Abstract
The purpose of the study was to determine whether Aβ1–42 and p-Tau181 cerebral spinal fluid (CSF) levels can predict progression from amnestic mild cognitive impairment (aMCI) to Alzheimer’s disease dementia (ADD) in a 3-year follow-up study. All participants were evaluated blindly by a behavioral neurologist and a neuropsychologist, and classified according to the Petersen criteria for aMCI and according to the Clinical Dementia Rating (CDR) scale. Individuals were also submitted to lumbar puncture at baseline. Levels of Aβ1–42 and p-Tau181 were measured by immunoenzymatic assay. Values were adjusted for age and sex. Thirty-one of 33 (93.9%) participants completed follow-up. Approximately 39% of aMCI individuals progressed to ADD. The relative risk of developing ADD in those with Aβ1–42 CSF levels lower than 618.5 pg/mL was 17.4 times higher than in those whose levels were higher than 618.5 pg/mL (P = 0.003). p-Tau181 alone did not predict progression to ADD (P = 0.101). The relative risk in those with a p-Tau181/Aβ1–42 ratio higher than 0.135 was 5.7 times greater (P < 0.001). Aβ1–42 and p-Tau181 explained 40.1% of the verbal memory test subscore of the Consortium to Establish a Registry for Alzheimer’s Disease (ΔCERADs) variance (P = 0.008). Aβ1–42 strongly predicted progression from aMCI to ADD. p-Tau181 alone, or its relation to Aβ1–42, was inferior than Aβ1–42 alone as a predictor of progression to ADD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The correct identification of people at risk to develop Alzheimer’s disease dementia (ADD) is crucial in terms of early diagnosis (prodromal Alzheimer), prognosis, and treatment. Research efforts to improve the positive predictive value of progression from amnestic mild cognitive impairment (aMCI) to ADD are needed, since many trials on disease modifying drugs fail because of low progression rates. In this sense, efforts to correctly identify individuals with prodromal AD are of utmost importance (McGeer and McGeer 2013; Molinuevo et al. 2014). Even so, it is often difficult to identify who among those with aMCI will progress to ADD based solely on neuropsychological evaluations (Petersen et al. 2014).
Different biomarkers demonstrated to be useful diagnostic tools in vivo, and are now gaining attention in the clinical practice after being incorporated into research criteria for AD (Albert et al. 2011; Dubois et al. 2014; Morris et al. 2014). The combination of decreased concentrations of beta amyloid protein (Aβ1–42) and increased concentrations of hyperphosphorylated Tau protein (p-Tau181) in the CSF is related to AD pathology and may predict the progression to ADD (Jack et al. 2010, 2013).
Many studies have evaluated the value of CSF biomarkers in predict progression from MCI to ADD (Ferreira et al. 2014b; Prestia et al. 2015; Ritchie et al. 2014; Tondelli et al. 2015; Vos et al. 2013). However, no study has evaluated the relative risk to develop ADD in an exclusively amnestic MCI patient sample in a 3-year follow-up. This study consisted of measuring the concentrations of both CSF Aβ1–42 and p-Tau181 proteins in a sample of exclusive amnestic MCI subjects and analyzing to which extent these biomarkers could predict progression to ADD in a 3-year follow-up. The relationship and agreement of these biomarkers with neuropsychological tests were then verified.
Materials and Methods
Participants
Thirty-three subjects diagnosed with aMCI from a memory clinic in Southern Brazil were included in the study. All individuals were evaluated by a behavioral/geriatric neurologist and the National Institute on Aging-Alzheimer’s Association (NIA-AA) criteria for MCI (Albert et al. 2011), corroborated by the Clinical Dementia Rating scale (CDR) (Morris 1993). aMCI diagnoses were based on criteria by Petersen (2004) and Petersen et al. (2014). We excluded those who had diagnosis of dementia, stroke, Parkinson’s disease, depression or other neurological conditions that could possibly cause cognitive impairment. Furthermore, we also excluded individuals with normal cognition. A questionnaire was applied in order to obtain sociodemographic data.
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by the local research ethics committee and is in accordance with the Declaration of Helsinki. All participants gave their written consent in order to participate in this study.
CSF Analyses
All participants underwent a fasted lumbar puncture to measure Aβ1–42 and p-Tau181 proteins in the CSF. CSF (5 mL) was collected in polypropylene tubes and immediately centrifuged (4000g × 10 min) at 4 °C and then stored at − 80 °C until the assay. Commercially available ELISA kits were used to determine the levels of Aβ1–42 (Innotest β-amyloid1–42, Fujirebio-Europe, Gent, Belgium) and p-Tau181 (Innotest Phospho-Tau181P, Fujirebio-Europe). Tests were performed in duplicate and the laboratory technician was blinded to any clinical and demographic information about the participants.
Neuropsychological Tests
At baseline, participants were submitted to a battery of neuropsychological evaluations performed by a certified neuropsychologist. We utilized the validated adaptation for Brazilian patients version of the verbal memory test subscore of the Consortium to Establish a Registry for Alzheimer’s Disease (CERADs) (Morris et al. 1989; Bertolucci et al. 1998). The final score was composed by a possible sum of 30 points for word list learning, 10 points for word list recall, and 10 points for word list recognition (total of 50 possible points) (Chandler et al. 2005). The Geriatric Depression Scale (GDS) 15-item version (Yesavage et al. 1982), which was translated and validated to Brazilian Portuguese (Almeida and Almeida 1999), was utilized to screen for depressive symptoms.
After 3 years of follow-up, subjects were reassessed in order to verify whether or not they progressed to ADD. Evaluation was performed blindly by the same behavioral neurologist and neuropsychologist, and included a structured clinical interview, a full neurological examination, and neuropsychological evaluation. At this time, they were analyzed by NIA-AA criteria for ADD (McKhann et al. 2011), corroborated by the CDR, and were classified in two groups: progressors (P), and non-progressors (NP) to ADD. The neuropsychological tests, besides those performed at baseline, included the Montreal Cognitive Assessment (MoCA) (Memória et al. 2013; Nasreddine et al. 2005), Boston Naming Test (BNT) (Kaplan et al. 1983; Miotto et al. 2010), verbal fluency test, animals category (VFT) (Brucki et al. 1997; Isaacs and Kennie 1973) and Constructional Praxis (CP) (Bertolucci et al. 2001; Rosen et al. 1984), all translated and validated to Brazilian Portuguese. Instead of MMSE, we preferred to utilize MoCA because it has been shown to be more accurate in detecting MCI than MMSE (Trzepacz et al. 2015).
Statistical Analyses
Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) version 20. To compare sex distribution, a Chi-square test was performed. Mann–Whitney tests were performed to compare clinical, neuropsychological, and biomarker data. Receiver operating characteristic (ROC) curve analyses were used to obtain the area under the curve (AUC) and other parameters. The optimal cutoff values were determined by calculating the maximal sensitivity and specificity [i.e., maximizing the Youden index (Youden 1950)]. The Generalized Estimating Equation Model with the Bonferrroni post hoc test was applied to verify the mean of CERADs score between groups, discriminated by time. Linear regressions were performed to evaluate the relationship among ΔCERADs and CSF biomarkers. Values were adjusted for age and sex. All results were showed as confidence intervals CI 95% and P values < 0.05 were considered significant.
Results
Thirty-one of 33 (93.9%) participants completed the follow-up. Of these, twelve (38.4%) progressed to ADD after 3 years. Mean age was 68.1 ± 5.1 years and 67.75% were female. No participants deceased during the follow-up. Table 1 lists the demographic characteristics and baseline neuropsychological scores between P and NP. There was no significant difference between groups when baseline characteristics were analyzed.
Table 2 lists the neuropsychological scores after 3 years of follow-up and CSF biomarker features. Aβ1–42 concentrations in CSF were significantly different between groups (P < 0.001), as opposed to p-Tau181 concentrations (P = 0.101). The p-Tau181/Aβ1–42 ratio also displayed a significant difference between groups (P < 0.001). The CERADs and MoCA tests differed significantly at endpoint as well (P = 0.006 and P = 0.025, respectively).
In Table 3, cutoff values were determined by Youden’s index, which stretches the maximum potential effectiveness of a biomarker. For Aβ1–42, the threshold value was 618.5 pg/mL, with 91.7% sensitivity (CI 95% 64.6–98.5) and 94.7% specificity (CI 95% 75.4–99.0). At this cutoff level, 29 of 31 individuals (93.5%) were correctly classified in P or NP. The relative risk of developing ADD in those directly exposed (Aβ1–42 < 618.5 pg/mL) was 17.4 times higher than among those who were not exposed (CI 95% 2.5–118.2; P = 0.003). In other words, those who had values above this concentration had their risk of developing ADD reduced by 94.3%. The optimal cutoff value for the p-Tau181/Aβ1–42 ratio was 0.135, with 66.7% sensitivity (CI 95% 39.0–86.2) and 100% specificity (CI 95% 83.2–100.0). At this cutoff value, 27 of 31 individuals (87%) were correctly classified as P or NP. The relative risk of developing ADD in those whose p-Tau181/Aβ1–42 ratio was higher than 0.135 was 5.7 times greater when compared to those with a lower p-Tau181/Aβ1–42 ratio (CI 95% 2.3–14.0; P < 0.001).
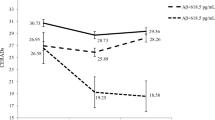
As measure of experimental validity, Fig. 1 displays the mean and standard errors of the CERADs score for each group discriminated by the baseline and follow-up times in years (baseline in 2013; follow-up in 2016). When the time was set and the groups were compared, there was no difference in the CERADs score of the NP group (P = 0.437), but there was a significant difference in the P group (P < 0.001). When the groups were fixed and times were compared, there was no difference between groups in 2013 (P = 0.575), but, in 2016, a significant difference between P and NP was observed (P = 0.004).
Line chart of the means and standard errors of the CERADs score of each group. There was an association between variables (P < 0.001). When the time was set and the groups were compared, there was no difference in the NP group (P = 0.437) but there was a significant difference in the P group (P < 0.001). When the groups were fixed and times were compared in 2013 (baseline), there was no difference between groups (P = 0.575). When times were compared in 2016 (follow-up), there was a statistical difference between groups (P = 0.004). P progressors, NP non-progressors, CERADs Consortium to Establish a Registry for Alzheimer’s Disease. Generalized Estimating Equation Model with the Bonferroni post hoc test was used
ΔCERADs was defined as the difference between CERADs score from 2016 to 2013. ΔCERADs was statistically different between P and NP groups (P = 0.002) and correlated with Aβ1–42 levels (P = 0.011), p-Tau181 levels (P = 0.045), and p-Tau181/Aβ1–42 ratio (P = 0.010). Aβ1–42 and p-Tau181 levels explained 40.1% of ΔCERADs variance (B: − 6.2; SE: 13.7; P = 0.008). As demonstrated in Fig. 2, Aβ1–42 alone explained 26.8% of ΔCERADs variance (P = 0.036), whereas p-Tau181 alone accounted for 27.5% of ΔCERADs variance (P = 0.032).
Discussion
In this study, 38.7% of aMCI individuals progressed to ADD, a rate which is consistent with previous studies (Schjønning Nielsen et al. 2016). CSF concentration of Aβ1–42 was significantly diminished in the P group (P < 0.001), whereas there was no difference in p-Tau181 levels between groups (P = 0.101). These results are in line with the model of dynamic pathophysiological biomarkers in AD (Jack et al. 2013), in which Aβ1–42 levels in the CSF becomes abnormal 5–10 years or before the diagnosis of dementia. A meta-analysis of fifty studies confirmed that Aβ1–42 is a good biomarker for discriminating ADD from MCI and other dementias (Ferreira et al. 2014a). Moreover, our group recently demonstrated that Aβ1–42, but not p-Tau181, is significantly associated with aMCI (Rizzi et al. 2018). Previous studies agree that p-Tau181 levels in the CSF become progressively abnormal, and have better predictive power only 0–5 years before progression to ADD (Buchhave et al. 2012). The p-Tau181/Aβ1–42 ratio also showed significant difference (P < 0.001) between patients that progressed to ADD versus those who did not. The literature supports the evidence that the combination of Aβ1–42 and p-Tau181 protein levels is a suitable measure for MCI prognosis (Ferreira et al. 2014a).
In the CSF, the Aβ1–42 concentration displayed a cutoff value of 618.5 pg/mL for aMCI cases, with greater sensitivity and specificity when compared to similar reports in the literature (Ferreira et al. 2014a; Ritchie et al. 2014). From this value, we can infer that the relative risk to develop ADD in those whose CSF Aβ1–42 levels were lower than 618.5 pg/mL was about 17.4 times higher than in those whose level was above this value. Other studies described different values associated with the relative risk of developing ADD from MCI. Michaud et al. (2015) found a hazard ratio of developing ADD among MCI patients with high-risk biomarker levels about 4 times greater than in MCI patients with low-risk biomarker values. Tondelli et al. (2015) established that Aβ1–42 was helpful to discriminate between MCI and dementia, with a relative risk of 1.01 in MCI patients. Here, we emphasize that our cutoff value was specific for amnestic MCI patients, even though our sample size was small. The interpretation of any comparative results should be made with caution due to the heterogeneity of the population that was evaluated in this study.
For the p-Tau181/Aβ1–42 ratio in CSF, the optimal cutoff value was 0.135, with less sensitivity than Aβ1–42 alone, but with 100% of specificity. The relative risk of developing ADD in those whose ratio was higher than 0.135 was 5.7 times greater than in those whose ration was lower than 0.135. Michaud and colleagues found that Aβ1–42 and p-Tau181 were the best combination among CSF biomarkers to predict the overall risk of developing ADD among MCI patients with an area under the curve of 0.77 (Michaud et al. 2015). In our study, Aβ1–42 alone (AUC: 0.961) performed better than the p-Tau181/Aβ1–42 ratio (AUC: 0.864) in predicting overall risk, but the combination of the two proteins showed a better positive predictive value (100%). Prestia et al. found that the best predictive accuracy was achieved by combinations of amyloidosis and neurodegeneration biomarkers (Prestia et al. 2015). Moreover, in a recent meta-review (Ferreira et al. 2014a), it was found that the p-Tau181/Aβ1–42 ratio was the most accurate CSF measure in predicting ADD progression from MCI. Our finding that Aβ1–42 levels alone is a superior, more accurate, measure when compared to the p-Tau181/Aβ1–42 ratio needs to be confirmed in further studies with larger samples sizes and a more heterogeneous population.
Although several different possible cutoff values were proposed, there is a lack of agreement on which would be the best discriminant value. This controversy might be due to the variability in CSF measurements between laboratories that utilize different techniques (Samtani et al. 2013; Tang et al. 2014). We decided to find the best cutoff values for our sample by utilizing the Youden test (1950), differently from other reports in which the threshold values were based on laboratory cutoff points that were based on different populations and methodologies (Ritchie et al. 2014). For instance, difficulties in predicting MCI progression to ADD could be influenced by the intrinsic heterogeneity of MCI individuals.
After 3 years of follow-up, only CERADs and MoCA tests reflected differences between the P and NP groups. These results agree with our baseline selection of exclusively aMCI cases, since episodic memory is the major cognitive domain impaired in this subtype of MCI patients (Gifford et al. 2015). There was no significant difference in the GDS test, although some patients who did not progress to ADD presented depressive symptoms after 3 years.
The CERADs test was performed at baseline and again after 3 years. As shown in Fig. 1, there was no difference between groups at baseline, but over time a significant decline in the episodic memory of those who progressed to ADD was observed. This pattern was not verified in the NP group, which performed similarly at baseline and follow-up. Additionally, ΔCERADs correlated with the two proteins alone, besides correlating with their combination ratio. This finding reinforces the early and specific involvement of episodic memory in the prodromal phase of AD. Haldenwanger et al. (2010) already emphasized the existence of a significant correlation between Aβ1–42 in the CSF and memory performance for aMCI patients, but not for non-amnesic MCI. Rami and collaborators also suggested that memory performance is first related with Aβ1–42 levels and then with t-Tau or p-Tau181 (Rami et al. 2011). Moreover, a recent study suggested that, in particular, Aβ1–42 protein is associated with a delayed memory performance CERAD (Haapalinna et al. 2016), agreeing with our results. Aβ1–42 and p-Tau181 levels explained 40.1% of ΔCERADs variance, better than each one alone.
Often, MCI represents an intermediate stage between normal cognition and dementia (Petersen et al. 2014). In 1 year, about 10–15% of MCI patients develop ADD. In 5 years, this number can raise up to 40–60% (Schjønning Nielsen et al. 2016; Tondelli et al. 2015). To our knowledge, this is the first study that evaluated the relative risk of both Aβ1–42 and p-Tau181/Aβ1–42 ratio in predicting progression from aMCI to ADD in an exclusively amnestic MCI sample, in a 3-year follow-up.
An important limitation of our study is the relatively small sample size. Another possible methodologic drawback is the absence of controls. Nonetheless, in this study, we did not aim to evaluate differences between individuals with aMCI and controls, but, instead, to analyze the role of CSF Aβ1–42 and p-Tau181 in predicting progression from aMCI to ADD. In another words, we tried to answer a common question that physicians find in everyday practice: what is the predictive value of analyzing Aβ1–42 and p-Tau181 in the CSF of individuals with aMCI?
Conclusion
Taken together, our results suggest that the CSF biomarkers analyzed herein provide predictive information about progression from aMCI to ADD. Aβ1–42 strongly predicted progression from aMCI to ADD, whereas p-Tau181 alone or its relation to Aβ1–42 was no better than Aβ1–42 alone as a progression to ADD predictor. However, the CSF p-Tau181/Aβ1–42 ratio did improve the specificity and negative predictive value as compared to CSF Aβ1–42 levels alone.
References
Albert, M. S., DeKosky, S. T., Dickson, D., Dubois, B., Feldman, H. H., Fox, N. C., Gamst, A., Holtzman, D. M., Jagust, W. J., Petersen, R. C., Snyder, P. J., Carrillo, M. C., Thies, B., & Phelps, C. H. (2011). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement, 7(3), 270–279.
Almeida, O., & Almeida, S. (1999). Confiabilidade da versão brasileira da Escala de Depressão em Geriatria (GDS) versão reduzida. Arq Neuro-Psiquiatr, 57, 421–426.
Bertolucci, P. H., Okamoto, I. H., Brucki, S. M., Siviero, M. O., Toniolo Neto, J., & Ramos, L. R. (2001). Applicability of the CERAD neuropsychological battery to Brazilian elderly. Arq Neuropsiquiatr, 59(3-A), 532–536.
Bertolucci, P. H. F., Okamoto, I. H., Toniolo Neto, J., Ramos, L. R., & Brucki, S. M. D. 1998. Desempenho da populacao Brasileira na bateria neuropsicologica do Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Revista de Psiquiatria Clínica, 25, 80–83.
Brucki, S. M., Malheiros, S. M., Okamoto, I. H., & Bertolucci, P. H. (1997). Normative data on the verbal fluency test in the animal category in our milieu. Arquivos de Neuro-psiquiatria, 55(1), 56–61.
Buchhave, P., Minthon, L., Zetterberg, H., Wallin, A. K., Blennow, K., & Hansson, O. (2012). Cerebrospinal fluid levels of β-amyloid 1–42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia. Archives of General Psychiatry, 69(1), 98–106.
Chandler, M. J., Lacritz, L. H., Hynan, L. S., Barnard, H. D., Allen, G., Deschner, M., Weiner, M. F., & Cullum, C. M. (2005). A total score for the CERAD neuropsychological battery. Neurology, 65(1), 102–106.
Dubois, B., Feldman, H. H., Jacova, C., Hampel, H., Molinuevo, J. L., Blennow, K., DeKosky, S. T., Gauthier, S., Selkoe, D., Bateman, R., Cappa, S., Crutch, S., Engelborghs, S., Frisoni, G. B., Fox, N. C., Galasko, D., Habert, M. O., Jicha, G. A., Nordberg, A., Pasquier, F., Rabinovici, G., Robert, P., Rowe, C., Salloway, S., Sarazin, M., Epelbaum, S., de Souza, L. C., Vellas, B., Visser, P. J., Schneider, L., Stern, Y., Scheltens, P., & Cummings, J. L. (2014). Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. The Lancet Neurology, 13(6), 614–629.
Ferreira, D., Perestelo-Pérez, L., Westman, E., Wahlund, L. O., Sarría, A., & Serrano-Aguilar, P. (2014a). Meta-review of CSF core biomarkers in Alzheimer’s disease: The state-of-the-art after the new revised diagnostic criteria. Frontiers in Aging Neuroscience, 6, 47.
Ferreira, D., Rivero-Santana, A., Perestelo-Pérez, L., Westman, E., Wahlund, L. O., Sarría, A., & Serrano-Aguilar, P. (2014b). Improving CSF biomarkers’ performance for predicting progression from mild cognitive impairment to Alzheimer’s disease by considering different confounding factors: A meta-analysis. Frontiers in Aging Neuroscience, 6, 287.
Gifford, K. A., Liu, D., Damon, S. M., Chapman, W. G., Iii, R., Samuels, R. R., Lu, L. R., Jefferson, Z., Initiative, A. L., A.s.D.N (2015). Subjective memory complaint only relates to verbal episodic memory performance in mild cognitive impairment. Journal of Alzheimer’s Disease, 44(1), 309–318.
Haapalinna, F., Paajanen, T., Penttinen, J., Kokki, H., Kokki, M., Koivisto, A. M., Hartikainen, P., Solje, E., Hänninen, T., Remes, A. M., & Herukka, S. K. (2016). Low cerebrospinal fluid amyloid-beta concentration is associated with poorer delayed memory recall in women. Dementia and Geriatric Cognitive Disorders Extra, 6(2), 303–312.
Haldenwanger, A., Eling, P., Kastrup, A., & Hildebrandt, H. (2010). Correlation between cognitive impairment and CSF biomarkers in amnesic MCI, non-amnesic MCI, and Alzheimer’s disease. Journal of Alzheimer’s Disease, 22(3), 971–980.
Isaacs, B., & Kennie, A. T. (1973). The Set test as an aid to the detection of dementia in old people. The British Journal of Psychiatry, 123(575), 467–470.
Jack, C. R., Knopman, D. S., Jagust, W. J., Petersen, R. C., Weiner, M. W., Aisen, P. S., Shaw, L. M., Vemuri, P., Wiste, H. J., Weigand, S. D., Lesnick, T. G., Pankratz, V. S., Donohue, M. C., & Trojanowski, J. Q. (2013). Tracking pathophysiological processes in Alzheimer’s disease: An updated hypothetical model of dynamic biomarkers. The Lancet Neurology, 12(2), 207–216.
Jack, C. R., Knopman, D. S., Jagust, W. J., Shaw, L. M., Aisen, P. S., Weiner, M. W., Petersen, R. C., & Trojanowski, J. Q. (2010). Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. The Lancet Neurology, 9(1), 119–128.
Kaplan, E., Goodglass, H., & Weintraub, S. (1983). Boston naming test. Philadelphia: Lea & Febiger.
McGeer, P. L., & McGeer, E. G. (2013). The amyloid cascade-inflammatory hypothesis of Alzheimer disease: Implications for therapy. Acta Neuropathology, 126(4), 479–497.
McKhann, G. M., Knopman, D. S., Chertkow, H., Hyman, B. T., Jack, C. R., Kawas, C. H., Klunk, W. E., Koroshetz, W. J., Manly, J. J., Mayeux, R., Mohs, R. C., Morris, J. C., Rossor, M. N., Scheltens, P., Carrillo, M. C., Thies, B., Weintraub, S., & Phelps, C. H. (2011). The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s and Dementia, 7(3), 263–269.
Memória, C. M., Yassuda, M. S., Nakano, E. Y., & Forlenza, O. V. (2013). Brief screening for mild cognitive impairment: Validation of the Brazilian version of the Montreal cognitive assessment. International Journal of Geriatric Psychiatry, 28(1), 34–40.
Michaud, T. L., Kane, R. L., McCarten, J. R., Gaugler, J. E., Nyman, J. A., Kuntz, K. M., & Initiative, A. S. D. N. (2015). Risk stratification using cerebrospinal fluid biomarkers in patients with mild cognitive impairment: An exploratory analysis. Journal of Alzheimer’s Disease, 47(3), 729–740.
Miotto, E. C., Sato, J., Lucia, M. C., Camargo, C. H., & Scaff, M. (2010). Development of an adapted version of the Boston Naming Test for Portuguese speakers. Revista Brasileira de Psiquiatria, 32(3), 279–282.
Molinuevo, J. L., Blennow, K., Dubois, B., Engelborghs, S., Lewczuk, P., Perret-Liaudet, A., Teunissen, C. E., & Parnetti, L. (2014). The clinical use of cerebrospinal fluid biomarker testing for Alzheimer’s disease diagnosis: A consensus paper from the Alzheimer’s Biomarkers Standardization Initiative. Alzheimer’s & Dementia, 10, 808–817
Morris, J. C. (1993). The clinical dementia rating (CDR): Current version and scoring rules. Neurology, 43(11), 2412–2414.
Morris, J. C., Blennow, K., Froelich, L., Nordberg, A., Soininen, H., Waldemar, G., Wahlund, L. O., & Dubois, B. (2014). Harmonized diagnostic criteria for Alzheimer’s disease: Recommendations. Journal of Internal Medicine, 275(3), 204–213.
Morris, J. C., Heyman, A., Mohs, R. C., Hughes, J. P., van Belle, G., Fillenbaum, G., Mellits, E. D., & Clark, C. (1989). The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology, 39(9), 1159–1165.
Nasreddine, Z. S., Phillips, N. A., Bédirian, V., Charbonneau, S., Whitehead, V., Collin, I., Cummings, J. L., & Chertkow, H. (2005). The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatric Society, 53(4), 695–699.
Petersen, R. C. (2004). Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine, 256(3), 183–194.
Petersen, R. C., Caracciolo, B., Brayne, C., Gauthier, S., Jelic, V., & Fratiglioni, L. (2014). Mild cognitive impairment: A concept in evolution. Journal of Internal Medicine, 275(3), 214–228.
Prestia, A., Caroli, A., Wade, S. K., van der Flier, W. M., Ossenkoppele, R., Van Berckel, B., Barkhof, F., Teunissen, C. E., Wall, A., Carter, S. F., Schöll, M., Choo, I. H., Nordberg, A., Scheltens, P., & Frisoni, G. B. (2015). Prediction of AD dementia by biomarkers following the NIA-AA and IWG diagnostic criteria in MCI patients from three European memory clinics. Alzheimer’s & Dementia, 11(10), 1191–1201.
Rami, L., Fortea, J., Bosch, B., Solé-Padullés, C., Lladó, A., Iranzo, A., Sánchez-Valle, R., & Molinuevo, J. L. (2011). Cerebrospinal fluid biomarkers and memory present distinct associations along the continuum from healthy subjects to AD patients. Journal of Alzheimer’s Disease, 23(2), 319–326.
Ritchie, C., Smailagic, N., Noel-Storr, A. H., Takwoingi, Y., Flicker, L., Mason, S. E., & McShane, R. 2014. Plasma and cerebrospinal fluid amyloid beta for the diagnosis of Alzheimer’s disease dementia and other dementias in people with mild cognitive impairment (MCI). Cochrane Database System Review, 6, CD008782.
Rizzi, L., Maria Portal, M., Batista, C. E. A., Missiaggia, L., & Roriz-Cruz, M. (2018). CSF Aβ1–42, but not p-Tau181, differentiates aMCI from SCI. Brain Research, 1678, 27–31.
Rosen, W. G., Mohs, R. C., & Davis, K. L. (1984). A new rating scale for Alzheimer’s disease. American Journal of Psychiatry, 141(11), 1356–1364.
Samtani, M. N., Raghavan, N., Shi, Y., Novak, G., Farnum, M., Lobanov, V., Schultz, T., Yang, E., DiBernardo, A., Narayan, V. A., & Initiative, A. s. D. N. (2013). Disease progression model in subjects with mild cognitive impairment from the Alzheimer’s disease neuroimaging initiative: CSF biomarkers predict population subtypes. The British Journal of Clinical Pharmacology, 75(1), 146–161.
Schjønning Nielsen, M., Simonsen, A. H., Siersma, V., Hasselbalch, S. G., & Høgh, P. (2016). Are CSF biomarkers useful as prognostic indicators in diagnostically unresolved cognitively impaired patients in a normal clinical setting. Dementia and Geriatric Cognitive Disorders Extra, 6(3), 465–476.
Tang, W., Huang, Q., Wang, Y., Wang, Z. Y., & Yao, Y. Y. (2014). Assessment of CSF Aβ42 as an aid to discriminating Alzheimer’s disease from other dementias and mild cognitive impairment: A meta-analysis of 50 studies. Journal of Neurology Science, 345(1–2), 26–36.
Tondelli, M., Bedin, R., Chiari, A., Molinari, M. A., Bonifacio, G., Lelli, N., Trenti, T., & Nichelli, P. (2015). Role of cerebrospinal fluid biomarkers to predict conversion to dementia in patients with mild cognitive impairment: A clinical cohort study. Clinical Chemistry Laboratory Medicine, 53(3), 453–460.
Trzepacz, P. T., Hochstetler, H., Wang, S., Walker, B., Saykin, A. J., & Initiative, A. S. D. N. (2015). Relationship between the Montreal Cognitive Assessment and Mini-mental State Examination for assessment of mild cognitive impairment in older adults. BMC Geriatric, 15, 107.
Vos, S. J., van Rossum, I. A., Verhey, F., Knol, D. L., Soininen, H., Wahlund, L. O., Hampel, H., Tsolaki, M., Minthon, L., Frisoni, G. B., Froelich, L., Nobili, F., van der Flier, W., Blennow, K., Wolz, R., Scheltens, P., & Visser, P. J. (2013). Prediction of Alzheimer disease in subjects with amnestic and nonamnestic MCI. Neurology 80(12), 1124–1132.
Yesavage, J. A., Brink, T. L., Rose, T. L., Lum, O., Huang, V., Adey, M., & Leirer, V. O. (1982). Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research, 17(1), 37–49.
Youden, W. J. (1950). Index for rating diagnostic tests. Cancer, 3(1), 32–35.
Acknowledgements
This study was supported by CAPES-CNPQ (Grant Number: 476387/2013-2) and FIPE (Process: 13-0009). All authors declare that their funding source had no role in the study design, in the collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the paper for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors hereby declare that there are no actual or potential conflicts of interest that may have affected the discussion presented.
Rights and permissions
About this article
Cite this article
Rizzi, L., Missiaggia, L. & Roriz-Cruz, M. CSF Aβ1–42, but not p-Tau181, Predicted Progression from Amnestic MCI to Alzheimer’s Disease Dementia. Neuromol Med 20, 491–497 (2018). https://doi.org/10.1007/s12017-018-8516-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-018-8516-8