Abstract
Dermatomyositis (DM) is a common idiopathic inflammatory myopathy. The pathogenesis is considered to be microangiopathy affecting skin and muscle. The cutaneous manifestations of DM are the most important aspect of this disease, and their correct evaluation is important for early diagnosis. The skin signs are various: Some are pathognomonic or highly characteristic, and others are compatible with DM. Recently, DM has been categorized into several disease subsets based on the various autoantibodies present in patients. Sometimes, characteristic cutaneous manifestations are strongly associated with the presence of specific autoantibodies. For example, anti-Mi-2 antibody is associated with the classic features of DM, including heliotrope rash, Gottron’s papules, the V-neck sign, the shawl sign, cuticular overgrowth, and photosensitivity. Frequent cutaneous features in anti-transcriptional intermediary factor 1 gamma (TIF1γ)-positive patients are diffuse photoerythema, including “dusky red face,” while skin ulcerations, palmar papules (inverse Gottron), diffuse hair loss, panniculitis, and oral pain and/or ulcers are sometimes associated with anti-melanoma differentiation-associated gene 5 product (MDA5) antibody. Here, we review important cutaneous manifestations seen in patients with DM, and we examine the relationship between the skin changes and myositis-associated autoantibodies. Correct evaluation of cutaneous manifestations and myositis-associated autoantibodies should help the clinician in the early diagnosis of DM, for a quick recognition of cutaneous signs that may be the symptom of onset before muscle inflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dermatomyositis (DM) is an idiopathic inflammatory myopathy with characteristic cutaneous lesions. Some patients manifest the skin disease but without clinical muscle symptoms, a condition called amyopathic DM. Although the etiology remains unknown, autoantibodies to various cellular constituents are found in sera from patients with DM such as systemic lupus erythematosus (SLE) and systemic scleroderma (SSc) [1, 2]. Recent studies on myositis-specific autoantibodies (MSAs) and myositis-associated autoantibodies (MAAs) have clarified that they are useful tools for identifying clinical subsets of patients with DM [3, 4]. Since some MSAs are not always specific to myositis but are sometimes associated with respiratory and/or dermatological disease, the recent review recommends that we regard all recently established autoantibodies as MAAs instead of distinguishing between MSAs and MAAs [5].
Although myopathy is required as a finding for diagnosis of DM according to conventional criteria [6–9], DM can be diagnosed by accurate evaluation of the cutaneous manifestations. This review article discusses the cutaneous signs of DM and their relationship to MAAs.
Cutaneous Manifestations of Dermatomyositis
The main cutaneous symptom of DM is erythema, although edema is also visible at lesion sites under slight skin tension and hyperkeratotic changes are discernable at skin sites that receive external irritation. We divide the cutaneous manifestations into those that are pathognomonic/highly suggestive and those that are characteristic/compatible with DM. Even for the former kind of presentation, only around 60∼75 % of patients with DM had such eruptions in a Japanese multicenter study [8]. The important strategy for accurate evaluation is (1) to comprehensively recognize the simultaneous presence of multiple skin symptoms in a patient and (2) to confirm the pathological findings of skin biopsy specimens.
Highly Diagnostic, Frequently Observed Skin Lesions of Dermatomyositis
Heliotrope Rash
Heliotrope rash is periorbital erythema that is often accompanied with symmetrically distributed edema (Fig. 1a, b). The upper eyelid is mostly involved, possibly from the stimulus of blinking. In very rare cases, only edema with little color change is seen or the erythema is asymmetrical. Heliotrope rash and periorbital erythema can be seen in many other conditions, including systemic lupus erythematosus (Table 1).
Gottron Papules and Gottron Sign
Gottron papules and Gottron sign are papules and erythema, respectively, at the joint extensor. They are erythematous-to-violaceous plaques often overlying the dorsolateral aspect of metacarpophalangeal joints, proximal interphalangeal joints, and/or distal interphalangeal joints (Fig. 1c–e). Gottron sign can be seen also over bony prominences, such as the elbows, the knees, and, less commonly, the medial malleoli (Fig. 2g, h). According to Dr. R. D. Sontheimer, since “Gottron sign” was originally used to describe acral skin changes of familial acrogeria, “Gottron sign of DM” is a more accurate designation of the lesions addressed in the current review [10]. The points of differential diagnosis of Gottron papules/sign are listed in Table 2.
Occasional Observed Skin Lesions of Dermatomyositis
Facial Erythema
Facial erythema in DM is frequently seen at the malar area, the forehead, the temples, the medial angle areas of eye and around the nose, and the preauricular area (Fig. 1f, g). Basically, the nasolabial folds are spared. The distribution sometimes resembles that of seborrheic dermatitis. The auricle is sometimes targeted at the helix (Fig. 1h).
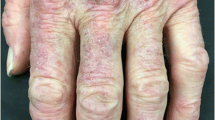
Periungual Changes
Periungual areas show erythema, dilated capillary loops, cuticular hemorrhages, and/or cuticular overgrowth (Fig. 1i, j), which can be also seen in patients with SSc and, rarely, in patients with SLE. As in SSc, the severity of telangiectasia and vessel dropout reflects the ongoing disease activity of DM, particularly in the skin [11].
Inverse Gottron Papules (Sign)
Papules or erythema overlying the palmar surfaces of the hand joints are less commonly seen (Fig. 1k, l) [12]. In a Japanese study, such eruptions were reported to be associated with acute/subacute interstitial lung disease (ILD) [13].
Mechanic’s Hands
Mechanic’s hands were reported to be the most characteristic cutaneous marker of anti-synthetase syndrome and consist of hyperkeratosis, scaling, and fissuring of the fingers [14, 15]. The lesions are distributed along the ulnar aspect of the thumb and radial aspect of the fingers and are most prominent on the index and middle fingers, with infrequent extension to the palm (Fig. 1m, n). Although mechanic’s hands are found commonly in the setting of anti-synthetase syndrome, they are also seen in cases of polymyositis (PM), classic DM, and amyopathic DM not associated with anti-synthetase antibody [10], according to previous reports and our experience.
V-Neck Sign
Violaceous erythema is sometimes seen in the V area of the neck and the upper chest, probably caused by photosensitivity. Approximately 50 % of patients with DM experience photosensitivity [16], and severe photosensitivity may cause erythroderma. With long-standing inflammatory periods, poikilodermatous changes commonly develop in this area (Fig. 2a, b).
Shawl Sign
Shawl sign is symmetrical violaceous erythema that extends across the nape of the neck to the posterior aspects of the shoulders and upper back (Fig. 2d).
Flagellate Erythema
The trunk should also be investigated when DM is suspected. Centripetal flagellate erythema, patchy violaceous erythema, and/or poikilodermatous changes appear less often on the back, lateral chest, and/or upper buttocks (Fig. 2c, d). Such manifestations can be seen in adult-onset Still’s disease, bleomycin-induced eruptions, and dermatitis from ingestion of shiitake mushrooms.
Vesiculo-Bullous Eruptions
Multiloculated vesicles or bullous lesions can develop, rarely, on the dorsal surfaces of the hands or forearms and on other areas (Fig. 2e). Such eruptions may be caused by liquefaction degeneration of the epidermis, subepidermal edema with mucin deposition in the upper dermis or complications from autoimmune bullous diseases. Although a Japanese study showed the incidence of internal malignancy to be much higher in patients with vesiculo-bullous DM than in patients without bullous lesions [17], no such statistical analysis has been reported since then.
Purpura
Purpura is very rarely found in Gottron sign lying over bony prominences or other lesions (Fig. 2f).
Alopecia
Mild to moderate nonscarring diffuse alopecia of the scalp in DM is relatively common (Fig. 3a, b). Even after the disease activity diminishes, the alopecia may persist.
Panniculitis
Erythematous, firm, tender areas of panniculitis develop on some patients with DM. Some lesions are followed by calcification (Fig. 3c, d). They often occur on the buttocks and upper thighs. Lipodystrophy is also known to have a strong association with juvenile-onset DM (JDM). It can be regarded as a result of longstanding panniculitis [18].
Calcinosis Cutis
Calcification frequently occurs in areas of panniculitis or in poikilodermatous areas and increases over the course of months or years (Fig. 3c, d). Widespread or joint-site calcification sometimes causes functional problems and severe pain. Extrusion of calcium through the skin is associated with ulceration and infection. Calcinosis, a major cause of morbidity in patients with JDM, occurs in up to 30 % of cases [19].
Skin Ulcers
Skin ulcers are associated with cutaneous vasculitis or with vascular damage due to panniculitis/calcification. Some ulcers occur at the site of Gottron sign (Fig. 3e, f), and some occur on the buttocks and thighs. Figure 3g shows skin ulcers of holster sign: Violaceous erythema underlies the general area where a pistol holster is worn at a belt on the waist.
Poikiloderma
Poikiloderma is circumscribed violaceous erythema containing telangiectasia, hypopigmentation, hyperpigmentation, and superficial atrophy (Fig. 3h). This disease is commonly found on the posterior shoulder, upper arm, back, buttocks, and V-neck area.
Pruritus
Severe pruritus is sometimes present, especially in the early course of the skin lesions from DM. It is often resistant to therapies of oral antihistamines and corticosteroid ointments.
Pathological Findings of Dermatomyositis Skin Biopsy
In the acute phase of dermatomyositis, the skin histological changes are indistinguishable from lupus erythematosus. The erythematous eruption shows slight hyperkeratosis and epidermal atrophy with effacement of the rate ridge (Fig. 4a). Basal cell liquefactive degeneration is typical, and cytoid bodies are sometimes found. There is upper dermal edema, and melanophages can be seen. Perivascular lymphocytic infiltration of activated T-lymphocytes and macrophages is present. Mucin deposits, which occur in the dermis, although not diagnostic, are suggestive of dermatomyositis.
Gottron papules are characterized by hyperkeratosis, acanthosis, and mild papillomatosis (Fig. 4b). The changes of interface dermatitis are seen as described above. Direct immunofluorescence of lesional skin may reveal granular deposits of immunoglobulin and complement at the dermal-epidermal junction. Magro and Crowson demonstrated C5b-9 deposition in blood vessel walls and at the dermal-epidermal junction in conjunction with a negative lupus band test with high specificity (94 %) and sensitivity (79 %) against lupus [20].
Cutaneous Manifestation of Dermatomyositis and Myositis-Associated Autoantibodies
Although elucidation of the association between MAAs and types of cutaneous manifestations would be useful for evaluating the etiology of MAAs, a strict association has rarely been established. There have not been many large cohort studies. The diverse results of the previous studies might also be due to racial differences or clinical backgrounds.
Anti-Mi-2 Antibody
The previous studies demonstrated that the anti-Mi-2 antibody is associated with the classic features of DM, including heliotrope rash, Gottron’s papules, the V-neck sign, the shawl sign, cuticular overgrowth, and photosensitivity [21–23]. A recent Japanese study showed that nailfold punctate hemorrhages are more frequently seen in patients with anti-Mi-2 (89 %) than in patients with anti-melanoma differentiation-associated gene 5 product (MDA5) (37 %) or anti-transcriptional intermediary factor 1 gamma (TIF1γ)/α (44 %) [24]. A recent Brazilian study showed that anti-Mi-2-positive DM patients are significantly more likely to have photosensitivity and the shawl sign by univariate analysis but that, by multivariate analysis, the anti-Mi-2 antibody was associated only with photosensitivity [25]. According to a North American collaborative study, JDM patients with the anti-Mi-2 antibody showed the V-neck sign, the shawl sign, and cuticular overgrowth significantly less than adult DM patients did [26].
Anti-ARS Antibody
Although mechanic’s hands are a well-known cutaneous sign associated with the anti-aminoacyl-tRNA synthetase (ARS) antibody [27], this sign can also be seen in various cases not associated with anti-ARS described above. Juvenile-onset patients with anti-ARS show mechanic’s hands less frequently than adult-onset patients (32 vs 71 %) [26].
Hamaguchi et al. investigated the clinical features of adult Japanese patients with six different kinds of anti-ARS [28]. Mechanic’s hands were shown in patients with anti-Jo-1 with the highest frequency (56 %), and the prevalence was significantly higher than in patients with anti-PL-12 (22 %), anti-KS (23 %), and anti-EJ (29 %). Heliotrope rash was found in 16 % patients with anti-ARS. Such manifestation was found predominantly in patients with anti-PL-7 (38 %) and anti-EJ (21 %). These frequencies were higher than those for patients with anti-Jo-1 (7 %). Gottron’s sign (hand, elbow, and/or knee) was found in 27–45 % in patients with anti-EJ, anti-PL-7, anti-PL-12, and anti-Jo-1, whereas this manifestation and heliotrope rash were rarely found in patients with anti-KS or anti-OJ, possibly due to the very small number of DM patients with anti-KS or anti-OJ.
Anti-TIF1γ/α Antibody (Anti-155/140 Antibody)
Anti-155/140 antibodies, which were originally nabbed by Kaji et al., are identical to anti-p155 antibodies [29, 30]. Autoantibodies against a 155 kDa polypeptide and a 140 kDa polypeptide target TIF1γ and TIF1α, respectively [31]. Anti-155/140 antibodies always recognize TIF1γ and sometimes recognize TIF1α. Although it remains obscure as to whether all anti-p155 antibodies recognize only p155 (TIF1γ) in published reports, I would like to discuss both studies on anti-155/140 antibodies and on anti-p155 antibodies simultaneously here.
In a JDM study, patients with these antibodies had significantly more cutaneous involvement, including Gottron’s papules (P = 0.003), skin ulcers (P = 0.005), and subcutaneous edema (P = 0.013) in a UK cohort [32]. In a US cohort, generalized lipodystrophy was reported to be associated with these antibodies [33]. In a subsequent report of US cohort, anti-TIF1γ/α antibody-positive patients showed classic JDM features, including Gottron papules, heliotrope and malar rashes, periungual abnormalities, photosensitivity, linear extensor erythema, cuticular overgrowth, V-neck sign, shawl sign, lipodystrophy, and erythroderma [26].
Fiorentino et al. analyzed cutaneous manifestations in patients with anti-TIF1γ in detail [34]. Significantly, frequent cutaneous features in the antibody-positive patients were diffuse photoerythema, including scalp rash, facial rash, V-neck rash and back rash, holster sign, and palmar papules. These palmar papules were hyperkeratotic and verruca-like papules, which were different from anti-MDA5-associated palmar papules, which were erythematous and often tender. The skin disease severity scored using the Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI) was significantly higher in the anti-TIF1γ-positive patients than in the anti-TIF1γ-negative patients.
We call the red face in DM patients with the anti-TIF1γ antibody “dusky red face” (Fig. 5). In such patients, a few cases develop erythroderma, which is not always associated with internal malignancy.
Anti-TIF1α Antibody
Since the anti-TIF1α antibody appears simultaneously with anti-TIF1γ antibodies, the specific clinical association of anti-TIF1α antibody should be considered by comparing patient groups with anti-TIF1α/γ antibody to those with anti-TIF1γ antibody alone. According to such a study performed by Fujimoto et al. [31], truncal erythema was the only skin manifestation found significantly more in patients with anti-TIF1α/γ antibody that in patients with anti-TIF1γ antibody alone (77 vs 33 %).
We showed that anti-Mi-2-positive patients simultaneously had the anti-TIF1α antibody [35]. Since anti-TIF1α-specific examination for a large number of anti-Mi-2-positive patients has not been performed, it has not been proven whether there are patients with monospecific anti-TIF1α antibody without anti-Mi-2 (and without anti-TIF1γ antibody).
Anti-MDA5 Antibody
Patients with the anti-MDA5 antibody have several distinguishable mucocutaneous features. Fiorentino et al. showed that skin ulcerations, palmar papules (inverse Gottron), mechanic’s hands, Gottron sign (elbow/knee), hand swelling, diffuse hair loss, panniculitis, and oral pain and/or ulcers were associated with this antibody [36]. They also demonstrated a strong association between location of skin ulcer at the digital pulp/periungual region and this antibody in a subsequent study [37]. Anti-MDA5 antibodies were associated with ILD largely in patients with skin ulcers. Also in a Japanese study, although the presence of skin ulcers was not a prognostic marker in patients with anti-MDA5, skin ulcers were more frequently found in them than in patients with anti-Mi-2 or anti-TIF1 [24]. Also, in a JDM study, the anti-MDA5 antibody was associated with skin ulcers (P = 0.03) and oral ulceration (P = 0.01) in a UK cohort [38].
Anti-NXP2 Antibody
The anti-NXP2 antibody is common in JDM, in which it is identifiable in 13–23 % of patients [39, 40]. In a study by the JDM Research Group of the UK, the anti-NXP2 antibody was shown to have a significant association with calcinosis (OR 2.10, 95 % CI 1.10–4.01) [41]. Forty-three percent of anti-NXP2-positive patients developed calcinosis. In US patients with adult-onset DM, this antibody was strongly associated with calcinosis in multivariate analysis (OR 15.52, 95 % CI 2.01–119.90) [42]. In an Italian study of adult DM/PM, heliotrope rash (P = 0.01) and calcinosis (P = 0.09) were found to be more frequent in patients with anti-MJ antibodies, which were the most frequent specificity [43]. Japanese studies found no association between this antibody and calcinosis [44, 45].
Anti-SAE Antibody
Most patients with anti-small ubiquitin-like modifier activating enzyme (SAE) antibodies present with skin lesions first and then develop myositis [46–48]. The initial studies showed that periungual eruptions appeared in all patients with the anti-SAE antibody and that mechanic’s hands were found in none of them [46, 47]. However, subsequent studies showed the absence of periungual eruptions and the presence of mechanic’s hands in some anti-SAE-positive patients [49, 50]. To date, fewer than 40 cases of anti-SAE antibody-positive patients have been reported, including our patients.
Anti-PM/Scl Antibody
Marie et al. noticed that anti-PM/Scl antibody-positive patients with PM/DM commonly had mechanic’s hands, a finding similar to that for anti-ARS-positive patients [51]. They found that two of 20 patients with PM/DM presented hyperkeratotic rhagadiform hand symptoms. Interestingly, we also found three anti-PM/Scl antibody-positive patients with DM, and two of them had mechanic’s hands in addition to distinctive sole hyperkeratotic rhagadiform symptoms [52].
Other MAAs
Cutaneous manifestations associated with the presence of the anti-TIF1β antibody, the anti-Ku antibody, anti-SRP, and anti-HMGCR have not been reported. We recently reported autoantibodies against DNA mismatch repair enzyme, including MLH1, MSH2, PMS1, and PMS2 in DM/PM; however, we were unable to find the characteristic DM cutaneous signs associated with these autoantibodies [53]. Table 3 summarizes the rough associations between cutaneous manifestations and MAA, except for heliotrope rash and Gottron papules/signs.
Concluding Remarks
Cutaneous involvement of DM is often found at areas that are stretched and/or rubbed. Mechanical stress associated with the stretching of skin over joints could cause such skin disease. Microtrauma and/or ischemia from stretching at these sites could be a local factor. Around orbital areas, blinking is constantly performed during waking hours by the movement of the upper eyelids, which could also be a pathogenic factor.
The involvement of the face and extensor sides of the arms may be related to sun exposure in some patients with DM. These environmental stresses on the skin are known to be provoking factors of the Köbner phenomenon (isomorphic response).
The Köbner phenomenon, which was first described in psoriatic skin by Dr. Heinlich Köbner in 1876, is seen not only in autoimmune inflammatory dermatitis but also in the skin of patients with systemic autoimmune disease (lupus, dermatomyositis, etc.) and sarcoidosis [54]. Although the pathophysiology of the Köbner phenomenon has not been clarified, environmental stimuli are known to cause many inflammatory substances, including various cytokines, stress proteins, and adhesion molecules, to be released in local tissues, suggesting a cause of Köbner phenomenon. Also, some autoantigens are released from intracellular areas [55] and could be fragmented [56]. Future studies will be necessary to investigate whether UV light or other stimuli affect expression, structure, or subcellular localization of autoantigens targeted by MAA in the skin [57].
References
von Mühlen CA, Tan EM (1995) Autoantibodies in the diagnosis of systemic rheumatic diseases. Semin Arthritis Rheum 24:323–358
Mahler M, Pierangeli S, Meroni PL, Fritzler MJ (2014) Autoantibodies in systemic autoimmune disorders. J Immunol Res 2014:263091
Casciola-Rosen L, Mammen AL (2012) Myositis autoantibodies. Curr Opin Rheumatol 24:602–608
Tansley SL, Betteridge ZE, McHugh NJ (2013) The diagnostic utility of autoantibodies in adult and juvenile myositis. Curr Opin Rheumatol 25:772–777
Tansley S, Gunawardena H (2014) The evolving spectrum of polymyositis and dermatomyositis—moving towards clinicoserological syndromes: a critical review. Clin Rev Allergy Immunol 47:264–273
Bohan A, Peter JB (1975) Polymyositis and dermatomyositis (first of two parts). N Engl J Med 292:344–347
Bohan A, Peter JB (1975) Polymyositis and dermatomyositis (second of two parts). N Engl J Med 292:403–407
Tanimoto K, Nakano K, Kano S, Mori S, Ueki H, Nishitani H, Sato T, Kiuchi T, Ohashi Y (1995) Classification criteria for polymyositis and dermatomyositis. J Rheumatol 22:668–674
Targoff IN, Miller FW, Medsger TA Jr, Oddis CV (1997) Classification criteria for the idiopathic inflammatory myopathies. Curr Opin Rheumatol 9:527–535
Sontheimer RD, Euwer RL, Costner MI (2004) Dermatomyositis. In: Sontheimer RD, Provost TT (eds) Cutaneous manifestations of rheumatic diseases, 2nd edn. Lippincott Williams & Wilkins, Philadelphia, pp 65–103
Christen-Zaech S, Seshadri R, Sundberg J, Paller A, Pachman LM (2008) Persistent association of nailfold capillaroscopy changes and skin involvement over thirty-six months with duration of untreated disease in juvenile dermatomyositis. Arthritis Rheum 58:571–576
Quinter SD, Chiu YE, Lyon VB, Holland KE, Ruggeri SY, Drolet BA (2012) Inverse Gottron’s papules: an unusual cutaneous manifestation of juvenile dermatomyositis. Pediatr Dermato 29:641–644
Kameda H, Nagasawa H, Ogawa H, Sekiguchi N, Takei H, Tokuhira M, Amano K, Takeuchi T (2005) Combination therapy with corticosteroids, cyclosporin A, and intravenous pulse cyclophosphamide for acute/subacute interstitial pneumonia in patients with dermatomyositis. J Rheumatol 32:1719–1726
Stahl NI, Klippel JH, Decker JL (1979) A cutaneous lesion associated with myositis. Ann Intern Med 91:577–579
Love LA, Leff RL, Fraser DD, Targoff IN, Dalakas M, Plotz PH, Miller FW (1991) A new approach to the classification of idiopathic inflammatory myopathy: myositis-specific autoantibodies define useful homogeneous patient groups. Medicine (Baltimore) 70:360–374
Dourmishev L, Meffert H, Piazena H (2004) Dermatomyositis: comparative studies of cutaneous photosensitivity in lupus erythematosus and normal subjects. Photodermatol Photoimmunol Photomed 20:230–234
Kubo M, Sato S, Kitahara H, Tsuchida T, Tamaki K (1996) Vesicle formation in dermatomyositis associated with gynecologic malignancies. J Am Acad Dermatol 34:391–394
Pope E, Janson A, Khambalia A, Feldman B (2006) Childhood acquired lipodystrophy: a retrospective study. J Am Acad Dermatol 55:947–950
Tansley SL, McHugh NJ (2014) Myositis specific and associated autoantibodies in the diagnosis and management of juvenile and adult idiopathic inflammatory myopathies. Curr Rheumatol Rep 16:464
Magro CM, Crowson AN (1997) The immunofluorescent profile of dermatomyositis: a comparative study with lupus erythematosus. J Cutan Pathol 24:543–552
Targoff IN, Reichlin M (1985) The association between Mi-2 antibodies and dermatomyositis. Arthritis Rheum 28:796–803
Love LA, Leff RL, Fraser DD, Targoff IN, Dalakas M, Plotz PH, Miller FW (1991) A new approach to the classification of idiopathic inflammatory myopathy: myositis-specific autoantibodies define useful homogeneous patient groups. Medicine (Baltimore 70:360–374
Komura K, Fujimoto M, Matsushita T, Kaji K, Kondo M, Hirano T, Orito H, Horikawa M, Hamaguchi Y, Hasegawa M, Takehara K, Sato S (2005) Prevalence and clinical characteristics of anti-Mi-2 antibodies in Japanese patients with dermatomyositis. J Dermatol Sci 40:215–217
Hamaguchi Y, Kuwana M, Hoshino K, Hasegawa M, Kaji K, Matsushita T, Komura K, Nakamura M, Kodera M, Suga N, Higashi A, Ogusu K, Tsutsui K, Furusaki A, Tanabe H, Sasaoka S, Muro Y, Yoshikawa M, Ishiguro N, Ayano M, Muroi E, Fujikawa K, Umeda Y, Kawase M, Mabuchi E, Asano Y, Sodemoto K, Seishima M, Yamada H, Sato S, Takehara K, Fujimoto M (2011) Clinical correlations with dermatomyositis-specific autoantibodies in adult Japanese patients with dermatomyositis: a multicenter cross-sectional study. Arch Dermatol 147:391–398
Cruellas MG, Viana Vdos S, Levy-Neto M, Souza FH, Shinjo SK (2013) Myositis-specific and myositis-associated autoantibody profiles and their clinical associations in a large series of patients with polymyositis and dermatomyositis. Clinics (Sao Paulo) 68:909–914
Rider LG, Shah M, Mamyrova G, Huber AM, Rice MM, Targoff IN, Miller FW, Childhood Myositis Heterogeneity Collaborative Study Group (2013) The myositis autoantibody phenotypes of the juvenile idiopathic inflammatory myopathies. Medicine (Baltimore) 92:223–243
Targoff IN (1992) Autoantibodies in polymyositis. Rheum Dis Clin N Am 18:455–482
Hamaguchi Y, Fujimoto M, Matsushita T, Kaji K, Komura K, Hasegawa M, Kodera M, Muroi E, Fujikawa K, Seishima M, Yamada H, Yamada R, Sato S, Takehara K, Kuwana M (2013) Common and distinct clinical features in adult patients with anti-aminoacyl-tRNA synthetase antibodies: heterogeneity within the syndrome. PLoS One 8, e60442
Kaji K, Fujimoto M, Hasegawa M, Kondo M, Saito Y, Komura K, Matsushita T, Orito H, Hamaguchi Y, Yanaba K, Itoh M, Asano Y, Seishima M, Ogawa F, Sato S, Takehara K (2007) Identification of a novel autoantibody reactive with 155 and 140 kDa nuclear proteins in patients with dermatomyositis: an association with malignancy. Rheumatology (Oxford) 46:25–28
Targoff IN, Mamyrova G, Trieu EP, Perurena O, Koneru B, O’Hanlon TP, Miller FW, Rider LG; Childhood Myositis Heterogeneity Study Group; International Myositis Collaborative Study Group (2006) A novel autoantibody to a 155-kd protein is associated with dermatomyositis. Arthritis Rheum 54:3682–3689
Fujimoto M, Hamaguchi Y, Kaji K, Matsushita T, Ichimura Y, Kodera M, Ishiguro N, Ueda-Hayakawa I, Asano Y, Ogawa F, Fujikawa K, Miyagi T, Mabuchi E, Hirose K, Akimoto N, Hatta N, Tsutsui K, Higashi A, Igarashi A, Seishima M, Hasegawa M, Takehara K (2012) Myositis-specific anti-155/140 autoantibodies target transcription intermediary factor 1 family proteins. Arthritis Rheum 64:513–522
Gunawardena H, Wedderburn LR, North J, Betteridge Z, Dunphy J, Chinoy H, Davidson JE, Cooper RG, McHugh NJ (2008) Juvenile dermatomyositis research group UK. Clinical associations of autoantibodies to a p155/140 kDa doublet protein in juvenile dermatomyositis. Rheumatology (Oxford) 47:324–328
Bingham A, Mamyrova G, Rother KI, Oral E, Cochran E, Premkumar A, Kleiner D, James-Newton L, Targoff IN, Pandey JP, Carrick DM, Sebring N, O’Hanlon TP, Ruiz-Hidalgo M, Turner M, Gordon LB, Laborda J, Bauer SR, Blackshear PJ, Imundo L, Miller FW, Rider LG, Childhood Myositis Heterogeneity Study Group (2008) Predictors of acquired lipodystrophy in juvenile-onset dermatomyositis and a gradient of severity. Medicine (Baltimore) 87:70–86
Fiorentino DF, Kuo K, Chung L, Zaba L, Li S, Casciola-Rosen L (2015) Distinctive cutaneous and systemic features associated with antitranscriptional intermediary factor-1γ antibodies in adults with dermatomyositis. J Am Acad Dermatol 72:449–455
Muro Y, Ishikawa A, Sugiura K, Akiyama M (2012) Clinical features of anti-TIF1-α antibody-positive dermatomyositis patients are closely associated with coexistent dermatomyositis-specific autoantibodies and anti-TIF1-γ or anti-Mi-2 autoantibodies. Rheumatology (Oxford) 51:1508–1513
Fiorentino D, Chung L, Zwerner J, Rosen A, Casciola-Rosen L (2011) The mucocutaneous and systemic phenotype of dermatomyositis patients with antibodies to MDA5 (CADM-140): a retrospective study. J Am Acad Dermatol 65:25–34
Narang NS, Casciola-Rosen L, Li S, Chung L, Fiorentino DF (2015) Cutaneous ulceration in dermatomyositis: association with anti-melanoma differentiation-associated gene 5 antibodies and interstitial lung disease. Arthritis Care Res. doi:10.1002/acr.22498
Tansley SL, Betteridge ZE, Gunawardena H, Jacques TS, Owens CM, Pilkington C, Arnold K, Yasin S, Moraitis E, Wedderburn LR, McHugh NJ, UK Juvenile Dermatomyositis Research Group (2014) Anti-MDA5 autoantibodies in juvenile dermatomyositis identify a distinct clinical phenotype: a prospective cohort study. Arthritis Res Ther 16:R138
Gunawardena H, Wedderburn LR, Chinoy H, Betteridge ZE, North J, Ollier WE, Cooper RG, Oddis CV, Ramanan AV, Davidson JE, McHugh NJ, Juvenile Dermatomyositis Research Group, UK and Ireland (2009) Autoantibodies to a 140-kd protein in juvenile dermatomyositis are associated with calcinosis. Arthritis Rheum 60:1807–1814
Espada G, Maldonado Cocco JA, Fertig N, Oddis CV (2009) Clinical and serologic characterization of an argentine pediatric myositis cohort: identification of a novel autoantibody (anti-MJ) to a 142-kDa protein. J Rheumatol 36:2547–2551
Tansley SL, Betteridge ZE, Shaddick G, Gunawardena H, Arnold K, Wedderburn LR, McHugh NJ, Juvenile Dermatomyositis Research Group (2014) Calcinosis in juvenile dermatomyositis is influenced by both anti-NXP2 autoantibody status and age at disease onset. Rheumatology (Oxford) 53:2204–2208
Valenzuela A, Chung L, Casciola-Rosen L, Fiorentino D (2014) Identification of clinical features and autoantibodies associated with calcinosis in dermatomyositis. JAMA Dermatol 150:724–729
Ceribelli A, Fredi M, Taraborelli M, Cavazzana I, Franceschini F, Quinzanini M, Tincani A, Ross SJ, Chan JY, Pauley BA, Chan EK, Satoh M (2012) Anti-MJ/NXP-2 autoantibody specificity in a cohort of adult Italian patients with polymyositis/dermatomyositis. Arthritis Res Ther 14:R97
Ichimura Y, Matsushita T, Hamaguchi Y, Kaji K, Hasegawa M, Tanino Y, Inokoshi Y, Kawai K, Kanekura T, Habuchi M, Igarashi A, Sogame R, Hashimoto T, Koga T, Nishino A, Ishiguro N, Sugimoto N, Aoki R, Ando N, Abe T, Kanda T, Kuwana M, Takehara K, Fujimoto M (2012) Anti-NXP2 autoantibodies in adult patients with idiopathic inflammatory myopathies: possible association with malignancy. Ann Rheum Dis 71:710–713
Ishikawa A, Muro Y, Sugiura K, Akiyama M (2012) Development of an ELISA for detection of autoantibodies to nuclear matrix protein 2. Rheumatology (Oxford) 51:1181–1187
Betteridge ZE, Gunawardena H, Chinoy H, North J, Ollier WE, Cooper RG, McHugh NJ, UK Adult Onset Myositis Immunogenetic Collaboration (2009) Clinical and human leucocyte antigen class II haplotype associations of autoantibodies to small ubiquitin-like modifier enzyme, a dermatomyositis-specific autoantigen target in UK Caucasian adult-onset myositis. Ann Rheum Dis 68:1621–1625
Fujimoto M, Matsushita T, Hamaguchi Y, Kaji K, Asano Y, Ogawa F, Yamaoka T, Fujikawa K, Tsukada T, Sato K, Echigo T, Hasegawa M, Takehara K (2013) Autoantibodies to small ubiquitin-like modifier activating enzymes in Japanese patients with dermatomyositis: comparison with a UK Caucasian cohort. Ann Rheum Dis 72:151–153
Muro Y, Sugiura K, Akiyama M (2013) Low prevalence of anti-small ubiquitin-like modifier activating enzyme antibodies in dermatomyositis patients. Autoimmunity 46:279–284
Bodoki L, Nagy-Vincze M, Griger Z, Betteridge Z, Szöllősi L, Dankó K (2014) Four dermatomyositis-specific autoantibodies-anti-TIF1γ, anti-NXP2, anti-SAE and anti-MDA5-in adult and juvenile patients with idiopathic inflammatory myopathies in a Hungarian cohort. Autoimmun Rev 13:1211–1219
Muro Y, Sugiura K, Nara M, Sakamoto I, Suzuki N, Akiyama M (2015) High incidence of cancer in anti-small ubiquitin-like modifier activating enzyme antibody-positive dermatomyositis. Rheumatology (Oxford) (in press)
Marie I, Lahaxe L, Benveniste O, Delavigne K, Adoue D, Mouthon L, Hachulla E, Constans J, Tiev K, Diot E, Levesque H, Boyer O, Jouen F (2010) Long-term outcome of patients with polymyositis/ dermatomyositis and anti-PM-Scl antibody. Br J Dermatol 162:337–344
Muro Y, Hosono Y, Sugiura K, Ogawa Y, Mimori T, Akiyama M (2015) Anti-PM/Scl antibodies are found in Japanese patients with various systemic autoimmune conditions besides myositis and scleroderma. Arthritis Res Ther 17:57
Muro Y, Nakashima R, Hosono Y, Sugiura K, Mimori T, Akiyama M (2014) Autoantibodies to DNA mismatch repair enzymes in polymyositis/dermatomyositis and other autoimmune diseases: a possible marker of favorable prognosis. Arthritis Rheumatol 66:3457–3462
Ueki H (2005) Koebner phenomenon in lupus erythematosus with special consideration of clinical findings. Autoimmun Rev 4:219–223
Casciola-Rosen LA, Anhalt G, Rosen A (1994) Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med 179:1317–1330
Casciola-Rosen L, Wigley F, Rosen A (1997) Scleroderma autoantigens are uniquely fragmented by metal-catalyzed oxidation reactions: implications for pathogenesis. J Exp Med 185:71–79
Burd CJ, Kinyamu HK, Miller FW, Archer TK (2008) UV radiation regulates Mi-2 through protein translation and stability. J Biol Chem 283:34976–34982
Acknowledgments
This study was supported by a grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan (26461656 to YM) and a grant from the Ministry of Health, Labour and Welfare of Japan (to YM). Informed consent was obtained from all individual participants in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muro, Y., Sugiura, K. & Akiyama, M. Cutaneous Manifestations in Dermatomyositis: Key Clinical and Serological Features—a Comprehensive Review. Clinic Rev Allerg Immunol 51, 293–302 (2016). https://doi.org/10.1007/s12016-015-8496-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-015-8496-5