of 1:2000 dilution for another 1 h. These immunoreactive-bands were detected by using the enhanced chemiluminescence (ECL) detection kit and the ImageQuant LAS-4000 mini system (GE Healthcare Life Sciences, Chicago, IL, USA). The intensities of these bands were normalized to the GAPDH intensity using Multi Gauge software (Fuji Film, Tokyo, Japan).
Apomorphine-Induced Rotation Test
An apomorphine-induced rotation test was performed to elucidate the hypersensitivity of the lesioned striatum, which was assessed by injecting 0.5 mg/kg of apomorphine subcutaneously. The rotation test was conducted first, after 2 weeks from the 6-OHDA injection and then after 21 days of drug treatment to the rats to evaluate their behavioral changes. Briefly, the rats were injected with apomorphine and placed into a white cylindrical area (Width 45 cm, Height 22 cm), and then recorded with its rotating behavior by a video camera for 1 h duration. Contralateral rotations induced by apomorphine were measured.
Isolation and Analysis of Active Compound
The dried plants material (400 g) was extracted twice with 4 L of 70% EtOH at 80 °C in water bath for 3 h and the solvent was evaporated in vacuo at 45 °C. The 70% EtOH extract (84 g) was chromatographed over Diaion HP-20 (ɸ6.8 × 30.5 cm) as stationary phase with a MeOH-H2O gradient (from 6:4 → 8:2 → 1:0 v/v; final stage, acetone 100%) as mobile phase to afford 14 fractions (Fr.1–Fr.14). Fraction 9 (1.8 g) was fractionated further by using a flash chromatography system using Redi Sep-C18 (130 g, MeOH-H2O, 1:1 to 4:1 v/v) cartridge to obtain 14 sub-fractions (Fr.9–1 to Fr.9–14). Fraction 10 (5.2 g) was chromatographed over silica gel CC as stationary phase with EtOAc/BuOH/MeOH/H2O mixture (3/4/1.5/1.5 v/v) as mobile phase to afford 7 sub-fractions (Fr.10–1 to Fr.10–7). Fraction 10–4 (1.2 g) was further divided by a flash chromatography system using Redi Sep-C18 (130 g, MeOH-H2O, 1:1 to 4:1 v/v) cartridge, yielding onjisaponin B (212.4 mg). Onjisaponin B was identified via the comparison of their NMR data with those of literatures and analysis of mass data (Sakuma and Shoji 1982).
SH-SY5Y Cell Culture, Treatments, and Cell Viability Assay
SH-SY5Y cell, the human neuroblastoma cell line, was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were grown in DMEM supplemented with 10% heat-inactivated FBS (v/v) and 100 units/mL penicillin and 100 mg/mL streptomycin in a humidified atmosphere of 5% CO2 and 95% air incubator at 37 °C. The medium was replaced every 3 days. Before the experiment, SH-SY5Y cells were seeded in 6-well plates (2 × 106 cells/well) and grew for 24 h and then treated with various concentrations of onjisaponin B (1.25, 2.5, 5, 10, and 20 μM) for 24 h incubation. For 6-OHDA-induced cellular toxicity study, 6-OHDA was prepared to treatment concentrations immediately before use from the 10 mM stock solution. SH-SY5Y cells were pre-treated with WIN-1001X (100, 200, and 400 μg/mL) for 17 h, followed by 50 μM 6-OHDA co-treatment for another 7 h. The cells were harvested at this point for protein extraction. For cell viability assay, 3-(4,5-dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide (MTT) solution was treated for 1 h and the absorbance was measured with a microplate reader (Power Wave XS, Bio-Tek, VT, USA) at a wavelength of 450 nm.
Cell Lysate for Western Blot Analysis
The whole cell lysates of SH-SY5Y cells were prepared by incubating the cells in radioimmunoprecipitation assay (RIPA) buffer (Cell Signaling, Danvers, MA, USA) supplemented with a protease inhibitor cocktail (Roche, Mannheim, Germany). And the whole protein was extracted according to the manufacturer’s instructions. Protein concentrations were determined using Bradford method and western blot analysis was conducted in accordance with the same procedure for the brain tissues from in vivo.
Statistical Analysis
Data were analyzed with Prism 5.0 software (GraphPad Software, Inc., San Diego, CA, USA) and were expressed as the mean ± S.E.M. Statistical analyses were performed using one-way analysis of variance (ANOVA) followed by the Student–Newman–Keuls test. Statistical significance was set at p < 0.05.
Results
WIN-1001X Treatment Exhibits Protective Effects on the Acute MPTP Injection-Induced PD Mouse Model
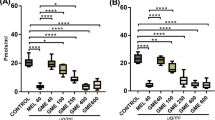
The total latency time for a mouse to stay on the rotating rod was measured to observe the motor coordination. Acute MPTP injection significantly decreased the latency measure to 124.7 ± 14.0 s compared with the control group three days after the MPTP injection (Fig. 1a, p < 0.05). However, the latency on the rod was significantly recovered in the WIN-1001X 200 mg/kg treatment group to 170.6 ± 14.3 s compared with the MPTP-injected vehicle group (Fig. 1a, p < 0.05), whereas ropinirole-treated group showed only a tendency of latency recovery.
WIN-1001X administration rescues behavioral impairments and TH-positive cells in SNpc and striatum from the acute MPTP injection-induced toxicity in mice. On 8-week-old C57BL/6 J mice, ropinirole (Ropi.; 5 mg/kg/day), WIN-1001X (100 or 200 mg/kg/day), or saline as vehicle was administered orally for 3 days (n = 10–13 per group). Then MPTP (20 mg/kg) was intraperitoneally injected 4 times at 2 h intervals on the third day after the drug administration. Daily drug administration continued for 3 more days and mice were tested for behavioral impairments and then sacrificed. a Rota-rod test was conducted with pre-trained mice and average latency of triplicated sessions on the accelerated rotating rod (from 2 to 40 rpm) of the apparatus was recorded (maximum 300 s). b During the pole test, time taken for the mouse to turn completely downward (T-turn) and time taken to reach the floor level (T-LA) were recorded. c Sacrificed mice brains were sectioned in paraffin (10 μm) to expose the SNpc and striatum area. IHC staining was processed using antibody against TH (1:1000) and slides were scanned at × 40 magnification for striatum, and × 100 magnification for SNpc. d, e TH-positive cells of the brain were counted at each SNpc and striatum regions and average cell density was compared with the ipsilateral side average of control group. Values are indicated as the mean ± S.E.M. *p < 0.05, **p < 0.01, and ***p < 0.001 show significance compared with the control group; #p < 0.05, ##p < 0.01, and ###p < 0.001 show significance compared with the MPTP-injected vehicle group
Pole test is one of the behavioral tests focusing on motor inhibitory symptoms that gathers consensus of detecting the signs of Parkinsonism more accurately. In the pole test, the T-turn (time to rotate the body) and T-LA (time to climb down the pole) values were all significantly extended to 74.9 ± 13.6 s and 89.1 ± 12.0 s in MPTP-injected vehicle group compared with the control group, respectively (Fig. 1b, p < 0.001). However, the T-turn value was significantly shortened in the WIN-1001X 100 mg/kg treatment group to 34.1 ± 11.8 s (Fig. 1b, p < 0.05) which was similar degree of latency with the ropinirole-treated group. With our interest, there were further shortening of latency in WIN-1001X 200 mg/kg treatment group down to 8.8 ± 2.8 s (T-turn) and 15.3 ± 2.3 s (T-LA) (Fig. 1b, p < 0.001).
Observing TH which plays a role in the rate limiting step in the production of dopamine precursor, l-dopa, is a well-accepted method to detect the dopaminergic or dopamine-related neurons in SNpc and striatum. The brain TH expressions in our experimental groups showed significant changes depending on the drug treatment. As shown in Fig. 1c, the bodies of the dopaminergic cells in the SNpc and striatum of the control mice were intensely stained with distinctive immune positivity. Compared with the control group, MPTP-injected vehicle group showed significantly decreased TH-positive cells and this decrease was rescued by WIN-1001X treatments. The density of TH-positive cells was counted for each group in both SNpc and striatum (Fig. 1d, e). The number of TH-positive cells in the SNpc and striatum after the MPTP injection significantly decreased by 66.2 ± 7.0% and 60.8 ± 6.8% of the control group, respectively (p < 0.01). Both 100 and 200 mg/kg of WIN-1001X treatments significantly sustained the TH-positive cells in SNpc (92.4 ± 5.3% and 90.6 ± 5.0%) and in striatum (81.0 ± 6.5% and 90.9 ± 6.0%) compared to the control group, respectively (p < 0.05). The ropinirole-treated group, however, exhibited no significant difference of TH-positive cell counts in both SNpc and striatum regions.
WIN-1001X Treatment Protects Dopaminergic Cells and Induce Autophagy in Sub-chronic MPTP Injection-Induced PD Mouse Model
We utilized sub-chronic MPTP injection-induced PD model for the observation of possible protective cellular mechanisms via WIN-1001X treatment in mice starting with the measurement of TH protein expression level in the SNpc and striatum region of the brain using western blot (Fig. 2a). The quantitative results in Fig. 2b, c showed significant reduction of TH protein expression in both SNpc (35.0 ± 4.8%) and striatum (36.9 ± 5.1%) of MPTP-injected vehicle group compared to the control group (p < 0.001). However, ropinirole treatment increased the TH protein expression levels in both SNpc (65.6 ± 9.2%; p < 0.01) and striatum (55.8 ± 9.3%; p < 0.05), significantly. Interestingly, WIN-1001X treatment even potentiated the increase of TH protein expression higher than ropinirole-treated group. WIN-1001X 100 and 200 mg/kg treatment significantly increased the TH protein expression up to 71.0 ± 8.6% (p < 0.01) and 82.9 ± 4.8% (p < 0.001) of the control group in the SNpc (Fig. 2b), and 70.4 ± 7.8% (p < 0.01) and 82.8 ± 2.8% (p < 0.001) of the control group in the striatum (Fig. 2c), respectively.
WIN-1001X administration attenuates the down-regulation of TH in SNpc and striatum induced by sub-chronic MPTP injection in mice. Twelve-week-old C57BL/6J mice were administered orally with ropinirole (5 mg/kg/day), WIN-1001X (100 or 200 mg/kg/day), or saline as vehicle for 3 days. Then MPTP (30 mg/kg/day) was intraperitoneally injected 1 h after daily drug administration for 5 consecutive days (n = 5–6 per group). Additional 7 days after the last drug administration, mouse brains were dissected for SNpc and striatum collection. a Western blotting was conducted to observe expression of TH protein in the brain regions of SNpc and striatum for each group. b, c The bar graphs display densitometry analyses of the TH protein expression ratio in the SNpc and striatum. Values are indicated as the mean ± S.E.M. ***p < 0.001 shows significance compared with the control group; #p < 0.05, ##p < 0.01, and ###p < 0.001 show significance compared with the MPTP-injected vehicle group
Next, we targeted the major contributors of autophagy mechanism to understand if WIN-1001X treatment can modulate the autophagy at the injured region of brain. Proteins from SNpc region of sub-chronic MPTP-injected mice brains were assayed by western blotting (Fig. 3a). The WIN-1001X treatment increased the conversion ratio of LC3-I to LC3-II up to 200.0 ± 20% (p < 0.05) at 200 mg/kg administration compared to the control group (Fig. 3b). Ropinirole treatment not only showed no change in LC3 conversion rate, but also beclin-1 protein expression level was not changed. However, WIN-1001X treatment both 100 and 200 mg/kg significantly increased the expression level of beclin-1 up to 144.3 ± 10.1% (p < 0.01) and 147.3 ± 15.1% (p < 0.05) compared to the control group, respectively (Fig. 3c). The level of mTOR protein expression was not much changed with MPTP injection when compared to the control group. However, ropinirole treatment significantly reduced its expression level down to 37.3 ± 5.3% (p < 0.01) of the control group (Fig. 3d). Even though the statistical significance was not observed, WIN-1001X-treated groups also showed reduced trend of mTOR protein expression in both 100 and 200 mg/kg treatment groups. For the p62 protein expression, MPTP injection exhibited decreased expression level down to 84.9 ± 2.3% (p < 0.05) of the control group (Fig. 3e). While ropinirole treatment maintained the similar expression level without statistical significance, WIN-1001X treatment at 100 and 200 mg/kg significantly further reduced the p62 protein expression level down to 53.5 ± 4.0% and 47.7 ± 3.7% of the control group, respectively (p < 0.001).
WIN-1001X administration promotes autophagy-regulating proteins in the SNpc of sub-chronical MPTP-injected mice. In the SNpc region of sub-chronic MPTP-injected (30 mg/kg/day) C57BL/6J mice (n = 5–6 per group), a autophagy-related protein expression levels were measured using western blotting. The bar graphs display densitometry analyses of the protein expression ratios of b LC3-II and LC3-I, c beclin-1, d mTOR, and e p62. Values are indicated as the mean ± S.E.M. *p < 0.05 shows significance compared with the control group; #p < 0.05, ##p < 0.01, and ###p < 0.001 show significance compared with the MPTP-injected vehicle group
WIN-1001X Treatment Recovers Behavioral Deficit and TH Activity as Well as Inducing Autophagy in 6-OHDA Injection-Induced PD Mouse Model
Challenge with amphetamine or dopamine receptor agonists, such as apomorphine, is the most frequently utilized test method to quantify rotational behavior in rats with unilateral depletion of striatal dopamine. We have challenged our experimental rats with apomorphine after 3 weeks of drug administration. 6-OHDA-injected vehicle group showed significantly increased average rotational behavior (312 ± 23 rotations; p < 0.001) compared to the sham-operated control group (0.7 ± 0.3 rotation). This rotating behavior was significantly suppressed down with the positive control agent, L-Dopa, treatment (219 ± 17 rotations; p < 0.01) as well as with WIN-1001X treatment of both 50 (210 ± 10 rotations; p < 0.001) and 100 (216 ± 11 rotations; p < 0.001) mg/kg reflecting symptomatic relief function of WIN-1001X against depletion of dopamine in the striatum (Online Resource 1).
We further analyzed the protein expression levels in the rat striatum to find out the effect of WIN-1001X on dopaminergic cells and autophagy pathway under the influence of 6-OHDA-induced neuronal toxicity (Fig. 4a). First, 6-OHDA injection significantly reduced the TH protein expression down to 15.9 ± 2.9% (p < 0.001) compared to the sham-operated control group (Fig. 4b). l-Dopa- and WIN-1001X-treated groups showed lesser reduction of TH protein expression but only WIN-1001X 50 mg/kg treatment group showed statistical significance against the 6-OHDA-injected vehicle group with value of 58.9 ± 18.6% (p < 0.05).
WIN-1001X administration attenuates the down-regulation of TH and promotes autophagy-regulating proteins in the striatum of 6-OHDA-injected rats. In the striatum region of 6-OHDA-injected SD rat brain samples (n = 4–6 per group), a western blotting was used to detect TH, LC3, beclin-1, and mTOR protein expressions. The bar graphs display densitometry analyses of the protein expression ratios of b TH, c LC3-II/LC3-I, d beclin-1, and e mTOR. Values are indicated as the mean ± SEM. ***p < 0.001 shows significance compared with the sham-operated control group; #p < 0.05, ##p < 0.01, and ###p < 0.001 show significance compared with the 6-OHDA-injected vehicle group
Neuronal survival or protection from the loss of function can both be achieved by autophagy activation. Positive regulations of autophagic proteins were also observed with the treatment of WIN-1001X against the 6-OHDA-induced toxicity in the striatum. LC3 conversion ratio and beclin-1 protein expression were not significantly modified with 6-OHDA injection and even with l-Dopa treatment. However, significant increases of LC3-II/LC3-I conversion ratio were observed in both WIN-1001X 50 (135.6 ± 6.9%; p < 0.05) and 100 (162.9 ± 10.6%; p < 0.001) mg/kg treatment groups compared to the 6-OHDA-injected vehicle group (Fig. 4c). Protein expression levels of beclin-1 were also significantly increased only in WIN-1001X 50 (113.3 ± 4.9%) and 100 (113.1 ± 3.7%) mg/kg treated groups compared to the 6-OHDA-injected vehicle group (Fig. 4d; p < 0.001). For the mTOR protein expression, 6-OHDA initiated reduction of expression level down to 81.6 ± 2.3% (p < 0.001) compared to the sham-operated control group (Fig. 4e). Interestingly, both L-Dopa and WIN-1001X treatments further down-regulated the mTOR protein expressions with significance against 6-OHDA-injected vehicle group. With WIN-1001X 50 and 100 mg/kg treatment groups showing 65.0 ± 3.3% (p < 0.01) and 35.4 ± 3.9% (p < 0.001) of the control group, l-Dopa-treated group also showed significantly reduced value of 46.5 ± 3.4% (p < 0.001).
WIN-1001X Treatment Protects SH-SY5Y Cells and Induces Autophagy Under 6-OHDA-Induced Cytotoxicity
The effect of WIN-1001X on mice with 6-OHDA injection was further confirmed using human neuroblastoma cell line as well. First, with 50 μM 6-OHDA treatment for 1 h, the viability of exposed SH-SY5Y cells decreased to 59.6 ± 1.1% of the normal control group. This cytotoxicity was, however, prevented by all tested concentrations of WIN-1001X pretreatment with statistical significances (Online Resource 2). TH protein expression was significantly down-regulated with 6-OHDA treatment, whereas 400 μg/mL of WIN-1001X pretreatment significantly alleviated the change (Fig. 5a). Incubation with 6-OHDA also significantly increased the protein expression of α-synuclein in SH-SY5Y cells. The cells pre-treated with WIN-1001X, however, suppressed the increment in a dose-dependent manner (Fig. 5b). Such protective protein modulations could be derived from autophagic mechanism. The marker of autophagosome formation, LC3-II/I, and beclin-1 proteins were both up-regulated with 6-OHDA exposure. This up-regulation was significantly potentiated in the groups with WIN-1001X pretreatment at all concentrations (Fig. 5c, d). Exposure to 6-OHDA significantly down-regulated the protein expression of NBR1, but WIN-1001X pretreatment alleviated such loss of protein expression in a dose-dependent manner (Fig. 5e). On the other hand, mTOR protein expression was unchanged after 6-OHDA exposure. But WIN-1001X pretreatment significantly down-regulated its expression in a dose-dependent manner as well (Fig. 5f).
WIN-1001X pretreatment attenuates the alterations of TH and α-synuclein, as well as promoting autophagy-regulating proteins in the SH-SY5Y cells under 6-OHDA co-treatment. SH-SY5Y cells were pre-treated with the indicated concentrations of WIN-1001X (100, 200, and 400 μg/mL) for 17 h and co-treated with 50 μM of 6-OHDA for another 7 h. Protein expression levels of TH, α-synuclein, LC3-II/I, Beclin-1, NBR1, mTOR (a–f), and GAPDH were assessed by western blotting and band intensities were quantified by densitometric analysis. GAPDH was used as an internal loading control. Values are indicated as the mean ± SEM (n = 3). **p < 0.01 and ***p < 0.001 show significance compared with the normal control group; ##p < 0.01 and ###p < 0.001 show significance compared with the 6-OHDA-co-treated control group
Chemical Structure of Onjisaponin B and Its Effect on LC3 Conversion Ratio In Vitro
As an active component candidate of WIN-1001X, onjisaponin B was identified via the comparison of its NMR data and analysis of mass data (Fig. 6a). With various concentrations of onjisaponin B treatments, human neuroblastoma SH-SY5Y cells exhibited significant increase of LC3-II/LC3-I conversion ratio in a dose-dependent manner even from the lowest concentration of 1.25 μM (Fig. 6b; p < 0.05).
Structure of onjisaponin B and its autophagic marker modulation in vitro. a The chemical structure of onjisaponin B isolated from WIN-1001X. SH-SY5Y human neuroblastoma cells were seeded in 6-well plates (2 × 106 cells/well) and then treated with various concentrations of onjisaponin B (1.25, 2.5, 5, 10, and 20 μM) for 24 h. b Western blotting was used to detect LC3-II/LC3-I protein expressions from the SH-SY5Y cell lysates. The bar graph displays densitometry analysis of the LC3-II/LC3-I protein expressions ratio in each treatment group. Values are indicated as the mean ± SEM. *p < 0.05 and ***p < 0.001 show significance compared with the control group
Discussion
The mixture of three individual herbs, Polygala tenuifolia, Angelica tenuissima, and Dimocarpus longan, was first introduced by our group mainly focused on its protective roles on PD via autophagy enhancement and anti-apoptotic effect (Bae et al. 2015; Li et al. 2015). The water extract of three natural products exhibited significant benefits of reducing Parkinsonism symptoms which correlated with traditional recording of its use. Along with our further elucidation on possible active components for such effects, other researchers also reported that onjisaponin B, which can be isolated from our mixture, exhibits autophagy induction in cells (Wu et al. 2013). On other aspect, recent report claims that tenuifolin, the metabolite of onjisaponin B, reduces neuroinflammation in animals with PD (Peng et al. 2020). Accounting ours and others previous reports, we have modified our original extract to contain more of the non-polar active components while maintaining the similar level of tenuifolin-family compounds by preparing WIN-1001X extracted with 20% of ethanol included in the extraction solvent. The LC–MS profiling data of WIN-1001X were obtained in correlation with our previous publication (Bae et al. 2015) and relative content result is presented in Online Resource 3. With WIN-1001X, we focused on symptomatic relief as well as finding autophagy inducing effect in animal models of PD which was not confirmed in vivo condition elsewhere.
Based on the observation from two behavioral tests on motor impairments, WIN-1001X treatment successfully ameliorated acute MPTP injection-induced Parkinsonism symptoms even compared with the ropinirole-administered group. Such restoration of behavioral deficits can be explained if the dopaminergic neurons in SNpc region were rescued from the acute MPTP toxicity and striatal dopamine level was protected from the depletion.
From the previously reported observations of TH-immunoreactive neurons reappearing in the damaged brains, TH changes are more likely to reflect the function of dopamine synthesis rather than the actual death or survival of dopaminergic neurons (Alam et al. 2017). However, delayed observation of TH expression level partially represents the survival status as well as the functioning of dopaminergic neurons after initial recovery from MPTP challenges. Compared to significantly suppressed TH expression in MPTP-injected vehicle group, WIN-1001X treatment exhibited increased level of TH-positive cell density reflecting the increased survival of dopaminergic neurons or at least the neurons with less compromised dopaminergic function after the acute challenge of MPTP.
Considering the slow progression of PD in clinical cases, there is a need for use of less acute model investigating the cellular injury process in PD. While acute MPTP-injection PD model delivers rapid dopaminergic cell death and depletion of dopamine leading to significant changes in behavioral symptoms, sub-chronic or chronic MPTP-injection PD models develop relatively slow progression of cellular injury and cell death pathways (Tatton and Kish 1997; Meredith and Rademacher 2011). In combination with the TH-positive cell observation in acute MPTP-injection model, WIN-1001X treatment maintained the decrease of TH to minimum in the brains of both acute and sub-chronic MPTP-induced PD models. While both the protection of dopaminergic cells from cell death and enhancement of TH activity in survived neurons can be the cause of such observations, autophagy may contribute to the general aspects of both predicted mechanisms.
The loss of autophagy seems to induce the general degeneration of neuronal system and dopaminergic neuronal degeneration is one of them. It is often considered that pathologic conditions themselves induce the autophagic mechanisms as a self-protective gesture. However, if the pathologic condition induces greater stress that exceeds the level of homeostatic balance, the autophagy mechanisms fail to be activated. Ropinirole treatment did not alter the autophagy-related protein expressions as expected, whereas WIN-1001X treatment successfully enhanced the multiple autophagy-regulating proteins suggesting that protective mechanism of WIN-1001X might be different from the effect of ropinirole.
LC3 is the most well-established autophagic marker protein that involves in the formation of autophagosome and being converted into its other form. WIN-1001X treatment significantly up-regulated this conversion rate in SNpc of sub-chronic MPTP-injected mice, whereas MPTP alone showed insignificant influence. In the formation of autophagosome, beclin-1 is also a key protein in initiating the stage of autophagy by forming the isolation membrane which engulfs the cytoplasmic materials. Beclin-1 expression level was also significantly up-regulated with WIN-1001X treatment, whereas only minimal changes were observed with MPTP and ropinirole treatment. The mTOR being the major negative regulator in the up-stream of autophagy mechanism, WIN-1001X treatment also down-regulated the mTOR protein expression in sub-chronical MPTP-injected mice. However, the down-regulation was not significant enough compared to the ropinirole where its function as a dopamine agonist seems to affect the mTOR protein expression significantly. Therefore, we further measured the WIN-1001X’s effect on another autophagic protein, p62, in mice. The p62 is a link connecting autophagy pathway and ubiquitin–proteasome system and it plays a role as an autophagy substrate which delivers ubiquitinated proteins for degradation. It is expected that with increased flux of autophagy, p62 proteins are likely to be degraded during the autophagy process. Indeed, the protein level of p62 significantly decreased in WIN-1001X-treated mice under sub-chronic MPTP injection, further confirming that autophagosomes being degraded through the autophagy process in SNpc.
The 6-OHDA injection is another classical and important chemically induced PD research model showing significant behavioral deficits and neuronal damages (Ungerstedt 1968; Agid et al. 1973). Since 6-OHDA is unable to cross the BBB by itself, it was injected intracranially and unilateral injection of 6-OHDA into the nigrostriatal tract caused turning asymmetry, in which the animals showed tendency to rotate toward the side of the lesion. Challenge with amphetamine or dopamine receptor agonists, such as apomorphine, is the most frequently utilized test method to quantify such rotational behavior in rats with unilateral depletion of striatal dopamine. WIN-1001X treatment significantly reduced the rotational behavior which the exact mechanisms involved in such function is not elucidated yet, but the protection against the extended 6-OHDA-induced cellular injury during the drug administration period and functional recovery of survived dopaminergic neurons may lead to such consequence. TH protein expression in the rat striatum also revealed positive effect of WIN-1001X treatment which correlated with possible rescue mechanism of autophagy involvement proved by protein expression changes of LC3, beclin-1, and mTOR favorable to enhancement of autophagy. It is highly likely that the protective effect of WIN-1001X could be distinct from l-dopa considering its differential autophagic protein regulation. Such observation was reconfirmed positively using in vitro experiment where a human neuroblastoma SH-SY5Y cell line was pre-treated with WIN-1001X and challenged under the 6-OHDA-induced toxicity.
Collectively, our data presented in this study suggest that WIN-1001X treatment can effectively relieve the Parkinsonism symptoms induced by the chemical toxin challenges even compared to the positive controls of ropinirole and l-dopa. Also, greater dopaminergic neuronal injury and loss of activity caused by MPTP and 6-OHDA toxicities were countered by autophagy induction due to the WIN-1001X treatment. With our further effort pursuing the active component of WIN-1001X on autophagy activation, onjisaponin B came as a strong candidate of such effect. Onjisaponin B has shown the neuroprotective effect and improvement of impaired cognition (Li et al. 2016, 2018a, b). Furthermore, onjisaponin B was previously noticed with its autophagy enhancing effect in vitro and recently reported for anti-oxidative and anti-inflammatory effects in the MPTP-induced mouse PD model (Wu et al. 2013; Peng et al. 2020). Considering our current study as well as the reports from peers, WIN-1001X may hold a position of new therapeutic candidate against the PD and further detailed mechanism studies would be beneficial in revealing PD treatment partially via modulation of autophagy.
References
Agid, Y., Javoy, F., & Glowinski, J. (1973). Hyperactivity of remaining dopaminergic neurones after partial destruction of the nigro-striatal dopaminergic system in the rat. Nature New Biology, 245(144), 150–151.
Alam, G., Edler, M., Burchfield, S., & Richardson, J. R. (2017). Single low doses of MPTP decrease tyrosine hydroxylase expression in the absence of overt neuron loss. Neurotoxicology, 60, 99–106. https://doi.org/10.1016/j.neuro.2017.03.008.
Bae, N., Chung, S., Kim, H. J., Cha, J. W., Oh, H., Gu, M. Y., et al. (2015). Neuroprotective effect of modified Chungsimyeolda-tang, a traditional Korean herbal formula, via autophagy induction in models of Parkinson’s disease. Journal of Ethnopharmacology, 159, 93–101. https://doi.org/10.1016/j.jep.2014.11.007.
Cheung, Z. H., & Ip, N. Y. (2009). The emerging role of autophagy in Parkinson’s disease. Molecular Brain, 2, 29. https://doi.org/10.1186/1756-6606-2-29.
Choi, M., Lee, Y., & Cho, S. H. (2018). Angelica tenuissima Nakai ameliorates cognitive impairment and promotes neurogenesis in mouse model of Alzheimer’s disease. Chinese Journal of Integrative Medicine, 24(5), 378–384. https://doi.org/10.1007/s11655-017-2812-2.
Chung, J. W., Choi, R. J., Seo, E. K., Nam, J. W., Dong, M. S., Shin, E. M., et al. (2012). Anti-inflammatory effects of (Z)-ligustilide through suppression of mitogen-activated protein kinases and nuclear factor-kappaB activation pathways. Archives of Pharmacal Research, 35(4), 723–732. https://doi.org/10.1007/s12272-012-0417-z.
Fan, Z., Liang, Z., Yang, H., Pan, Y., Zheng, Y., & Wang, X. (2017). Tenuigenin protects dopaminergic neurons from inflammation via suppressing NLRP3 inflammasome activation in microglia. Journal of Neuroinflammation, 14(1), 256. https://doi.org/10.1186/s12974-017-1036-x.
Guo, C., Shen, J., Meng, Z., Yang, X., & Li, F. (2016). Neuroprotective effects of polygalacic acid on scopolamine-induced memory deficits in mice. Phytomedicine, 23(2), 149–155. https://doi.org/10.1016/j.phymed.2015.12.009.
Iida, M., Miyazaki, I., Tanaka, K., Kabuto, H., Iwata-Ichikawa, E., & Ogawa, N. (1999). Dopamine D2 receptor-mediated antioxidant and neuroprotective effects of ropinirole, a dopamine agonist. Brain Research, 838(1–2), 51–59. https://doi.org/10.1016/s0006-8993(99)01688-1.
Kirik, D., Rosenblad, C., & Bjorklund, A. (1998). Characterization of behavioral and neurodegenerative changes following partial lesions of the nigrostriatal dopamine system induced by intrastriatal 6-hydroxydopamine in the rat. Experimental Neurology, 152(2), 259–277. https://doi.org/10.1006/exnr.1998.6848.
Kopin, I. J. (1987). MPTP: An industrial chemical and contaminant of illicit narcotics stimulates a new era in research on Parkinson’s disease. Environmental Health Perspectives, 75, 45–51.
Kopin, I. J., & Markey, S. P. (1988). MPTP toxicity: Implications for research in Parkinson’s disease. Annual Review of Neuroscience, 11, 81–96. https://doi.org/10.1146/annurev.ne.11.030188.000501.
Kuboyama, T., Hirotsu, K., Arai, T., Yamasaki, H., & Tohda, C. (2017). Polygalae radix extract prevents axonal degeneration and memory deficits in a transgenic mouse model of Alzheimer’s disease. Frontiers in Pharmacology, 8, 805. https://doi.org/10.3389/fphar.2017.00805.
Kunworarath, N., Rangkadilok, N., Suriyo, T., Thiantanawat, A., & Satayavivad, J. (2016). Longan (Dimocarpus longan Lour.) inhibits lipopolysaccharide-stimulated nitric oxide production in macrophages by suppressing NF-kappaB and AP-1 signaling pathways. Journal of Ethnopharmacology, 179, 156–161. https://doi.org/10.1016/j.jep.2015.12.044.
Lee, D. S., Choi, J., Kim, S. H., & Kim, S. (2014). Ameliorating effects of HX106N, a water-soluble botanical formulation, on Abeta25-35-induced memory impairment and oxidative stress in mice. Biological &/and Pharmaceutical Bulletin, 37(6), 954–960.
Li, G., Yu, J., Zhang, L., Wang, Y., Wang, C., & Chen, Q. (2018a). Onjisaponin B prevents cognitive impairment in a rat model of D-galactose-induced aging. Biomedicine & Pharmacotherapy, 99, 113–120. https://doi.org/10.1016/j.biopha.2018.01.006.
Li, H., Lin, S., Qin, T., Li, H., Ma, Z., & Ma, S. (2017). Senegenin exerts anti-depression effect in mice induced by chronic un-predictable mild stress via inhibition of NF-kappaB regulating NLRP3 signal pathway. International Immunopharmacology, 53, 24–32. https://doi.org/10.1016/j.intimp.2017.10.001.
Li, H., Park, G., Bae, N., Kim, J., Oh, M. S., & Yang, H. O. (2015). Anti-apoptotic effect of modified Chunsimyeolda-tang, a traditional Korean herbal formula, on MPTP-induced neuronal cell death in a Parkinson’s disease mouse model. Journal of Ethnopharmacology, 176, 336–344. https://doi.org/10.1016/j.jep.2015.11.013.
Li, X., Cui, J., Yu, Y., Li, W., Hou, Y., Wang, X., et al. (2016). Traditional Chinese nootropic medicine radix polygalae and its active constituent onjisaponin B reduce beta-amyloid production and improve cognitive impairments. PLoS ONE, 11(3), e0151147. https://doi.org/10.1371/journal.pone.0151147.
Li, X., Sun, Y., Wei, Y., Zhou, L., Liu, L., Yin, P., et al. (2018b). Onjisaponin B (OB) is neuroprotective during cognitive loss through immune-mediated and SIRT1 pathways. Curr Neurovasc Res, 15(2), 94–102. https://doi.org/10.2174/1567202615666180528071520.
Lin, A. M., Wu, L. Y., Hung, K. C., Huang, H. J., Lei, Y. P., Lu, W. C., et al. (2012). Neuroprotective effects of longan (Dimocarpus longan Lour.) flower water extract on MPP+-induced neurotoxicity in rat brain. Journal of Agricultural and Food Chemistry, 60(36), 9188–9194. https://doi.org/10.1021/jf302792t.
Lynch-Day, M. A., Mao, K., Wang, K., Zhao, M., & Klionsky, D. J. (2012). The role of autophagy in Parkinson’s disease. Cold Spring Harb Perspect Med, 2(4), a009357. https://doi.org/10.1101/cshperspect.a009357.
Meredith, G. E., & Rademacher, D. J. (2011). MPTP mouse models of Parkinson’s disease: an update. Journal of Parkinsons Diseases, 1(1), 19–33. https://doi.org/10.3233/JPD-2011-11023.
Ogawa, N., Hirose, Y., Ohara, S., Ono, T., & Watanabe, Y. (1985). A simple quantitative bradykinesia test in MPTP-treated mice. Research Communications in Chemical Pathology and Pharmacology, 50(3), 435–441.
Parvez, S., Winkler-Stuck, K., Hertel, S., Schönfeld, P., & Siemen, D. (2010). The dopamine-D2-receptor agonist ropinirole dose-dependently blocks the Ca2+-triggered permeability transition of mitochondria. Biochimica et Biophysica Acta (BBA), 1797(6), 1245–1250.
Peng, F., Lu, L., Wei, F., Wu, D., Wang, K., & Tang, J. (2020). The onjisaponin B metabolite tenuifolin ameliorates dopaminergic neurodegeneration in a mouse model of Parkinson’s disease. NeuroReport, 31(6), 456–465. https://doi.org/10.1097/wnr.0000000000001428.
Sakuma, S., & Shoji, J. (1982). Studies on the constituents of the root of Polygala tenuifolia Willdenow. II. On the Structures of Onjisaponins A, B and E. Chemical & Pharmaceutical Bulletin, 30(3), 810–821. https://doi.org/10.1248/cpb.30.810.
Sauer, H., & Oertel, W. H. (1994). Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6-hydroxydopamine: A combined retrograde tracing and immunocytochemical study in the rat. Neuroscience, 59(2), 401–415.
Shim, E. B., Lee, S., Kim, J. Y., & Earm, Y. E. (2008). Physiome and Sasang constitutional medicine. Journal of Physiological Science, 58(7), 433–440. https://doi.org/10.2170/physiolsci.RV004208.
Tatton, N. A., & Kish, S. J. (1997). In situ detection of apoptotic nuclei in the substantia nigra compacta of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice using terminal deoxynucleotidyl transferase labelling and acridine orange staining. Neuroscience, 77(4), 1037–1048.
Tseng, H. C., Wu, W. T., Huang, H. S., & Wu, M. C. (2014). Antimicrobial activities of various fractions of longan (Dimocarpus longan Lour. Fen Ke) seed extract. International Journal of Food Sciences and Nutrition, 65(5), 589–593. https://doi.org/10.3109/09637486.2014.886181.
Ungerstedt, U. (1968). 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. European Journal of Pharmacology, 5(1), 107–110.
Weeratunga, P., Uddin, M. B., Kim, M. S., Lee, B. H., Kim, T. H., Yoon, J. E., et al. (2016). Interferon-mediated antiviral activities of Angelica tenuissima Nakai and its active components. Journal of Microbiology, 54(1), 57–70. https://doi.org/10.1007/s12275-016-5555-4.
Wu, A. G., Wong, V. K., Xu, S. W., Chan, W. K., Ng, C. I., Liu, L., et al. (2013). Onjisaponin B derived from Radix Polygalae enhances autophagy and accelerates the degradation of mutant alpha-synuclein and huntingtin in PC-12 cells. International Journal of Molecular Sciences, 14(11), 22618–22641. https://doi.org/10.3390/ijms141122618.
Wu, A. G., Wong, V. K., Zeng, W., Liu, L., & Law, B. Y. (2015). Identification of novel autophagic Radix Polygalae fraction by cell membrane chromatography and UHPLC-(Q)TOF-MS for degradation of neurodegenerative disease proteins. Science Report, 5, 17199. https://doi.org/10.1038/srep17199.
Acknowledgements
The authors would like to thank Lori I. Won for assisting with revision of manuscript.
Funding
This work was funded and supported by the Bio-Synergy Research Project (NRF-2012M3A9C4048793) of the Ministry of Science, ICT, and Future Planning through the National Research Foundation of the Republic of Korea. This work also was supported by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant Number: HI18C1860).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare there is no conflict of interest regarding current study.
Ethical Approval
All the animal protocols used in current study was approved and in accordance with the Korea Institute of Science and Technology Animal Care Committee guidelines.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, H., Kim, J., Tran, H.N.K. et al. Extract of Polygala tenuifolia, Angelica tenuissima, and Dimocarpus longan Reduces Behavioral Defect and Enhances Autophagy in Experimental Models of Parkinson’s Disease. Neuromol Med 23, 428–443 (2021). https://doi.org/10.1007/s12017-020-08643-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-020-08643-x