Abstract
Lilium brownii (L. brownii) is a plant that can be used for both medicine and food. Its bulbs are commonly used to treat neurological disorders like depression, insomnia, and Parkinson’s disease (PD). However, the mechanism by which it treats PD is not yet fully understood. This study aims to investigate the possible mechanism of L. brownii extract in treating PD and to compare the efficacy of ethanol and aqueous extracts of L. brownii. In this study, mice with PD induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine hydrochloride (MPTP) were given L. brownii extracts for 30 days, and the effects of both extracts were then evaluated. Our study demonstrated that both extracts of L. brownii effectively improved motor dysfunction in PD mice induced by MPTP. Additionally, they increased the number of neurons in the substantia nigra region of the mice. Moreover, both extracts reduced levels of malondialdehyde (MDA) and ferrous ion (Fe2+), while increasing levels of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) in serum. They also influenced the expression of proteins associated with the p62-Keap1-Nrf2 pathway. Interestingly, while both extracts had similar behavioral effects, the ethanol extract appeared to have a more significant impact on individual proteins in the p62-Keap1-Nrf2 pathway compared to the aqueous extract, possibly due to its higher phenolic acid glyceride content. In conclusion, L. brownii shows promise as an effective and safe treatment for PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder globally (Sharma et al. 2022), with prevalence rates varying by region and age group. In Europe and the United States, about 1% of individuals over 60 years old have PD, increasing to over 4% in those over 80. In China, the prevalence is 1.7% in individuals over 65, and it is projected to rise significantly by 2030 (Li et al. 2019b). PD is characterized by both motor symptoms (like tremors, muscle rigidity and gait disorder) and non-motor symptoms (such as depression, insomnia and constipation) (Fu et al. 2016; Ma et al.2017). The disease is primarily linked to the loss of dopamine (DA) neurons and the formation of Lewy bodies containing misfolded α-Synuclein (α-Syn) (Dickson et al. 2010; Simon et al. 2019). While the exact causes of PD are not fully understood, factors like oxidative stress, neuroinflammation and the misfolding and aggregation of α-Syn are believed to play a role (Bloem et al. 2021). Current treatment options focus on symptom management through medications like levodopa and surgical interventions like deep brain stimulation (Meissner et al. 2011; Fox et al. 2018). Research has demonstrated that long-term levodopa use in many patients results in complications including unstable symptoms and abnormal involuntary movements (Meissner et al. 2011). Moreover, existing medications can only alleviate PD symptoms but are unable to reverse the disease progression. Consequently, the development of new drugs that can effectively prevent or treat PD is of considerable practical importance, contributing to social advancement and human welfare.
Traditional Chinese Medicine, known for its natural composition, lower toxicity and powerful synergistic effects, holds great potential for the long-term prevention and treatment of PD. Various formulas like Baihe Zhimu decoction, Baihe Dihuang decoction and Ganmai Dazao decoction, as well as individual herbs such as Bai-he, Gastrodia elata and Ginkgo biloba have neuroprotective effects and can effectively alleviate the symptoms of PD (Kaur et al. 2017; Lee et al. 2024). Studies have shown that active components in these herbs can mitigate PD symptoms through anti-inflammatory, anti-apoptotic, anti-oxidative stress mechanisms and by activating autophagy pathways (Chen et al. 2022). For instance, phenols like resveratrol have antioxidant and anti-inflammatory effects that can reduce motor and cognitive impairments in PD mice (Bjørklund et al. 2022; Kung et al. 2021). Terpenoids, such as andrographolide, have anti-aging and antioxidant effects, protecting neurons and improving cognitive function (Prasertsuksri et al. 2023; Lu et al. 2019). Alkaloids like rhynchophylline have anti-inflammatory and antioxidant effects, aiding in reducing motor deficits in PD mice (Hu et al.2018). As a traditional Chinese medicine, the crude drug “Bai-he” prepared from the bulbs of several Lilium species, including Lilium lancifolium Thunb., Lilium brownii F. E. Brown var. viridulum Baker (L. brownii) and Lilium pumilum DC. (Zhang et al. 2022). The reported phytochemical components of L. brownii include steroidal saponins, phenolics, flavonoids, alkaloids and polysaccharides (Ma et al. 2023; Su et al. 2021). These bioactive compounds have demonstrated with sedative-hypnotic, antidepressant, anti-PD, antioxidant and anti-inflammatory potentials (Wu et al. 2020; Luo et al. 2017). Studies have shown that both aqueous and ethanol extracts of Bai-he can enhance sleep quality in mice, possibly by increasing serotonin levels in the brain and reducing stress in the hypothalamic-pituitary-adrenal axis (Oh et al. 2019; Wang et al. 2015). Compounds like Liliumtide A and Regaloside A isolated from L. brownii exhibit hypnotic and sedative properties (Li et al. 2023; Zhang et al. 2022). L. brownii and its formulations have been clinically used and recognized for the treatment of PD (Guan et al. 2020).
However, it is still uncertain whether the aqueous and ethanol extracts of L. brownii have the same effectiveness or show differences in treating PD, as well as their mechanism of action. Therefore, in light of the potential therapeutic benefits of L. brownii for the central nervous system, we conducted an experiment to explore the effects of both ethanol and aqueous extracts of L. brownii on mice with MPTP-induced PD. The aim was to investigate the efficacy and possible mechanisms of L. brownii on PD and to provide a basis for the development and utilisation of L. brownii.
Materials and methods
Animals
Healthy male C57BL/6J mice aged 6–8 weeks were purchased from Beijing Viton Lihua Laboratory Animal Technology Co., Ltd., with the license number SCXK (Beijing)-2021-001 and animal qualification certificate number 110,011,231,104,369,548. The mice were accommodated in an SPF-level animal facility at the Research Center of Henan University of Chinese Medicine for a week. All animal experimental studies were approved by the Experimental Animal Management Committee of Henan University of Chinese Medicine (Ethics Approval Document No. 202,303,014). Maintenance conditions included a temperature of (22 ± 2) ℃ and a humidity of 55–65%, along with a 12-hour light/dark cycle, the mice had free access to food and water. All feeding, handling and sample collection of animal experiments are strictly in accordance with the principles of the Laboratory Animal Ethics Committee and the International Standards for the Conduct of Animal Experiments.
Drugs
In August 2022, the fresh bulbs of L. brownii were obtained from Longhui County, Hunan Province. They were confirmed by Prof. Guo Tao from Henan University of Chinese Medicine to be the underground scale bulbs of Lilium brownii F. E. Brown var. viridulum Baker. The sample has been stored at Henan University of Chinese Medicine in Room BN912, with the reference number 20,220,862 A.
Two batches of 4 kg fresh bulbs from L. brownii were processed by being extracted twice with 80% ethanol for 2 h and three times with water for 1 h, respectively. The resulting extracts were then filtered and concentrated, yielding 500 g of ethanol extract and 312.5 g of aqueous extract with concentrations of 12.5% and 7.8125%, respectively.
Madopar (product code: YT0317) was obtained from Shanghai Roche Pharmaceuticals.
Instruments and reagents
The mouse rotor fatigue instrument (XR-6 C) and open field instrument (XR-XZ301) were provided by Shanghai Xinsoft Information Technology Co., Ltd. The vertical electrophoresis instrument (BV-2) and transfer electrophoresis instrument (BT-2) were obtained from Wuhan Saiweier Biotechnology Co., Ltd. The Infinite F50 microplate reader was supplied by Tecan (Switzerland). Olympus BX53 upright fluorescence microscope was supplied by Olympus Corporation (Japan). The BCD-272WDGD Refrigerator was from Qingdao Haier Co., Ltd. The 3K15 High-Speed Cryogenic Centrifuge was provided by Sigma (Germany) and the ultra-low temperature storage box (-80 °C) was supplied by Haire Biomedical Co., Ltd. The Uv-2600 UV spectrophotometer was produced by Shimadzu (Japan).
The MPTP reagent (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine hydrochloride, HY-15,608) and Regaloside A (114420-66-5, ≥ 99%) were purchased from MCE Inc. The PBS reagent (P1020) was obtained from Beijing Solaibao Co., Ltd. Normal saline (DG0242) was sourced from Henan Kelun Pharmaceutical Co., Ltd. The 4% paraformaldehyde fixative solution (BL539A) was provided by Beijing Lanjieke Technology Co., Ltd. The following reagents were supplied by Wuhan Saiweier Biotechnology Co., Ltd: RIPA lysate (G2002), 50×Cocktail protease inhibitor (G2006), PMSF (100mmol/L, G2008), phosphorylated protease inhibitor (G2007), BCA protein quantification kit (G2026), Protein reduction loading buffer (G2075), SDS-PAGE gel preparation kit (G2003), Protein marker (G2083) and PVDF membrane 0.45 μm (WGPVDF45) and antibodies including TH (GB11181), BDNF (GB11559), α-Syn (GB11404), p62 (GB11531), Keap1 (GB113747), Nrf2 (GB113808), HO-1 (GB12104), GPX4 (GB113745), ACSL4 (GB115608) and Actin (GB12001). The MDA kit (A003-1-2), SOD kit (A001-1-2), GSH-Px kit (A005-1-2) and tissue iron kit (A039-2-1) were obtained from Nanjing Jiancheng Co., Ltd.
Determination of the content of phenolic acid glyceride components in L. brownii extracts
Initially, a standard curve was created using the absorbance values of various concentrations of Regaloside A detected at 310 nm. Following this, the absorbance at 310 nm was measured at 0.125 mg/ml for the ethanolic and aqueous extracts of L. brownii, respectively. The content of phenolic acid glyceride components present in these extracts were then calculated by referencing the standard curve (Ran et al. 2022).
Establishment of a mouse model of PD
To ensure that the mice are free of any physical obstacles, they undergo experimental tests such as open field, rotarod and pole climbing after an acclimatization period of 7 days. Subsequently, except for those in the control group, all selected mice received daily intraperitoneal injections of MPTP solution (30 mg/kg) for a duration of 7 consecutive days to induce the PD.
Grouping and administration
The L. brownii extracts and Madopar dosages given to the mice were adjusted to match the adult clinical equivalent, as reported in previous studies (Wang et al. 2020; Ma et al. 2016). Following the identification of mice displaying PD symptoms through open field, rotarod, and pole climbing tests, the mice were randomly allocated into different groups based on existing literature, with each group consisting of 8 mice: MPTP group (Rui et al. 2020; Haque et al. 2021), Madopar group (Mad, 107.14 mg/kg) (Huang et al. 2020, 2021), L. brownii ethanol extract at low and high dose groups (LEE-L, 1.25 g/kg; LEE-H, 2.5 g/kg), and L. brownii aqueous extract at low and high dose groups (LAE-L, 0.78125 g/kg; LAE-H, 1.5625 g/kg). All groups, except for the control and MPTP groups, received the designated drugs via gavage for a period of 30 days. Throughout this time, the body weights of all mice were measured and documented every two days. The open field, rotarod, and pole climbing tests were repeated at the end of the treatment period, and samples were collected after the behavioral assessments were completed.
Behavioural tests
As described in the literature (Kheirbek et al. 2009), the mice were tested on open field (XR-XZ301), rotarod (XR-6 C) and climbing pole (homemade). Following each testing session with a mouse, it was necessary to clean the instruments with 75% alcohol to prevent any potential interference with the subsequent mouse evaluations.
Histological observation
Lesions within the substantia nigra region of the mouse brain were examined through Hematoxylin and Eosin (H&E) staining. The substantia nigra tissue was initially fixed in paraformaldehyde, followed by dehydration using a series of graded ethanol concentrations ranging from 50 to 100%. Subsequently, the tissue was cleared with xylene and embedded in paraffin. The tissue was then sectioned into 5 μm slices, with some of the sliced samples stained with H&E to assess pathological changes under a 200x magnification light microscope. Other samples underwent deparaffinization, rehydration, antigen retrieval, and serum blocking, followed by overnight incubation with the corresponding primary antibody at 4 °C, and subsequently with the secondary antibody for 1 h at room temperature. The sections were stained with diaminobenzidine and hematoxylin, dehydrated, washed, examined under a microscope, and their average optical density values were measured using Image J version 1.44.
Biochemical factor testing
Blood samples were collected from the eyeballs and subsequently centrifuged at 10,000 rpm for 10 min. Subsequently, the levels of malondialdehyde (MDA), glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD) were measured in the serum according to the kit’s instructions. The head was then decapitated using a guillotine, cooled on ice, and the cerebral cortex was carefully extracted using precise forceps. The cerebral cortex was homogenized, and the resulting homogenate was centrifuged at 4000 revolutions per minute for 15 min to obtain the supernatant. The ferrous iron (Fe2+) levels in the mouse cerebral cortex were determined in accordance with the instructions provided in the kit.
Western blot analysis
Following homogenization of the entire mouse brain with a combination of RIPA lysis buffer and a 50x protease inhibitor, the resulting mixture was centrifuged at 14,000 rpm for 20 min to collect the supernatants for quantification of protein levels utilizing the BCA assay. Subsequently, protein samples (each containing 40 µg) were fractionated on 10% polyacrylamide SDS-PAGE gels, transferred onto polyvinylidene fluoride membranes (0.22 μm), and blocked with 5% skimmed milk for 1 h at 37 °C. The membranes were then exposed to primary antibodies overnight at 4 °C, followed by three washes with TBST. Subsequent to this, the membranes were treated with the appropriate horseradish peroxidase (HRP)-conjugated secondary antibody for 1 h at room temperature. The gray value of the target bands was subsequently analyzed using Alpha software.
Statistical analysis
All datas were from three independent replicate trials and presented as mean ± standard deviation (x̄ ± s). Statistical analysis was performed by one-way ANOVA using SPSS 26.0 software, where #P < 0.05, ##P < 0.01 indicates statistical significance compared to the control group, and *P < 0.05, **P < 0.01 denotes statistical significance compared to the MPTP group.
Results
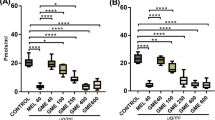
The content of phenolic acid glyceride components in L. brownii extracts
The linear regression equation of Regaloside A was y = 20.623x + 0.0689, R2 = 0.9995 (Fig. 1A). According to the Regaloside A, the content of phenolic acid glycerides components in ethanol extract and aqueous extract were 182.392 and 12.686 mg/g, respectively (Fig. 1B).
Effects of L. brownii extracts on body weight of MPTP-induced PD mice
As shown in Fig. 2, the mice lost weight after injection of MPTP solution and gradually gained weight over time. Except for the control group, no difference in the body weight was found between mice in MPTP group and those in other groups.
Effects of L. brownii extracts on motor function in MPTP-induced PD mice
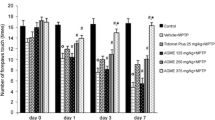
As shown in Fig. 3, compared to the control group, the distance traveled, speed of movement, central residence time and residence time on the rod of mice in the MPTP group significant decreased while the duration of pole climbing and head turning notably increased. Given the L. brownii extracts, there was a noticeable increase in distance travelled, speed of movement, time spent in the central and time spent on the pole, and a marked decrease in the duration of pole-climbing and head-turning. Further, no difference was observed between the ethanol and aqueous extract treatment groups.
Effects of L. brownii extracts on motor function in MPTP-induced PD mice. A Five-minute movement trajectories of mice. B Total distance traveled by mice in the open field test. C Average speed of mouse movement in the open field test. D Duration of mice’s stay in the center area during the open field test. E Mouse activity duration in the rotarod test. F Climbing duration of mice in the pole climbing test. G Turning duration of mice in the pole climbing test. (Con: normal saline; MPTP: normal saline; Mad: 107.14 mg/kg; LEE-L: 1.25 g/kg; LEE-H: 2.5 g/kg; LAE-L: 0.78125 g/kg; LAE-H: 1.5625 g/kg, #P < 0.05, ##P < 0.01, compared with the control group; *P < 0.05, **P < 0.01, compared with the MPTP group.)
Histopathological observation
As depicted in Fig. 4, the control mice exhibited a significant quantity of well-organized neurons in the substantia nigra region. In contrast, the MPTP group demonstrated pronounced neuronal damage in the substantia nigra area, characterized by a significant reduction in cell count, disorganized structures, shrinkage of cell bodies, gaps around cells and noticeably widened intercellular spaces. It was seen that the number of neurons remarkably increased and the cell gap reduced in the groups of ethanol and aqueous extracts of L. brownii, and there was no difference between them.
Histopathological analysis of substantia nigra sections in MPTP-induced PD mice, stained with Hematoxylin and Eosin (H&E, 200×magnification). Con: normal saline, MPTP: normal saline, Mad:107.14 mg/kg, LEE-L: 1.25 g/kg, LEE-H: 2.5 g/kg, LAE-L: 0.78125 g/kg, LAE-H: 1.5625 g/kg. (#P < 0.05, ##P < 0.01, compared with the control group; *P < 0.05, **P < 0.01, compared with the MPTP group, the arrows represent the neuronal region.)
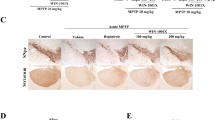
Effects of L. brownii extracts on the expression of TH and α-Syn neurons in the substantia nigra of MPTP-induced PD mice
As shown in Fig. 5, MPTP solution caused a significant reduction in the TH-positive dopaminergic neurons and a significant increase in α-Syn-positive neurons in the substantia nigra in mice. Notably, L. brownii extracts significantly protected MPTP-induced dopaminergic neuron loss.
Effects of L. brownii extracts on the expression of TH and α-Syn the substantia nigra of MPTP-induced PD mice. A TH-stained sections of the substantia nigra (200×magnification). B α-Syn-stained sections of the substantia nigra (200×magnification). C Average optical density of TH in the substantia nigra. D Average optical density of α-Syn in the substantia nigra. (Con: normal saline, MPTP: normal saline, Mad: 107.14 mg/kg, LEE-L: 1.25 g/kg, LEE-H: 2.5 g/kg, LAE-L: 0.78125 g/kg, LAE-H: 1.5625 g/kg. #P < 0.05, ##P < 0.01, compared with the control group; *P < 0.05, **P < 0.01, compared with the MPTP group.)
Effects of L. brownii extracts on biochemical factors in MPTP-induced PD mice
Compared with the control group, the levels of MDA in serum (Fig. 6A) and Fe2+ in cortex (Fig. 6D) markedly increased while the levels of SOD and GSH-Px in serum (Fig. 6B, C) markedly decreased in the MPTP group. After the administration of L. brownii extracts, the levels of these indicators converged to those of the control group.
Effects of L. brownii extracts on biochemical factors in MPTP-induced PD mice. A MDA levels in mouse serum. B SOD levels in mouse serum. C GSH-Px levels in mouse serum. D Fe2+ levels in mouse cortex. (#P < 0.05, ##P < 0.01, compared with the control group; *P < 0.05, **P < 0.01 compared with the MPTP group. Con: normal saline; MPTP: normal saline; Mad: 107.14 mg/kg; LEE-L: 1.25 g/kg; LEE-H: 2.5 g/kg; LAE-L: 0.78125 g/kg; LAE-H: 1.5625 g/kg)
Effects of L. brownii extracts on the p62-Keap1-Nrf2 pathway in MPTP-induced PD mice
As shown in Fig. 7, α-Syn protein expression in the brains of mice dramatically enhanced while tyrosine hydroxylase (TH) and brain-derived neurotrophic factor (BDNF) proteins expression significantly reduced in MPTP group. Compared with the MPTP group, a remarkable increase in the expression of TH protein in the low dose group of both ethanol and aqueous extracts and the expression of BDNF protein in the extract groups, and a decrease in the expression of α-Syn protein in all L. brownii extracts groups were found.
Effects of L. brownii extracts on basal protein expression in the whole brain of MPTP-induced PD mice. A Expression of α-Syn, BDNF and TH as indicator proteins. B Quantification of α-Syn expression. C Quantification of TH expression. D Quantification of BDNF expression. (#P < 0.05, ##P < 0.01, compared with the control group; *P < 0.05, **P < 0.01 compared with the MPTP group. Con: normal saline; MPTP: normal saline; Mad: 107.14 mg/kg; LEE-L: 1.25 g/kg; LEE-H: 2.5 g/kg; LAE-L: 0.78125 g/kg; LAE-H: 1.5625 g/kg)
In addition, compared to the control group, the expression of p62, nuclear factor erythroid 2-related factor 2 (Nrf2), heme oxygenase 1 (HO-1) and glutathione peroxidase 4 (GPX4) proteins significantly reduced in the MPTP group while that of Kelch-like ECH-associated protein 1 (Keap1) and acyl-CoA synthetase long-chain family member 4 (ACSL4) proteins significantly increased (Fig. 8). Moreover, compared to MPTP group, the expression of Nrf2 and GPX4 proteins remarkable raised in all extract groups while that of ACSL4 remarkable decreased (Fig. 8D, F, G). Additionally, the expression of Keap1 protein significantly decreased in the ethanol extract group (Fig. 8C).
Effects of L. brownii extracts on the p62-Keap1-Nrf2 pathway in the whole brain of MPTP-induced PD mice. A Proteins of the p62-Keap1-Nrf2 pathway. B Quantification of p62 expression. C Quantification of Keap1 expression. D Quantification of Nrf2 expression. E Quantification of HO-1 expression. F Quantification of GPX4 expression. G Quantification of ACSL4 expression. (#P < 0.05, ##P < 0.01, compared with the control group; *P < 0.05, **P < 0.01 compared with the MPTP group. Con: normal saline; MPTP: normal saline; Mad: 107.14 mg/kg; LEE-L: 1.25 g/kg; LEE-H: 2.5 g/kg; LAE-L: 0.78125 g/kg; LAE-H: 1.5625 g/kg)
Discussion
L. browniiis a traditional medicinal plant known for its effectiveness in treating depression, insomnia, Alzheimer’s, and various neurological disorders. While Bai-he Dihuang decoction has shown efficacy in treating PD (Peng et al. 2018), the specific impact and mechanism of the single herb L. brownii in the treatment of PD remains unclear. Therefore, MPTP was selected as an inducer of PD to assess the impact and potential mechanism of L. brownii extract on PD.
In this study, we noted significant changes in the behavior and physiology of mice injected with MPTP. After injection of MPTP solution, all mice exhibited significant distress such as weight loss, raised fur, stiff tail and dry feces. All these manifestations were attenuated or completely ceased after L. brownii intervention. These findings suggest that L. brownii extracts attenuate MPTP-induced PD symptoms.
PD is a neurodegenerative disorder impacting motor abilities, and evaluating motor function can serve as a valuable indicator to gauge the disease’s progression in an individual (Shulman et al. 2011). Compared with the control group, mice in the MPTP group exhibited significant reductions in distance, speed and time spent in the central area. Furthermore, they often moved in the opposite direction of training and paused for long periods of time in the course of the movement. All indications pointed towards the conclusion that the administration of the MPTP solution not only hindered the motor abilities of the mice, but also influenced their cognitive functions. Following the administration of L. brownii extracts, mice demonstrated increased activity levels, as evidenced by enhanced distance traveled, speed, and time spent exploring the central area, along with a decrease in moving in the opposite direction of training. Consequently, it can be inferred that L. brownii extracts possess the capacity to ameliorate motor dysfunction in mice.
The analysis of the substantia nigra area in mice yielded important findings regarding the potential therapeutic effects of L. brownii extracts in PD. The research revealed that the administration of L. brownii extracts led to an increase in the quantity of neurons, particularly in the expression of TH neurons, while reducing the presence of α-Syn neurons. These results indicate that L. brownii extracts may enhance the production of TH, a key enzyme in dopamine synthesis, inhibit α-Syn aggregation, and consequently support the development of dopamine-producing neurons.
Current research highlights the association of PD development with oxidative stress and iron accumulation (Cai et al. 2020; Gerlach et al. 2006). SOD and GSH-Px are integral components of the body’s antioxidative defense system. They play a pivotal role in neutralizing reactive oxygen species (ROS), oxygen radicals and hydroxyl radicals. MDA serves as an indicator of lipid peroxidation, reflecting the extent of this process within the body. An increase in lipid peroxidation results in heightened production of oxygen free radicals, prompting an increase in antioxidant enzymes like SOD and GSH-Px to maintain a balance between oxidative and antioxidative processes (Chi et al. 2015). The bioactive constituents of L. brownii such as polysaccharides, saponins, phenols and flavonoids, are effective in scavenging superoxide ions and hydroxyl radicals (Su et al. 2021). Our research revealed significant rises in SOD and GSH-Px levels in serum of mice treated with L. brownii extracts, accompanied by a noticeable decrease in MDA levels (Fig. 6A-C). The extracts of L. brownii effectively mitigate the excessive accumulation of ROS and MDA, thereby aiding in maintaining a balance between oxidation and antioxidation in mice.
Iron ions play a crucial role in various biological functions such as DNA synthesis, gene expression, myelination, neurotransmission, and mitochondrial activity. Imbalances in iron levels within the brain can interfere with regular cellular processes, potentially resulting in the death of neuronal cells (Sun et al. 2023). Particularly in the iron-rich substantia nigra, oxidative toxicity of DA can be exacerbated. Our study revealed a notable decrease in iron levels in the cerebral cortex of mice treated with L. brownii extracts. This finding suggests that L. brownii extracts may have a protective effect by reducing iron accumulation, thus safeguarding dopamine neurons from iron-induced harm.
The abnormal aggregation of α-Syn is a critical pathological factor in the progression of PD, leading to the accelerated death of DA neurons (Lin et al. 2023). BDNF plays a vital role in promoting neuronal survival and growth, offering protection against the decline of nigrostriatal dopaminergic neurons (Yang et al. 2023; Moffat et al. 2023). TH is indispensable for dopamine synthesis, and a decrease in its expression results in reduced dopamine production (Bobrovskaya et al. 2006). Consequently, elevated TH activity is associated with increased DA levels in dopaminergic cells. The levels of BDNF and TH proteins in the brains of mice in the MPTP group significantly diminished, while the expression of α-Syn protein elevated. Following the administration of L. brownii extracts, the levels of TH, BDNF and α-Syn showed recovery towards those of the control group. These findings indicate that L. brownii extracts have the potential to enhance TH and BDNF production while reducing α-Syn aggregation.
The p62-Keap1-Nrf2 signaling cascade is pivotal in regulating oxidative stress and redox homeostasis (Zhang et al. 2021). In PD, dysregulation of the autophagy pathway can lead to the accumulation of abnormal proteins (Zhu et al. 2019). The p62 as a key protein in the pathogenesis of autophagy in PD, also regulates the Keap1/Nrf2 pathway (Komatsu et al. 2010). In normal conditions, Keap1 binds to Nrf2 in the cytoplasm and promotes the ubiquitination and proteasomal degradation of Nrf2. However, upon exposure to certain chemicals or ROS, the ubiquitin E3 ligase activity of the Keap1–Cul3 complex declines, leading to the stabilization of Nrf2. The stabilized Nrf2 accumulates in the nucleus and activates its target genes (Kobayashi et al. 2004; McMahon et al. 2006; Li et al. 2021).GPX4, a transcriptional target of Nrf2, plays a crucial role in inhibiting ferroptosis by reducing ROS levels (Ma et al. 2021). ACSL4 is involved in the production of lipid peroxides, which are key regulators of ferroptosis (Sha et al. 2021; Li et al. 2019a). In the study, the expression of p62, Nrf2, HO-1 and GPX4 proteins in the brains of mice treated with LB extracts significantly increased, while the expression of Keap1 and ACSL4 proteins notably decreased. These results suggest that extracts from L. brownii may potentially mitigate PD symptoms through modulation of the p62-Keap1-Nrf2 signaling pathway.
In the context of extracting Chinese herbal medicine, water is commonly utilized for decoction. However, studies have shown that there are still a large number of active components in the residue after water extraction (Zheng et al. 2016). The selection of extraction solvent significantly impacts the extraction efficiency and preservation of active constituents. Presently, ethanol solutions are increasingly employed in the extraction process of Chinese herbal medicine. In the study, both ethanol and aqueous extracts showed promising efficacy in treating PD, with no significant differences observed in improving motor dysfunction and increasing neuronal count in the PD mouse model, and only some differences in the expression of individual proteins. The ethanol extract demonstrated a more pronounced effect on the p62-Keap1-Nrf2 pathway compared to the aqueous extract.
The Regaloside components belong to the phenolic acid glyceride, which are the characteristic components of Bai-he and have antioxidant, anti-inflammatory and antidepressant activities (Fu et al. 2023; Weng et al. 2023). Additionally, studies have indicated that Regaloside components exhibit the ability to promote neuronal cell survival and provide neuroprotective advantages (Tang et al. 2023). These compounds primarily function by circulating within the bloodstream (Wu et al. 2021). We found that the content of phenolic acid glyceride components in ethanol extract was higher than that in aqueous extract. It is plausible to infer that ethanol extract demonstrated a more pronounced effect on individual proteins in the p62-Keap1-Nrf2 pathway correlates with higher phenolic acid glyceride components.
Conclusion
In conclusion, the obtained results demonstrated that both ethanol and aqueous extracts of L. brownii improved motor function in PD mice, protected neuronal cells, as well as reduced the levels of peroxidation and iron death through the p62-Keap1-Nrf2 pathway, thus exerting neuroprotective and anti-PD effects. Interestingly, the ethanol extracts demonstrated superior regulation of individual proteins on this pathway compared to the aqueous extracts, possibly due to their higher content of phenolic acid glyceride components. It indicated that the phenolic acid glyceride components may be the potential pharmacological substance basis for the treatment of PD with L. brownii.
During subsequent research, it is possible to further investigate the therapeutic properties of L. brownii in the treatment of PD, as well as its possible application in the treatment of other neurological disorders like Alzheimer’s disease and stroke. This exploration could provide a deeper understanding of the neuroprotective benefits associated with L. brownii treatment.
Data availability
No datasets were generated or analysed during the current study.
References
Bjørklund G, Shanaida M, Lysiuk R, Butnariu M, Peana M, Sarac I, Strus O, Smetanina K, Chirumbolo S (2022) Natural compounds and products from an anti-aging perspective. Molecules 27(20):7084. https://doi.org/10.3390/molecules27207084
Bloem BR, Okun MS, Klein C (2021) Parkinson’s disease. Lancet 397(10291):2284–2303. https://doi.org/10.1016/S0140-6736(21)00218-X
Bobrovskaya L, Gilligan C, Bolster EK, Flaherty JJ, Dickson PW, Dunkley PR (2006) Sustained phosphorylation of tyrosine hydroxylase at serine 40: a novel mechanism for maintenance of catecholamine synthesis. J Neurochem 100(2):479–489. https://doi.org/10.1111/j.1471-4159.2006.04213.x
Cai LJ, Tu L, Li T, Yang XL, Ren YP, Gu R, Zhang Q, Yao H, Qu X, Wang Q, Tian JY (2020) Up-regulation of microRNA-375 ameliorates the damage of dopaminergic neurons, reduces oxidative stress and inflammation in Parkinson’s disease by inhibiting SP1. Aging 12(1):672–689. https://doi.org/10.18632/aging.102649
Chen P, Zhang J, Wang C, Chai YH, Wu AG, Huang NY, Wang L (2022) The pathogenesis and treatment mechanism of Parkinson’s disease from the perspective of traditional Chinese medicine. Phytomedicine 100:154044. https://doi.org/10.1016/j.phymed.2022.154044
Chi A, Li H, Kang C, Guo H, Wang Y, Guo F, Tang L (2015) Anti-fatigue activity of a novel polysaccharide conjugates from Ziyang Green Tea. Int J Biol Macromol 80:566–572. https://doi.org/10.1016/j.ijbiomac.2015.06.055
Dickson DW, Braak H, Duda JE, Duyckaerts C, Gasser T, Halliday GM, Hardy J, Leverenz JB, Del Tredici K, Wszolek ZK, Litvan I (2010) Neuropathological assessment of Parkinson’s disease: refining the diagnostic criteria. Lancet Neurol 8(12):1150–1157. https://doi.org/10.1016/S1474-4422(09)70238-8
Fox SH, Katzenschlager R, Lim SY, Barton B, de Bie RMA, Seppi K, Coelho M, Sampaio C, Movement Disorder Society Evidence-Based Medicine Committee (2018) International Parkinson and movement disorder society evidence-based medicine review: update on treatments for the motor symptoms of Parkinson’s disease. Mov Disord 33(8):1248–1266. https://doi.org/10.1002/mds.27372
Fu YC, Zhou TT, Wang J, Zheng MM, Wang QQ, Wang MY (2016) Effects of green tea polyphenols on motor, learning and memory functions of model mice of Parkinson’s disease. Chin J Clin Pharmacol Ther 21(08):904–908. https://doi.org/10.7666/d.y1745086
Fu CY, Liu L, Bai ZY, Zhang F, Liu YH, Peng L (2023) Establishment of HighPerformance Liquid Chromatography fingerprint of Lilium Brownii and determination of 3 phenolic glyceride glycoside active components. Phys Test Chem Anal (Part B: Chem Analysis) 59(07):825–832. https://doi.org/10.11973/lhjy-hx202307013
Gerlach M, Double KL, Youdim MB, Riederer P (2006) Potential sources of increased iron in the substantia nigra of Parkinsonian patients. J Neural Transm Suppl 70:133– 42. https://doi.org/10.1007/978-3-211-45295-0_21.
Guan GX, Li SR, Tian FL, Zhang LY (2020) Clinical efficacy observation of Baihe Dihuang combined with trazodone hydrochloride in the treatment of insomnia associated with Yin deficiency-type depressive disorder. China Practical Med 15(08):165–167. https://doi.org/10.14163/j.cnki.11-5547/r.2020.08.074
Haque ME, Azam S, Akther M, Cho DY, Kim IS, Choi DK (2021) The neuroprotective effects of GPR4 inhibition through the attenuation of caspase mediated apoptotic cell death in an MPTP induced mouse model of Parkinson’s Disease. Int J Mol Sci 22(9):4674. https://doi.org/10.3390/ijms22094674
Hu S, Mak S, Zuo X, Li H, Wang Y, Han Y (2018) Neuroprotection against MPP+-induced cytotoxicity through the activation of PI3-K/Akt/GSK3β/MEF2D signaling pathway by rhynchophylline, the major tetracyclic oxindole alkaloid isolated from Uncaria rhynchophylla. Front Pharmacol 9:768. https://doi.org/10.3389/fphar.2018.00768
Huang LP, Zhong XQ, Luo Q, Zhang QX, Deng MZ (2020) Autophagic activity of piperine on small intestine in dementia model mice with Parkinson’s disease. China J Chin Materia Med 45(21):5238–5247. https://doi.org/10.19540/j.cnki.cjcmm.20200624.408
Huang K, Bao CL, Chen WW, Tu JY, Luo LE (2021) Effect of electroacupuncture on mitochondrial function in mice with Parkinson’s disease. Acupunct Res 46(01):21–26. https://doi.org/10.13702/j.1000-0607.200266
Kaur K, Gill JS, Bansal PK, Deshmukh R (2017) Neuroinflammation - A major cause for striatal dopaminergic degeneration in Parkinson’s disease. J Neurol Sci 381:308–314. https://doi.org/10.1016/j.jns.2017.08.3251
Kheirbek MA, Britt JP, Beeler JA, Ishikawa Y, McGehee DS, Zhuang X (2009) Adenylyl cyclase type 5 contributes to corticostriatal plasticity and striatum-dependent learning. J Neurosci 29(39):12115–12124. https://doi.org/10.1523/JNEUROSCI.3343-09.2009
Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, Igarashi K, Yamamoto M (2004) Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol 24(16):7130–7139. https://doi.org/10.1128/MCB.24.16.7130-7139.2004
Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, Sou YS, Ueno I, Sakamoto A, Tong KI, Kim M, Nishito Y, Iemura S, Natsume T, Ueno T, Kominami E, Motohashi H, Tanaka K, Yamamoto M (2010) The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol 12(3):213–223. https://doi.org/10.1038/ncb2021
Kung HC, Lin KJ, Kung CT, Lin TK (2021) Oxidative stress, mitochondrial dysfunction, and neuroprotection of polyphenols with respect to Resveratrol in Parkinson’s Disease. Biomedicine 9(8):918. https://doi.org/10.3390/biomedicines9080918
Lee B, Yu MS, Song JG, Lee HM, Kim HW, Na D (2024) Corydalis ternata Nakai alleviates cognitive decline in Alzheimer’s disease by reducing β-Amyloid and neuroinflammation. Rejuvenation Res. https://doi.org/10.1089/rej.2023.0069
Li Y, Feng D, Wang Z, Zhao Y, Sun R, Tian D et al (2019a) Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ 26(11):2284–2299. https://doi.org/10.1038/s41418-019-0299-4
Li G, Ma J, Cui S, He Y, Xiao Q, Liu J, Chen S (2019b) Parkinson’s disease in China: a forty-year growing track of bedside work. Transl Neurodegener 8:22. https://doi.org/10.1186/s40035-019-0162-z
Li H, Shang Z, Liu X, Qiao Y, Wang K, Qiao J (2021) Clostridium butyricum alleviates enterotoxigenic escherichia coli K88-induced oxidative damage through regulating the p62-Keap1-Nrf2 signaling pathway and remodeling the cecal microbial community. Front Immunol 12:771826. https://doi.org/10.3389/fimmu.2021.771826
Li Q, Shi YH, Zhu Y, Li XL, Hou CW, Liu XD et al (2023) Resource distribution and modern research progress of medicinal and food homologous lily. Chin Wild Plant Resour 42(03):87–95. https://doi.org/10.3969/j.issn.1006-9690.2023.03.014
Lin Z, Huang L, Cao Q, Luo H, Yao W, Zhang JC (2023) Inhibition of abnormal C/EBPβ/α-Syn signaling pathway through activation of Nrf2 ameliorates Parkinson’s disease-like pathology. Aging Cell 22(10):e13958. https://doi.org/10.1111/acel.13958
Lu J, Ma Y, Wu J, Huang H, Wang X, Chen Z, Chen J, He H, Huang C (2019) A review for the neuroprotective effects of andrographolide in the central nervous system. Biomed Pharmacother. 2019 117:109078. https://doi.org/10.1016/j.biopha.2019.109078
Luo LM, Pei G, Tan L, Zhou XJ, Zhan JH, Liao N, Chen NH (2017) Research progress on chemical constituents and pharmacological effects of medicinal lilium plants. Traditional Chin Drug Res Clin Pharmacol 28(06):824–837. https://doi.org/10.19378/j.issn.1003-9783.2017.06.022
Ma Y, Jin G, Jiang F, Zhan QL, Li XG (2016) The therapeutic effect of combination dopamine and pramipexole in patients with Parkinson’s disease, the influence of blood uric acid level and life quality Chinese. J Difficult Complicated Cases 15(07):682–685. https://doi.org/10.3969/j.issn.1671-6450.2016.07.006
Ma LL, Yao XM, Wang J, Xu SN (2017) Advances in postural control in Parkinson’s disease. Chin J Rehab Med 32(03):373–376. https://doi.org/10.3969/j.issn.1001-1242.2017.03.028
Ma L, Xu A, Kang L, Cong R, Fan Z, Zhu X et al (2021) LSD1-demadhylated LINC01134 confers oxaliplatin resistance through SP1-induced p62 transcription in HCC. Hepatology 74(6):3213–3234. https://doi.org/10.1002/hep.32079
Ma WX, Zhou BY, Zhang JY, Meng LY, Gao F (2023) The research progress on the effects of active ingredients of Red Ginseng, lily and Rhizoma Anemarrhenae on immune function. Chin J Public Health Eng 22(02):282–286. https://doi.org/10.19937/j.issn.1671-4199.2023.02.044
McMahon M, Thomas N, Itoh K, Yamamoto M, Hayes JD (2006) Dimerization of substrate adaptors can facilitate cullin-mediated ubiquitylation of proteins by a tethering mechanism: a two-site interaction model for the Nrf2-Keap1 complex. J Biol Chem 281(34):24756–24768. https://doi.org/10.1074/jbc.M601119200
Meissner WG, Frasier M, Gasser T, Goetz CG, Lozano A, Piccini P et al (2011) Priorities in Parkinson’s disease research. Nat Rev Drug Discovery 10(5):377–393. https://doi.org/10.1038/nrd3430
Moffat JJ, Sakhai SA, Hoisington ZW, Ehinger Y, Ron D (2023) The BDNF Val68Met polymorphism causes a sex specific alcohol preference over social interaction and also acute tolerance to the anxiolytic effects of alcohol, a phenotype driven by malfunction of BDNF in the ventral hippocampus of male mice. Psychopharmacology 240(2):303–317. https://doi.org/10.1007/s00213-022-06305-3
Oh DR, Kim Y, Jo A, Choi EJ, Oh KN, Kim J, Kang H, Kim YR, Choi CY (2019) Sedative and hypnotic effects of Vaccinium bracteatum Thunb. through the regulation of serotonegic and GABAA-ergic systems: involvement of 5-HT1A receptor agonistic activity. Biomed Pharmacother 109:2218–2227. https://doi.org/10.1016/j.biopha.2018.10.003
Peng XJ, Yang XJ, Chen YB, Lu L, Xu HY, Xu HY et al (2018) Action mechanism of Baihe Dihuang decoction on depression based on integrative pharmacology of traditional Chinese medicine. China J Chin Materia Med 43(07):1338–1344. https://doi.org/10.19540/j.cnki.cjcmm.20180115.021
Prasertsuksri P, Kraokaew P, Pranweerapaiboon K, Sobhon P, Chaithirayanon K (2023) Neuroprotection of andrographolide against neurotoxin MPP+-Induced apoptosis in SH-SY5Y cells via activating mitophagy, autophagy, and antioxidant activities. Int J Mol Sci 24(10):8528. https://doi.org/10.3390/ijms24108528
Ran JF, Yu HY, Li QY et al (2022) Determination of dendrobium officinale polysaccharide by ultraviolet-visible spectrophotometry. Shandong Chem Ind 51(23):150–153. https://doi.org/10.19319/j.cnki.issn.1008-021x.2022.23.001
Rui W, Li S, Xiao H, Xiao M, Shi J (2020) Baicalein attenuates neuroinflammation by inhibiting NLRP3/caspase-1/GSDMD pathway in MPTP induced mice model of Parkinson’s disease. Int J Neuropsychopharmacol 23(11):762–773. https://doi.org/10.1093/ijnp/pyaa060
Sha R, Xu Y, Yuan C, Sheng X, Wu Z, Peng J et al (2021) Predictive and prognostic impact of ferroptosis-related genes ACSL4 and GPX4 on breast cancer treated with neoadjuvant chemotherapy. EBioMedicine 71:103560. https://doi.org/10.1016/j.ebiom.2021.103560
Sharma V, Bedi O, Gupta M, Deshmukh R (2022) Traditional herbs and remedies impacting pathogenesis of Parkinson’s disease. Naunyn Schmiedebergs Arch Pharmacol 395(5):495–513. https://doi.org/10.1007/s00210-022-02223-5
Shulman JM, De Jager PL, Feany MB (2011) Parkinson’s disease: genetics and pathogenesis. Annu Rev Pathol 6:193–222. https://doi.org/10.1146/annurev-pathol-011110-130242
Simon DK, Tanner CM, Brundin P (2019) Parkinson disease epidemiology, pathology, genetics, and pathophysiology. Clin Geriatr 36(1):1–12. https://doi.org/10.1016/j.cger.2019.08.002
Su Q, Wu P, Xia BH, Li YM, Lin Y, Lin LM, Liao DF (2021) Advance in research on chemical constituents and pharmacological activities of lilium. Chin Pharm J 56(11):875–882. https://doi.org/10.11669/cpj.2021.11.004
Sun J, Lin XM, Lu DH, Wang M, Li K, Li SR et al (2023) Midbrain dopamine oxidation links ubiquitination of glutathione peroxidase 4 to ferroptosis of dopaminergic neurons. J Clin Investig 133(10):e165228. https://doi.org/10.1172/JCI165228
Tang L, Zhao HQ, Yang H, Hu C, Ma SJ, Xiao WZ, Qing YH, Yang L, Zhou RR, Liu J, Zhang SH (2023) Spectrum-effect relationship combined with bioactivity evaluation to discover the main anxiolytic active components of Baihe Dihuang decoction. Ethnopharmacol 319(Pt1):117090. https://doi.org/10.1016/j.jep.2023.117090
Wang T, Huang H, Zhang Y, Li X, Li H, Jiang Q, Gao W (2015) Role of effective composition on antioxidant, anti-inflammatory, sedative-hypnotic capacities of 6 common edible Lilium varieties. J Food Sci 80(4):H857–H868. https://doi.org/10.1111/1750-3841.12787
Wang XM, Xu KY, Wu HR, Wei XX, Zhang HN, Li K (2020) Clinical application and dosage of lily bulb. J Changchun Univ Chin Med 36(04):637–639. https://doi.org/10.13463/j.cnki.cczyy.2020.04.008
Weng XJ, Tan Y, Chen MC, Gao XF (2023) Study on the active components and mechanism of Lilii bulbus in anti-anxiety and anti-depression. J Zhejiang Chin Med Univ 47(11):1243–1254. https://doi.org/10.16466/j.issn1005-5509.2023.11.002
Wu CH, Zhou XX, Xie M, Lin JS, Gao LL (2020) Expert consensus for TCM preventive treatment of diseases: Parkinson depression and/or anxiety. Chin J Inform Traditional Chin Med 27(01):1–5. https://doi.org/10.3969/j.issn.1005-5304.201909018
Wu H, Liu R, Wang J, Li T, Sun Y, Feng X, Bi Y, Zhang C, Sun Y (2021) Liquid chromatography-mass spectrometry in-depth analysis and in silico verification of the potential active ingredients of Baihe Dihuang decoction in vivo and in vitro. J Sep Sci 44(21):3933–3958. https://doi.org/10.1002/jssc.202100434
Yang YX, Wu CB, Yang BZ (2023) The effectiveness of pramipexole in improving neurological function in patients with Parkinson’s disease. China Foreign Med Treat 42(07):5–9. https://doi.org/10.16662/j.cnki.1674-0742.2023.07.005
Zhang H, Chu MH, Wang SQ, Li ZS, Song XL, Shi XR, Wang YL (2021) Protective effect of Xiangsha Yuyang decoction on oxidative injury in rats with gastric ulcer based on p62/Keap1/Nrf2 signal pathway. Chin J Experimental Traditional Med Formulae 27(04):56–63. https://doi.org/10.13422/j.cnki.syfjx.20210438
Zhang H, Jin L, Zhang JB, Niu T, Guo T, Chang J (2022) Chemical constituents from the bulbs of Lilium davidii var. Unicolor and anti-insomnia effect. Fitoterapia 161:105252. https://doi.org/10.1016/j.fitote.2022.105252
Zheng XJ, Wu QK, Wei ZQ, Xi YL, Dong HH (2016) Research progress on extraction methods of Chinese herbal medicine. J Jilin Med Univ 37(04):290–293. https://doi.org/10.13845/j.cnki.issn1673-2995.2016.04.022
Zhu Z, Yang C, Iyaswamy A, Krishnamoorthi S, Sreenivasmurthy SG, Liu J, Wang Z, Tong BC, Song J, Lu J, Cheung KH, Li M (2019) Balancing mTOR signaling and autophagy in the treatment of Parkinson’s disease. Int J Mol Sci 20(3):728. https://doi.org/10.3390/ijms20030728
Funding
2022 Ministry-provincial joint project “Zhang Zhongjing” Inheritance and Innovation Special Programme (GZY-KJS2022-048-3). Henan provincial Science and Technology Research and Development Programme Joint Fund Projects (222301420075).
Author information
Authors and Affiliations
Contributions
Chengcheng Hui and Tao Guo contributed to the study conception and design. Material preparation was performed by Jinghui Jin, Mengshan Ji, Yanpo Si Data collection and analysis were performed by Haibo Wang, Xiaowei Wang, Jianping Ma, Ya Wang, Suiqing Chen. The first draft of the manuscript was written by Chengcheng Hui and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Henan University of Traditional Chinese Medicine (Date2023.5/No. 202303014).
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hui, C., Jin, J., Ji, M. et al. Neuroprotective properties of the Lilium brownii extracts in the experimental model of Parkinson’s disease. Metab Brain Dis 39, 1085–1097 (2024). https://doi.org/10.1007/s11011-024-01397-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-024-01397-6