Abstract
Since the beginning of the Coronavirus disease (COVID)-19 pandemic in December 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been responsible for more than 600 million infections and 6.5 million deaths worldwide. Given the persistence of SARS-CoV-2 and its ability to develop new variants, the implementation of an effective and long-term herd immunity appears to be crucial to overcome the pandemic. While a vast field of research has focused on the role of humoral immunity against SARS-CoV-2, a growing body of evidence suggest that antibodies alone only confer a partial protection against infection of reinfection which could be of high importance regarding the strategic development goals (SDG) of the United Nations (UN) and in particular UN SDG3 that aims towards the realization of good health and well being on a global scale in the context of the COVID-19 pandemic.
In this review, we highlight the role of humoral immunity in the host defense against SARS-CoV-2, with a focus on highly neutralizing antibodies. We summarize the results of the main clinical trials leading to an overall disappointing efficacy of convalescent plasma therapy, variable results of monoclonal neutralizing antibodies in patients with COVID-19 but outstanding results for the mRNA based vaccines against SARS-CoV-2. Finally, we advocate that beyond antibody responses, the development of a robust cellular immunity against SARS-CoV-2 after infection or vaccination is of utmost importance for promoting immune memory and limiting disease severity, especially in case of (re)-infection by variant viruses.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since December 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic [1] is being responsible for more than 609 million infections and 6.5 million deaths as of September 2022, according to the Johns Hopkins Coronavirus Resource Center [2]. Pneumonia is the most severe presentation of SARS-CoV-2 infection, leading to acute respiratory failure in up to 20% of symptomatic patients at the beginning of the pandemic before vaccines development and the emergence of variants [3]. However, SARS-CoV-2 infection is also responsible for a wide range of clinical presentations including coagulopathy, cardiac or kidney injury, thrombotic events suggesting that endothelial injury and inflammation may be the cornerstone of the disease [4–6]. SARS-CoV-2 is an enveloped single-stranded RNA betacoronavirus. It belongs to the same family than the SARS-CoV, also responsible for an outbreak between 2002 and 2004 with no re-emergence since then [7]. It is also related to the Middle East Respiratory Syndrome (MERS) coronavirus, which causes mild to severe acute respiratory illness with up to 35% case-fatality rate and is responsible for outbreaks since 2012. In addition to these viruses responsible for severe diseases, four different coronaviruses (HCoV-OC43, HCoVHKU1, HCoV-NL63, and HCoV-229E) are commonly responsible for common colds.
The clearance of cytopathic viruses is highly mediated by the humoral immune response, which also prevents reinfection [8]. Thus, individuals recovering from viral infections typically develop virus-specific antibody responses that provide protective immunity against re-exposure. However, waning immunity or immune escape may increase the risk of reinfection [9]. While SARS-CoV-2 infection seems to confer an effective but not lasting immunity in most individuals [10], the role played by the antibodies in this protection remains unclear. Since the beginning of the recent Coronavirus disease 2019 (COVID-19) outbreak, SARS-CoV-2 humoral immunity has been extensively studied [11, 12]. Understanding what protection confer antibodies to SARS-CoV-2 and how long lasts this protection is a major issue with implications for public health politics and vaccine or therapeutic antibodies development. To answer to this question, our strategy of literature review used Pubmed and Bioxiv as data bases and the following keywords: SARS-CoV-2, with either Vaccine, Antibodies, Serology or Convalescent Plasma.
Overall, while the vaccine anti-SARS-CoV-2 appear to be very efficient [13], we highlight in this work the lack of definitive evidence that natural antibodies produced after SARS-CoV-2 infection are fully protective by themselves. This reinforces the need to a better understanding of the immune response to SARS-CoV-2 infection and, as requested by the SDG of the UN and the SDG3, the need to increase the worldwide distribution of an efficient vaccine to reach the herd immunity.
There is No Clear Association Between a SARS-CoV-2 Serologic Response and a Protection Against Severe Cases
Coronaviruses have four structural proteins: the spike protein (S), the nucleocapsid (N), the envelope protein (E), and the membrane protein (M). The S protein allows the virus entry into the cell by interacting with the cell receptor angiotensin-converting enzyme 2 (ACE2). The S protein is composed by two sub-units: the S1 protein, which contains the highly immunogenic receptor-binding domain (RBD) and the S2 protein, which mediates cell membrane fusion [14–16]. The N protein is also immunogenic and participate in genome encapsidation and enhance viral transcription and assembly [17, 18]. Serological responses to SARS-CoV-2 infection against S and N proteins have been extensively studied. In the literature, the large majority of patients develop S and N-specific immunoglobulins (Ig) G, IgM and IgA within 15 to 20 days after symptoms onset [19–21]. An interesting point is that the three types of antibodies are almost developed at the same time following SARS-CoV-2 infection [22, 23]. The IgG and IgM levels have been found to be higher in severe SARS-CoV-2 patients than in asymptomatic or mild cases (Fig. 1a) [19, 20] This is a major difference with the infections caused by SARS-CoV and MERS where delayed and weak antibodies responses seemed to be associated with more severe outcomes [24–26].
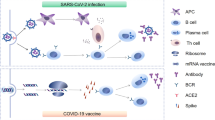
How protective are antibodies to SARS-CoV2? a) Viral load and levels of natural antibodies are not correlated with the severity of the disease. b) Patients with agammaglobulinemia do not have more severe SARS-CoV2 clinical presentation. Moreover, convalescent plasma therapy does not protect nor treat patients with SARS-CoV2 infection. c) mRNA vaccines induce 95% of protection in adults-vaccinated patients, by inducing the production of subsets of super-antibodies displaying increased neutralization potency and/or by other aspects of immune response as T-cell responses
According to the literature, the median time to detection by Enzyme Linked Immunoabsorbent Assay (ELISA) across different antibodies in SARS-CoV-2 infection is 11 days (Interquartile range 7–14 days) and severity appears to be associated with a longer time to detection of IgG [20, 27]. However, the consequence of this increased persistence of the antibodies after severe infections is difficult to interpret as pathogen-specific antibodies might be produced in response to infection without conferring any direct protection. In the case of the SARS-CoV-2 infections several arguments could favor this hypothesis. First, high titers of IgG antibodies in patients’ sera have not been reported to have a faster recovery compared to borderline antibody response [28]. Second, several studies have reported that viral clearance did not differ between asymptomatic and symptomatic patients and was not correlated to antibodies titers [21, 29]. Third, SARS-CoV-2 RNA could be detected for a very long period in patients who produce virus-specific IgG antibodies, with two cases for up to 50 days [30]. In the same study, Wang et al. also reported the case of a patient who cleared the virus in 46 days, although no anti-SARS-CoV-2 IgG was detected in his plasma. These data may suggest that humoral immunity is neither sufficient nor essential to obtain viral clearance and clinical recovery [30]. Furthermore, in patients with agammaglobulinemia (lacking of B lymphocytes), mild disease course with full recovery has been described confirming that B-cell responses and antibodies participate but are not mandatory to recover from a SARS-CoV-2 infection (Fig. 1b) [31, 32].
To better determine the protection induced by natural antibodies against SARS-CoV-2, retroviral particles and pseudovirus-based neutralizing assays have been performed in several studies [12, 20, 33]. Rijkers et al. showed that in 24 COVID-19 non-hospitalized healthcare professionals with mild symptoms, 75% of the population produced antibodies to SARS-CoV-2. However, neutralization assays demonstrated low or absent virus neutralization [23]. In contrast, a stronger neutralizing antibody response was present in severe COVID-19 patients. Wang et al. found the same result and demonstrated that patients with a worse clinical classification had higher neutralizing antibody titers [34].
There is No Clear Evidence in Favor of the Use of Convalescent Plasma for the Treatment of the Infection Caused By the SARS-CoV-2
Trying to correlate clinical protection and the neutralizing abilities of the natural antibodies against the S-protein and SARS-CoV-2 has been a major driver behind the use of convalescent plasma as a potential therapeutic against SARS-CoV-2 infections.
Therefore, the transfusion of plasma collected from individuals who recovered from COVID-19 to currently infected patients has been widely tested at a time when no vaccine against the virus was available. Some initial reports may have seemed positive, however, this therapeutic never reached the level of expected success.
The first published series of 5 critically-ill patients with COVID-19 and acute respiratory distress syndrome treated with convalescent plasma containing neutralizing antibodies showed an improvement in their clinical status [35], however no control group was included in this initial study. Another uncontrolled case series suggested that late administration of convalescent plasma treatment could end the SARS-CoV-2 shedding with no significant effect on mortality in critically end-stage COVID-19 patients [36]. Since these preliminary reports, different randomized controlled trials or controlled non-randomized trials analyzed the clinical effects of convalescent plasma in severe COVID-19 patients [37–42]. In randomized trials of convalescent plasma in patients with COVID-19 focusing on hospitalized patients who were already moderately to severely ill, no evidence of improvement in clinical condition nor mortality has been proved after the administration of convalescent plasma therapy. These results were confirmed in a Cochrane meta-analysis including 19 studies, which demonstrated uncertainty whether convalescent plasma decreased mortality and highlighted little to no difference in improvement of clinical symptoms at day 7 [43]. However, when focusing on older adults with mild SARS-CoV-2 symptoms, Libster et al. reported that early administration (within 72 h following the onset of symptoms) of high-titer convalescent plasma against SARS-CoV-2 might reduce the progression of COVID-19, with severe respiratory disease seemingly less developed in patients who received the convalescent plasma (relative risk 0.52 -95% confidence interval (CI) 0.29–0.94- p = 0.03) [42].
Results from the major clinical trial RECOVERY, that used only plasma with a cut-off of antibodies targeting the S glycoprotein with neutralizing antibody titers of ≥ 1:100, confirmed previous data regarding the inefficacy of convalescent plasma therapy in SARS-CoV-2 patients. The trial was conducted at 177 National Health Service (NHS) hospital organizations in the United Kingdom. In this study, 5795 patients were randomly allocated to receive convalescent plasma and 5763 received usual care alone. No significant difference in 28-day mortality between the two groups was found: 1398 (24%) in the treated group and 1408 (24%) in the standard of care died within 28 days (rate ratio 1.00 -95% CI 0.93–1.07- p = 0.95) [44].
In a recent multicenter, double-bind, randomised trial including 1225 outpatients, of whom more than 80% were unvaccinated, Sullivan et al. demonstrated that patients receiving plasma therapy within 9 days after the onset of symptoms had a 54% relative risk reduction of hospitalization compared to those having received placebo [45]. However, numerous other studies have proven no effect of convalescent plasma therapy in COVID-19 patients [46–48] (Table 1 and Fig. 1b). While the time of the convalescent plasma administration, the antibodies titers and the severity of the disease might all be factors influencing the efficacy of the convalescent plasma therapy approach, overall, these studies did not reinforce the hypothesis that natural antibodies against SARS-CoV-2 had a protective role on their own.
Yet, convalescent plasma therapy could be an efficient therapy in specific populations. Szwebel et al. reported that an HIV-1 infected patient treated with anti-CD20 monoclonal antibody for a B-cell lymphoma and diagnosed with protracted SARS-CoV-2 infection was successfully treated with convalescent plasma therapy with full clinical recovery and SARS-CoV-2 plasma clearance [49]. Moreover, Hueso et al. described in two studies that in patients with B-cell lymphopenia or neoplasm, convalescent plasma with anti-SARS-CoV-2 antibodies might be a promising and beneficial therapy [50, 51].
There is Only a Small Subpopulation of Highly Neutralizing Antibodies Generated After Infection By the SARS-CoV-2
These results reported from the plasma therapy led us to consider the possibility that while antibodies naturally produced after being infected by the SARS-CoV-2 might not be as protective as expected, a subpopulation within these antibodies might be significantly more protective. Being able to identify and characterize these highly neutralizing antibodies could help at different levels. First, in our understanding of the disease pathophysiology: the huge variability of the clinical pattern for patients infected by the SARS-CoV-2 may be related to the host immune response and its ability to generate this subpopulation of antibodies. Second, in optimizing the design of anti-SARS-CoV-2 vaccines.
Indeed, Robbiani et al. reported that most convalescent plasma samples obtained from individuals having recovered from a SARS-CoV-2 infection (median 39 days following infection) did not contain high levels of neutralizing activity [52]. Interestingly, the authors found through antibody sequencing that closely related antibodies were expressed in different individuals. These rare populations of RBD-antibodies displayed efficient antiviral activity with a neutralizing activity raising from 1:50 to 1:5000. Thus, the authors suggested that designing a vaccine which could specifically elicit the clonal expansion of these antibodies would be significantly more effective than the natural immune response [52]. This subpopulation of highly neutralizing antibodies was reported in different other studies. Liu et al. isolated nineteen potent SARS-CoV-2-neutralizing antibodies in vitro, obtained from five severe COVID-19 patients. Of those, only nine antibodies, directed against the RBD or the S protein, displayed extremely high neutralization with very low 50% virus-inhibitory concentrations [53]. Seydoux et al. described how they isolated S-specific B cells from one COVID-19 patient and generated 45 S-specific monoclonal antibodies. Only two antibodies seemed to efficiently neutralize the virus with the more potent directed against the RBD, suggesting that monoclonal antibodies targeting the RBD could be more efficient in preventing or treating SARS-CoV-2 infection [54]. Kreer et al. in a longitudinal study also isolated from 12 SARS-CoV-2 patients 255 antibodies of which 28 potently neutralized SARS-CoV-2. More importantly, they highlighted that adequate vaccination could quickly elicit highly effective antibodies production as naïve B cells from healthy individuals sampled before the pandemic displayed potential precursor sequences of these neutralizing antibodies [55]. Finally, Rogers et al. used a high-throughput antibody generation pipeline to isolate potent neutralizing antibodies, mostly directed against the RBD. Two of the neutralizing antibodies were tested in Syrian hamsters and provided protection against SARS-CoV-2 infection [56].
All these results support that highly effective natural antibodies could be developed as monoclonal antibodies or that COVID-19 vaccines could also aim to elicit these antibodies production to be broadly effective in the population (Fig. 1c). These studies also enlighten that all antibodies are not equal and that only low amounts of them seem to be very efficient and protective during SARS-CoV-2 infection.
The Role of Anti-SARS-CoV-2 IgM and IgA
Seow et al. demonstrated that S and RBD-IgM and IgA could neutralize SARS-CoV-2 even before an IgG response was detected by ELISA [33]. Indeed, secretory IgA are the first immune defense in protecting mucosal surfaces by neutralizing respiratory or intestinal viruses or preventing their adhesion to the epithelium. In mice, intranasal vaccination by SARS-CoV proteins provides a better protection and decreases the viral load in the lungs compared to intramuscular administrations, suggesting that mucosal-induced IgA could mediate local protection against viruses [57]. Usually, IgM are the first antibodies to be produced during the humoral response. However, in different studies on COVID-19, IgA were dominant at the early phase of the SARS-CoV-2-specific humoral response [58–61]. According to Klingler et al. serum IgA1 exhibited neutralizing properties at a lower potency than IgG1 and IgM [58], but these results were not confirmed in Sterlin et al. study. The authors demonstrated that anti-RBD IgA had a higher virus neutralization activity than IgG. However, anti-RBD IgA decreased quickly in the plasma and persisted for a longer time in the saliva. In this study, IgA were also detected in broncho-alveolar lavages of severe COVID-19 patients and exhibited neutralizing properties [60]. Cervia et al. highlighted that SARS-CoV-2-exposed healthcare workers with negative SARS-CoV-2-specific IgA and IgG serum titers had detectable SARS-CoV-2-specific IgA antibodies in their nasal fluids and tears, suggesting the mounting of an efficient local immune response [59].
However, different authors also suggested that IgA response might be responsible for the severity of the COVID-19. In Cervia et al. study, IgA were significantly increased in severe COVID-19 patients and increased production correlated with severe acute respiratory distress syndrome [60]. Yu et al. described a significant positive association between SARS-CoV-2 specific IgA level and the APACHE-II score (severity score) in COVID-19 critically-ill patients. The authors hypothesized that severe COVID-19 might be at least in part an IgA-mediated disease related to IgA deposition and vasculitis, and that IgA deposition could participate in the pathophysiology of the disease and the occurrence of complications as myocardial ischemia, acute pulmonary embolism, kidney injury [61]. In another cohort of 64 patients with COVID-19, elevated anti-cardiolipin IgA and anti-β2-Glucoprotein-1 IgA were detected in severe SARS-CoV-2 patients, suggesting that autoimmunity in COVID-19 patients might be triggered by IgA-response [62].
The Autoimmunity Caused By the SARS-CoV-2 Infection
Pathogenic autoantibodies targeting phospholipids and phospholipid-binding proteins (aPL antibodies) have been detected in patients by the SARS-CoV-2, a study even found that half of patients hospitalized with COVID-19 become at least transiently positive for aPL antibodies and that these autoantibodies are potentially pathogenic [63]. However, the clinical relevance of the aPL antibodies antibodies and their absence of specificity is now clearly demonstrated. Indeed, a recent study by another group found that patients with COVID-19 had an increased prevalence in lupus anticoagulant (LAC) positivity but in this case this positivity while associated with biologic markers of inflammation was not associated with higher risks of venous thromboembolism and/or in-hospital mortality [64]. These results have been confirmed and reviewed by APS-ACTION, an international aPL research network [65]. Antibody-mediated procoagulant platelets could be another explanation of the increased thromboembolic risk found in ICU COVID-19 patients. Indeed, immunoglobulin G fractions that could induce an Fcγ receptor IIA-dependent platelet apoptosis was found in COVID-19 patients [66]. Another important class of autoantibodies has been described during the SARS-CoV-2 infections: the autoantibodies against type I interferons [67]. These autoantibodies were found in 101 patients of 987 with life-threatening infection by SARS-CoV-2, while they were absent from 663 patients with non-severe infections. Interestingly, these autoantibodies were also found to be associated with an increased risk of critical influenza pneumonia [68].
The Place of Neutralizing Monoclonal Antibodies in the Treatment Against SARS-CoV-2 Infections
Fully human monoclonal antibodies (MAbs) may represent a promising class of therapeutics against SARS-CoV-2. To date, numerous studies have described the characterization of potent neutralizing MAbs, usually targeting the S-protein of the virus [53, 69, 70]. A cocktail of two fully human antibodies REGN10933 (Casirivimab) and REGN10987 (Imdevimab), called REGN-COV2, binding to distinct and non-overlapping regions of the RBD displayed potent neutralizing activity and did not result in the outgrowth of escape mutants. According to the authors, using a cocktail of antibodies decreases the risk of mutants selection as escape would need multiple and simultaneous viral mutation occurring in distinct genetic sites [71]. The firm Regeneron obtained the FDA approval to use this antibody cocktail in COVID-19 patients. In an interim analysis of an ongoing, double-blind, phase 1–3 trial REGN-COV2 antibody cocktail seemed able to reduce the viral load, however, clinically, time to alleviation of symptoms was not strongly associated with the treatment [72]. A randomized, placebo-controlled trial published in August 2021 found that a subcutaneous administration of 1200 mg of REGEN-COV (previously REGN-COV2) could prevent symptomatic and asymptomatic SARS-CoV-2 in previously non-infected participants with household COVID-19 contacts (relative risk reduction 81.4%; P < 0.001) [73]. In a phase 1, randomized, placebo-controlled trial, a monthly subcutaneous administration of Casirivimab and Imdevimab for a maximum of 6 months in non-infected volunteers significantly decreased the risk for COVID-19 infection compared to placebo during the treatment period (relative risk reduction 92.4%; odds ratio 0.07- 95% CI 0.01–0,27- nominal P-value < 0.001) [74]. LY-CoV555 (Bamlanivimab) developed by Lilly, a potent anti-S protein neutralizing antibody that binds with high affinity to the RBD, was derived from a convalescent plasma obtained from a hospitalized patient with COVID-19 [75]. The results of an interim analysis of the ongoing phase 2 trial involving outpatients with mild or moderate SARS-CoV-2 infection have been published [76]. Chen et al. included 467 patients (150 placebo and 317 LY-CoV555) and tested different doses of LY-CoV555 (700 mg, 2800 mg, 7000 mg). Each patient received a single infusion of antibody. Only the 2800 mg dose appeared to accelerate the viral load decline over time [76]. Another study included 314 hospitalized patients without invasive ventilation nor end-stage organ failure. 163 patients received a single infusion of 7000 mg of LY-CoV555 and 151 patients received placebo. In this study, hospitalized patients with COVID-19 who received LY-CoV555 did not have better clinical outcomes at day 5 than those who received placebo [77]. In a recent published paper, it has been demonstrated that having received an administration of Bamlanivimab not significantly decreased anti-S antibodies production following COVID-19 vaccination, with minimal reduction in inhibitory potency of endogenous antibodies [78]. However, all these studies were mainly conducted when the Omicron variant was not the predominant one. Since then, the WHO strongly advised against Casirivimab-Imdevimab and Sotrovimab for COVID-19 patients, as they are not likely to work against currently circulating variants [79]. Thus, different studies demonstrated that the BA.1 and BA.2 lineages of the Omicron variant exhibited decreased sensitivity to monoclonal antibodies than previous variants [80, 81]. However, Bebtelovimab, a RBD-specific antibody, has been shown to display potent neutralizing activity against all known SARS-CoV-2 variants [81–83].
Thus, MAbs might be a promising therapy, however inconsistencies and the emergence of new variants may damper the initial enthusiasm and their use in daily clinical practice.
The Emergence of New Vaccines and New SARS-CoV-2 Variants
To overcome this pandemic, safe and efficient vaccines remain the best way to build protection and reduce disease spread. To date, 199 candidate vaccines are in pre-clinical phase and 172 in clinical phase, according to the World Health Organization (WHO) COVID-19 vaccine tracker [84].
Different strategies have been considered for the development of vaccines against SARS-CoV-2. One approach to vaccine development has been the use of viral vector, like the Oxford-AstraZeneca ChAdOx1-S vaccine. In the literature, the efficacy of this vaccine is about 70% [85, 86]. However, this vaccine can result in the rare development of immune thrombotic thrombocytopenia mediated by platelet-activating antibodies against PF4, which clinically mimics autoimmune heparin-induced thrombocytopenia, mostly in young adults [87–90]. Others adenovirus-derived vaccines against the SARS-CoV-2 infection were also associated with vaccine-induced immune thrombotic thrombocytopenia: the Janssen vaccine Ad26.COV2.S [91] and more recently the Sputnik V vaccine that uses a heterologous recombinant adenovirus approach in two injections (a prime by rAD26 and booster with rAd5) [92].
In parallel, a new approach has emerged: nucleic acid mRNA based-vaccines, like the Pfizer-BioNTech’s mRNA BNT162b2 and the Moderna mRNA-1273 vaccines. This technology has been shown in a recent meta-analysis to have a 94.6% (95% CI 0.936–0.954) efficacy [93] in a total of 34.041 cases in phase II/III randomized controlled trials [94–98]. These results have been confirmed in very large or nationwide studies [13, 99]. Although antibodies production has been investigated as the major mechanism of protection in pre-clinical and clinical studies, it has also be shown that mRNA vaccines could elicit efficient T cell-responses, which participate in severe infection protection [100, 101] (Fig. 1c).
Parallel to this unique success in vaccine development, a new threat seemed to have emerged at the end of 2020, approximatively one year after the first reports of the disease: the emergence and dissemination of variant that may be more fit, more able to escape to the immune response and may also be associated with an increased mortality. In microbiology, the emergence of mutants more fit, more virulent and more resistant to either the immune response or the antimicrobial drugs is often considered as the worst case scenario.
The first major variant reported was the variant D614G, which became dominant compared to the wild type virus both in primary airway epithelial cells and in an animal model because of more efficient infection and replication [102]. In hamsters, this variant increased the viral load in the upper respiratory tract and enhanced transmission [102, 103].
The second major variant, the alpha variant (B1.1.7) was first reported after animal studies and showed increased infectivity in mice. Increased virulence was secondary to the rapid emergence of adaptive mutations [104]. After spreading in the UK, this variant has then been detected in different parts of the world [105], was estimated to be associated with 50–70% more transmissibility [106] and might also be associated with more lethality [107]. However, this variant did not appear able to escape the immune response [108] but showed modest resistance to convalescent plasma (threefold) and vaccinee sera (twofold) [109]. Another study reported 39 SARS-CoV-2 breakthrough infections, mostly mild or asymptomatic, among 1497 fully vaccinated healthcare workers with the B.1.1.7 (alpha) variant found in 85% of samples tested [110].
By contrast, the beta variant (B.1.351) first described in South Africa emerged independently of N501Y.V1. It shares some RBD mutations with the N501.V1 variant and contains also additional mutations. It seems to have significant abilities to escape neutralizing antibodies including from plasma of convalescent patients and vaccinated sera, including mRNA based vaccine [111–113] and against the Mabs developed by Regeneron [114]. In addition to these in vitro data, a multicenter, double-blind, randomized, placebo-controlled trial was conducted in South Africa to assess the safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222). It included 2026 HIV-negative adults and demonstrated an efficacy against the V2 variant (analyzed as a secondary end point) of 10.4% (95% CI, -76.8 to 54.8) [115].
The delta variant (B.1.617 lineage), known as the indian variant, has been originally described in India in October 2020. Once again, the delta variant exhibited enhanced resistance to convalescent sera and vaccinated sera, although vaccinated sera still could neutralize the variant [116, 117]. In the United Kingdom, the effectiveness of two doses of BNT162b2 was 88% (95%CI: 85.3 to 90.1) and with ChAdOx1, the two doses effectiveness was 67% (95%CI: 61.3 to 71.8) [118], with significant decrease in hospital admission for both vaccines [119].
However, the B1.617 variant seem also able to confer escape from HLA-A24-restricted cellular immunity [120]. As COVID-19 vaccines efficacy seems to be partially driven by cellular immunity, these resistance mechanisms could lead to viral evolution and new pandemic cycles [120, 121].
The omicron variant (B.1.1.529) is the last one declared variant of concern by the WHO on November 26, 2021. It presents with a high amount of mutations, most likely responsible for its high infectivity and transmissibility [122, 123]. According to the WHO, omicron is currently the dominant variant worldwide, representing over 98% of the sequences shared on GISAID (Global Initiative on Sharing Avian Influenza Data) after February 2022 [124]. However, this new variant does not seem to induce more mortality, but this might be due to vaccination [125–127]. Numerous sublineages of Omicron have been described since its appearance [128], and these subvariants are under close monitoring by the WHO. These subvariants do not display the same fitness [129], infectivity, exhibit different resistance profiles to vaccination and monoclonal antibodies [130, 131]. In a recent study, including 134 435 participants vaccinated with two doses of vaccine, 16 087 symptomatic patients had a RT-PCR positive for Omicron. The estimated vaccine effectiveness against symptomatic omicron cases was 36% (95% CI 24%-45%) until two months following the second dose then decreased to 1% (95% -8%-10%). However, in this study, the third dose of mRNA vaccine induced, after seven days, an increase in protection up to 61% (95%CI 56%-65%) [132]. Moreover, Amano et al. demonstrated in 32 health care workers at risk of developing severe COVID-19 that a fourth dose of BNT162b2 could restore the neutralizing activity of vaccinated sera against BA.2 and BA.5 omicron subvariants, however, it could not increase the humoral response induced by the third dose [133].
Finally, the duration of vaccines’ effectiveness has also to be understood. In a company-funded study, the BNT162b2 vaccine’s effectiveness was the strongest at 96.2%, between one week and two months and then declined with a vaccine efficacy from 4 to 6 months at 83.7% (95% CI [74.7–89.9]) [134]. Buchan et al. confirmed the weaning of protection against symptomatic omicron cases from 36 to 1% after two months following the second dose of vaccine [132]. These findings were then confirmed in other studies for the different types of vaccines [135, 136].
Regarding the evolution of the antibody levels, a study on anti-SARS-CoV-2 Spike RBD IgG levels in 587 healthcare workers who completed BNT162b2 vaccination found that the average antibody titer 3 weeks after the first dose in COVID-19-naïve participants was 873.5 AU/mL (median). This was followed with a significant increase after 1 month (median 9927.2 AU/mL). However, exponential decreases were found at 3 and 6 months after complete vaccination (median 2976.7 AU/mL and 966.0 AU/mL respectively) [135].
A Possible Reason for the Inconsistent Protection Efficacy of the Convalescent Plasma and the Synthesized Monoclonal Antibodies: The Cellular Immunity Has a Crucial Role in the Protection Against the SARS-CoV-2 Infections
Owing to variable levels of affinity, serum antibody titers only partially reflect the extent of protection against SARS-CoV-2. Conversely, low or waning concentrations of plasma antibodies after SARS-CoV-2 infection or vaccination do not necessarily mean an absence of immune memory.
While antibodies against SARS-CoV-2 represent the most easily measurable part of the humoral immune response, their production relies on the development of an efficient B cell response, which itself requires support from an efficient T-cell response in order to produce antibodies with the highest affinity. In addition, the induction of SARS-CoV-2-specific effector and memory B-cells and T-cells is essential for short and long-term protection [137]. As such, the development of a robust cellular immune response is essential for the onset of an efficient defense against active infection and for the implementation of long-term protection against reinfection.
The establishment of a durable protective immunity after SARS-CoV-2 infection or vaccination requires the generation of affinity-matured plasma cells and memory B cells. This process relies on the formation of germinal centers (GC) within lymphoid organs [138]. This so-called “GC response” gives rise to affinity-matured memory B cells and long-lived plasma cells (Fig. 2).
B and T cell responses following SARS-CoV-2 infection and vaccination. Infection of respiratory epithelial cells by SARS-CoV-2 or vaccination are followed by the uptake of antigens by antigen-presenting cells (APCs). After migration to draining lymph nodes, presentation of antigens to B- and T-cells initiates T-cell differentiation and B-cell maturation through the germinal center (GC) response. The induction of type I interferons promotes differentiation of effector T-cells and T follicular helper (TFH) cells, which stimulate B-cell differentiation. B cells that encounter their cognate antigen become activated and initiate the formation of GCs. Second signals provided by follicular helper T-cells (TFH) trigger B-cell division and somatic hypermutation, finally giving rise to affinity-matured memory B-cells and long-lived plasma cells
The infection of respiratory epithelial cells by SARS-CoV-2 is followed by the uptake of antigens by antigen-presenting cells (APCs). After migration to draining lymph nodes, presentation of antigens to B- and T-cells initiates the GC response. B cells that encounter their cognate antigen become activated. Second signals provided by follicular helper T-cells (TFH) trigger B-cell division and somatic hypermutation, finally giving rise to memory B-cells and plasma cells. Memory B-cells migrate to mucosal tissues and persist for several months after infection [139]. Upon antigen re-encounter, memory B cells differentiate into antibody-producing plasma cells mediating early viral clearance, or re-enter GCs to undergo further affinity maturation. SARS-CoV-2-specific plasma cells derived from the GC response are terminally differentiated cells which migrate to the bone marrow where they persist between 7 and 11 months after infection and serve as a first line of defense against reinfection through rapid and constitutive secretion of antibodies [139] (Fig. 2).
SARS-Cov-2 infection induces a T cell response, the amplitude of which is important for the control of primary infection [140]. The induction of type I interferons by infection promotes differentiation of effector T-cells producing cytokines and cytotoxic mediators, and CD4 + TFH cells, which stimulate the differentiation of B cells into plasma cells secreting high affinity antibodies (Fig. 2). SARS-CoV-2-specific T cells persist and remain polyfunctional for at least 6 months following infection [141]. Of note, T cell responses restimulation are minimally impacted by variant mutations, suggesting that T-cell immunity might contribute to limit disease severity after infection by variant viruses [142, 143].
Similar to infection, both mRNA- and adenovirus-based vaccines have been shown to be effective in inducing T- and B-cell responses [142]. Single-stranded RNA contained within lipid nanoparticles of mRNA-based vaccines acts both as immunogen (encoding viral proteins and stimulating B-cell responses), and adjuvant owing to the intrinsic immunostimulatory properties of RNA. Engulfment of nanoparticles by dendritic cells lead to the production and presentation of spike antigens to T cells for activation of the adaptive immune response [143]. The viral particles of adenovirus-based vaccines also contain inherent adjuvant properties and induce secretion of type I interferon by antigen presenting cells through stimulation of multiple pattern-recognition receptors [144].
Conclusion
The development and distribution of an efficient anti SARS-CoV-2 vaccine has been a major focus to fight the COVID-19 pandemic with the hope to be able to reach herd immunity. A few states were considering letting the natural infection by the virus of a majority of their population to get to this point but the cost of human lives would have been significantly greater than the current one without any insurance that the herd immunity would have been reached. The success of the vaccines based on a new mRNA based approach are very promising [13, 145]. Moreover, a recent report analyzing the incidence of SARS-CoV-2 infection in vaccinated and unvaccinated nursing home residents demonstrated that the incidence of infections decreased over time in both vaccinated and unvaccinated residents suggesting a protection of unvaccinated residents by a robust vaccine-coverage among residents and staff [146].
Other approaches, vector based for instance, were also able to reach the main goal of their phase 3 clinical trial [85, 147].
Other benefits have been liked to the COVID-19 vaccination, in particular the decrease morbidities associated with both the venous [148] and arterial thromboses [149].
The main threats for the vaccine strategy are either biological, with the emergence of variants, or technical, mainly the inability to vaccinate the population due to distribution issues, lack of compliance or inequal access to vaccination.
In this review, we opted for a less than usual optimistic approach point of view, underlining the inconsistence of protection conferred by natural antibodies against SARS-CoV-2 reported to date. Combining the clinical success of the vaccine against the COVID-19 and the hypothesis that natural antibodies might not be as protective as expected against SARS-CoV-2 might appear to constitute a paradox, but we propose that it might also be a key to get a better understanding of the clinical successes that were registered.
Several mechanisms of actions could be involved. First, neutralizing antibodies (produced either by vaccination of after a natural infection) may be more efficient as prophylactic agents than therapeutic ones. This hypothesis could explain the lack of success of convalescent plasma therapy and the mitigated one with the monoclonal antibodies. Second, antibodies raised by these vaccines may be similar to the super-neutralizing antibodies discussed in this review and that are only rarely found after a natural infection. Thus, Cavanaugh et al. demonstrated in a case–control study that being unvaccinated was associated with 2.34 times the odds of reinfection with SARS-CoV-2 compared with being fully vaccinated [150]. Finally, other components of the immune system could be triggered by the vaccination. A successful T-cell response for instance was consistently reported and exploring further this response may be of major importance in developing new therapeutic or preventive strategies (Fig. 1c) [151].
Overall, by showing and discussing reports about the potential lack of clinical protection conferred by natural antibodies against SARS-CoV-2, this review mainly aims to highlight the need to have a better understanding of (i) the immune response generated during and after the SARS-CoV-2 infection and of (ii) the mechanism of action of the anti-COVID-19 vaccines, in particular if new variants lead to new pandemic cycles.
Data Availability
Not applicable.
References
W.H.O. WHO characterizes COVID-19 as a pandemic. Available: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020
University JH. John Hopkins COVID-19 Map of the Johns Hopkins Coronavirus Resource Center. Available: https://coronavirus.jhu.edu/map.htm
Zhu, J., Ji, P., Pang, J., Zhong, Z., Li, H., He, C., … Zhao, C. (2020) Clinical characteristics of 3,062 COVID-19 patients: a meta-analysis. Journal of Medical Virology, 92(10), 1902–1914. https://doi: 10.1002/jmv.25884
Smadja, D. M., Mentzer, S. J., Fontenay, M., Laffan, M. A., Ackermann, M., Helms, J., … Griffioen, A. W. (2021). COVID-19 is a systemic vascular hemopathy: Insight for mechanistic and clinical aspects. Angiogenesis, 24, 755–788.
Debuc, B., & Smadja, D. M. (2021). Is COVID-19 a New Hematologic Disease? Stem Cell Reviews and Reports, 17, 4–8.
Pons, S., Fodil, S., Azoulay, E., & Zafrani, L. (2020). The vascular endothelium: The cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Critical Care, 24, 353. https://doi.org/10.1186/s13054-020-03062-7
Zhong, N. S., Zheng, B. J., Li, Y. M., Null, P., Xie, Z. H., & Chan, K. H. (2003) Epidemiology and cause of severe acute respiratory syndrome (SARS). Guangdong, People’s Republic of China, in February. Lancet, 362(9393), 1353–8. https://doi.org/10.1016/s0140-6736(03)14630-2
Dörner, T., & Radbruch, A. (2007). Antibodies and B Cell Memory in Viral Immunity. Immunity, 27, 384–392.
Hangartner, L., Zinkernagel, R. M., & Hengartner, H. (2006). Antiviral antibody responses: The two extremes of a wide spectrum. Nature Reviews Immunology, 6, 231–243.
Wadman, M. (2021). Having SARS-CoV-2 once confers much greater immunity than a vaccine—but no infection parties, please. Sciencemagorg. https://doi.org/10.1126/science.abm1207. Available: https://www.science.org/content/article/having-sars-cov-2-once-confers-much-greater-immunity-vaccine-vaccination-remains-vital
Ni, L., Ye, F., Cheng, M.-L., Feng, Y., Deng, Y.-Q., Zhao, H., … Dong, C. (2020). Detection of SARS-CoV-2-Specific humoral and cellular immunity in COVID-19 convalescent individuals. Immunity, 52(971–7), 3.
Brochot, E., Demey, B., Touzé, A., Belouzard, S., Dubuisson, J., Schmit, J.-L., … Helle, F. (2020). Anti-spike, anti-nucleocapsid and neutralizing antibodies in SARS-CoV-2 inpatients and asymptomatic individuals. Frontiers in Microbiology, 11, 584251.
Baden, L. R., El Sahly, H. M., Essink, B., Kotloff, K., Frey, S., & Novak, R. (2021). Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. New England Journal of Medicine, 384, 403–416.
Premkumar, L., Segovia-Chumbez, B., Jadi, R., Martinez, D. R., Raut, R., & Markmann, A. (2020). The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Science Immunology, 5, 8413.
Graham, N. R., Whitaker, A. N., Strother, C. A., Miles, A. K., Grier, D., & McElvany, B. D. (2020). Kinetics and isotype assessment of antibodies targeting the spike protein receptor-binding domain of severe acute respiratory syndrome-coronavirus-2 in COVID-19 patients as a function of age, biological sex and disease severity. Clinical & Translational Immunology, 9(10), e1189.
Duan, L., Zheng, Q., Zhang, H., Niu, Y., Lou, Y., & Wang, H. (2020). The SARS-CoV-2 Spike Glycoprotein Biosynthesis, Structure, Function, and Antigenicity: Implications for the Design of Spike-Based Vaccine Immunogens. Frontiers in Immunology, 11, 576622.
McBride, R., Zyl, M., & Fielding, B. (2014). The Coronavirus Nucleocapsid Is a Multifunctional Protein. Viruses, 6, 2991–3018.
Bai, Z., Cao, Y., Liu, W., & Li, J. (2021). The SARS-CoV-2 Nucleocapsid Protein and Its Role in Viral Structure, Biological Functions, and a Potential Target for Drug or Vaccine Mitigation. Viruses, 13, 1115.
Zhao, J., Yuan, Q., Wang, H., Liu, W., Liao, X., Su, Y., … Zhang, Z. (2020). Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clinical Infectious Diseases, 71(16), 2027–2034.
Qu, J., Wu, C., Li, X., Zhang, G., Jiang, Z., Li, X., … Liu, L. (2020). Profile of immunoglobulin G and IgM antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clinical Infectious Diseases, 71(16), 2255–2258. https://doi.org/10.1093/cid/ciaa489
Long, Q. X., Tang, X. J., Shi, Q. L., Li, Q., Deng, H. J., & Yuan, J. (2020). Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nature Medicine, 26(8), 1200–1204.
Zhang, G., Nie, S., Zhang, Z., & Zhang, Z. (2020). Longitudinal Change of Severe Acute Respiratory Syndrome Coronavirus 2 Antibodies in Patients with Coronavirus Disease 2019. Journal of Infectious Diseases, 222, 183–188.
Rijkers, G., Murk, J.-L., Wintermans, B., van Looy, B., van den Berge, M., Veenemans, J., … Reimerink, J. (2020). Differences in antibody kinetics and functionality between severe and mild severe acute respiratory syndrome coronavirus 2 infections. Journal of Infectious Diseases, 222, 1265–1269.
Ko, J.-H., Müller, M. A., Seok, H., Park, G. E., Lee, J. Y., Cho, S. Y., … Peck, K. R. (2017). Serologic responses of 42 MERS-coronavirus-infected patients according to the disease severity. Diagnostic Microbiology and Infectious Disease, 89, 106–111.
Park, W. B., Perera, R. A. P. M., Choe, P. G., Lau, E. H. Y., Choi, S. J., Chun, J. Y., … Oh, M.-D. (2015). Kinetics of serologic responses to MERS coronavirus infection in humans, South Korea. Emerging Infectious Diseases, 21, 2186–2189.
Cao, W. C., Liu, W., Zhang, P. H., Zhang, F., & Richardus, J. H. (2007). Disappearance of antibodies to SARS-associated coronavirus after recovery. New England Journal of Medicine, 357, 1162–1163.
Huang, A. T., Garcia-Carreras, B., Hitchings, M. D. T., Yang, B., Katzelnick, L. C., Rattigan, S. M., … Cummings, D. A. T. (2020). A systematic review of antibody mediated immunity to coronaviruses: Kinetics, correlates of protection, and association with severity. Nature Communications, 11, 4704.
McAndrews, K. M., Dowlatshahi, D. P., Dai, J., Becker, L. M., Hensel, J., Snowden, L. M., … Kalluri, R. (2020). Heterogeneous antibodies against SARS-CoV-2 spike receptor binding domain and nucleocapsid with implications for COVID-19 immunity. JCI Insight, 5(18), e142386. https://doi.org/10.1172/jci.insight.142386
Hung, I. F.-N., Cheng, V. C.-C., Li, X., Tam, A. R., Hung, D. L.-L., Chiu, K. H.-Y., … Yuen, K.-Y. (2020). SARS-CoV-2 shedding and seroconversion among passengers quarantined after disembarking a cruise ship: A case series. The Lancet Infectious Diseases, 20, 1051–1060.
Wang, B., Wang, L., Kong, X., Geng, J., Xiao, D., Ma, C., … Wang, P. (2020). Long-term coexistence of SARS-CoV-2 with antibody response in COVID-19 patients. Journal of Medical Virology, 92, 1684–1689.
Soresina, A., Moratto, D., Chiarini, M., Paolillo, C., Baresi, G., Focà, E., … Badolato, R. (2020). Two X‐linked agammaglobulinemia patients develop pneumonia as COVID‐19 manifestation but recover. Pediatric Allergy and Immunology, 31(5), 565–569. https://doi.org/10.1111/pai.13263
Quinti, I., Lougaris, V., Milito, C., Cinetto, F., Pecoraro, A., Mezzaroma, I., … Plebani, A. (2020). A possible role for B cells in COVID-19? Lesson from patients with agammaglobulinemia. The Journal of Allergy and Clinical Immunology, 146(211–3), 4.
Seow, J., Graham, C., Merrick, B., Acors, S., Pickering, S., Steel, K. J. A., … Doores, K. J.(2020). Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nature Microbiology, 5, 1598–1607.
Wang, P., Liu, L., Nair, M. S., Yin, M. T., Luo, Y., Wang, Q., … Ho, D. D.(2020). SARS-CoV-2 neutralizing antibody responses are more robust in patients with severe disease. Emerging Microbes & Infections, 9, 2091–2093.
Shen, C., Wang, Z., Zhao, F., Yang, Y., Li, J., Yuan, J., … Liu, L. (2020). Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA, 323, 1582.
Zeng, Q.-L., Yu, Z.-J., Gou, J.-J., Li, G.-M., Ma, S.-H., Zhang, G.-F., … Liu, Z.-S. (2020). Effect of convalescent plasma therapy on viral shedding and survival in patients with coronavirus disease 2019. Journal of Infectious Diseases, 222, 38–43.
Agarwal, A., Mukherjee, A., Kumar, G., Chatterjee, P., Bhatnagar, T., & Malhotra, P. (2020). Convalescent plasma in the management of moderate covid-19 in adults in India: Open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ, 371, m3939.
Li, L., Zhang, W., Hu, Y., Tong, X., Zheng, S., Yang, J., … Liu, Z. (2020). Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: A randomized clinical trial. JAMA, 324(5), 460.
Rogers, R., Shehadeh, F., Mylona, E. K., Rich, J., Neill, M., Touzard-Romo, F., … Mylonakis, E. (2020). Touzard-Romo F. Convalescent plasma for patients with severe COVID-19: a matched cohort study. Clinical Infectious Diseases, 73(1), e208–e214.
Hegerova, L., Gooley, T. A., Sweerus, K. A., Maree, C., Bailey, N., Bailey, M., … Pagel, J. M. (2020). Use of convalescent plasma in hospitalized patients with COVID-19: Case series. Blood, 136, 759–762.
Simonovich, V. A., Burgos Pratx, L. D., Scibona, P., Beruto, M. V., Vallone, M. G., Vázquez, C., … PlasmAr Study Group. (2020) A randomized trial of convalescent plasma in covid-19 severe pneumonia. The New England Journal of Medicine, 384(7), 619–629. https://doi.org/10.1056/NEJMoa2031304
Libster, R., Pérez Marc, G., Wappner, D., Coviello, S., Bianchi, A., Braem, V., … Polack, F. P. (2021). Early High-Titer Plasma Therapy to Prevent Severe Covid-19 in Older Adults. New England Journal of Medicine, 384, 610–618.
Chai, K. L., Valk, S. J., Piechotta, V., Kimber, C., Monsef, I., Doree, C., … Skoetz, N. (2020) Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: A living systematic review. Cochrane Database of Systematic Reviews, 10, CD013600. https://doi.org/10.1002/14651858.CD013600.pub3
Abani, O., Abbas, A., Abbas, F., Abbas, M., Abbasi, S., Abbass, H., … Zyengi, S. (2021). Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): A randomised controlled, open-label, platform trial. The Lancet, 397(10289), 2049–2059. https://doi.org/10.1016/S0140-6736(21)00897-7
Sullivan, D. J., Gebo, K. A., Shoham, S., Bloch, E. M., Lau, B., Shenoy, A. G., … Hanley, D. F. (2022). Early outpatient treatment for covid-19 with convalescent plasma. New England Journal of Medicine, 386, 1700–1711.
Alemany, A., Millat-Martinez, P., Corbacho-Monné, M., Malchair, P., Ouchi, D., Ruiz-Comellas, A., … CONV-ERT Group. (2022). High-titre methylene blue-treated convalescent plasma as an early treatment for outpatients with COVID-19: A randomised, placebo-controlled trial. The Lancet Respiratory Medicine, 10, 278–288.
K, B., Tn, G., M, V., F, L., R, S., C, B. (2022). Convalescent plasma in the treatment of moderate to severe COVID-19 pneumonia: a randomized controlled trial (PROTECT-Patient Trial. Scientific Report. 12:2552.
Ortigoza, M. B., Yoon, H., Goldfeld, K. S., Troxel, A. B., Daily, J. P., & Wu, Y. (2022). Efficacy and Safety of COVID-19 Convalescent Plasma in Hospitalized Patients: A Randomized Clinical Trial. JAMA Internal Medicine, 182, 115.
Szwebel, T. A., Veyer, D., Robillard, N., Eshagh, D., Canoui, E., & Bruneau, T. (2021). Usefulness of Plasma SARS-CoV-2 RNA Quantification by Droplet-based Digital PCR to Monitor Treatment Against COVID-19 in a B-cell Lymphoma Patient. Stem Cell Reviews and Reports, 17, 296–299.
Hueso, T., Pouderoux, C., Péré, H., Beaumont, A. L., Raillon, L. A., Ader, F. (2020), Convalescent plasma therapy for B-cell depleted patients with protracted COVID-19 disease. Blood. 136(20): 2290–2295. doi: 10.1182/blood.2020008423
Hueso, T., Godron, A. S., Lanoy, E., Pacanowski, J., Levi, L. I., & Gras, E. (2022). Convalescent plasma improves overall survival in patients with B-cell lymphoid malignancy and COVID-19: A longitudinal cohort and propensity score analysis. Leukemia, 36(4), 1025–1034. https://doi.org/10.1038/s41375-022-01511-6
Robbiani, D. F., Gaebler, C., Muecksch, F., Lorenzi, J. C. C., Wang, Z., & Cho, A. (2020). Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature, 584, 437–442.
Liu, L., Wang, P., Nair, M. S., Yu, J., Rapp, M., & Wang, Q. (2020). Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature, 584, 450–456.
Seydoux, E., Homad, L. J., MacCamy, A. J., Parks, K. R., Hurlburt, N. K., & Jennewein, M. F. (2020). Analysis of a SARS-CoV-2-Infected Individual Reveals Development of Potent Neutralizing Antibodies with Limited Somatic Mutation. Immunity, 53(98–105), 5.
Kreer, C., Zehner, M., Weber, T., Ercanoglu, M. S., Gieselmann, L., & Rohde, C. (2020). Longitudinal Isolation of Potent Near-Germline SARS-CoV-2-Neutralizing Antibodies from COVID-19 Patients. Cell, 182(843–54), 12.
Rogers, T. F., Zhao, F., Huang, D., Beutler, N., Burns, A., & He, W. (2020). Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science, 369, 956–963.
See, R. H., Zakhartchouk, A. N., Petric, M., Lawrence, D. J., Mok, C. P. Y., & Hogan, R. J. (2006). Comparative evaluation of two severe acute respiratory syndrome (SARS) vaccine candidates in mice challenged with SARS coronavirus. Journal of General Virology, 87, 641–650.
Klingler, J., Weiss, S., Itri, V., Liu, X., Oguntuyo, K. Y., & Stevens, C. (2021). Role of Immunoglobulin M and A Antibodies in the Neutralization of Severe Acute Respiratory Syndrome Coronavirus 2. Journal of Infectious Diseases, 223, 957–970.
Cervia, C., Nilsson, J., Zurbuchen, Y., Valaperti, A., Schreiner, J., & Wolfensberger, A. (2021). Systemic and mucosal antibody responses specific to SARS-CoV-2 during mild versus severe COVID-19. The Journal of Allergy and Clinical Immunology, 147(545–57), 9.
Sterlin, D., Mathian, A., Miyara, M., Mohr, A., Anna, F., & Claër, L. (2021). IgA dominates the early neutralizing antibody response to SARS-CoV-2. Science Translational Medicine, 13, 2223.
Yu, H., Sun, B., Fang, Z., Zhao, J., Liu, X., & Li, Y. (2020). Distinct features of SARS-CoV-2-specific IgA response in COVID-19 patients. European Respiratory Journal, 56, 2001526.
Hasan Ali, O., Bomze, D., Risch, L., Brugger, S. D., Paprotny, M., Weber, M., … Flatz, L. (2020). Severe COVID-19 is associated with elevated serum IgA and antiphospholipid IgA-antibodies. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, 73(9):e2869–e2874. https://doi.org/10.1093/cid/ciaa1496
Zuo, Y., Estes, S. K., Ali, R. A., Gandhi, A. A., Yalavarthi, S., Shi, H., et al. (2020). Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Science Translational Medicine, 12(570), eabd3876. https://doi.org/10.1126/scitranslmed.abd3876
Gendron, N., Dragon-Durey, M. A., Chocron, R., Darnige, L., Jourdi, G., Philippe, A., et al. (2021). Lupus anticoagulant single positivity during the acute phase of COVID-19 is not associated with venous thromboembolism or in-hospital mortality. Arthritis & Rhematology, 73(11), 1976–1985.
Wang, X., Gkrouzman, E., Andrade, D. C. O., Andreoli, L., Barbhaiya, M., Belmont, H. M., et al. (2021). COVID-19 and antiphospholipid antibodies: A position statement and management guidance from AntiPhospholipid Syndrome Alliance for Clinical Trials and InternatiOnal Networking (APS ACTION). Lupus, 30(14), 2276–2285.
Althaus, K., Marini, I., Zlamal, J., Pelzl, L., Singh, A., Haberle, H., et al. (2021). Antibody-induced procoagulant platelets in severe COVID-19 infection. Blood, 137(8), 1061–1071.
Bastard, P., Rosen, L. B., Zhang, Q., Michailidis, E., Hoffmann, H. H., Zhang, Y., et al. (2020). Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science, 370(6515), eabd4585. https://doi.org/10.1126/science.abd4585
Zhang, Q., Pizzorno, A., Miorin, L., Bastard, P., Gervais, A., Le Voyer, T., et al. (2022). Autoantibodies against type I IFNs in patients with critical influenza pneumonia. Journal of Experimental Medicine, 219(11), e20220514. https://doi.org/10.1084/jem.20220514
Pinto, D., Park, Y. J., Beltramello, M., Walls, A. C., Tortorici, M. A., & Bianchi, S. (2020). Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature, 583, 290–295.
Cao, Y., Su, B., Guo, X., Sun, W., Deng, Y., & Bao, L. (2020). Potent Neutralizing Antibodies against SARS-CoV-2 Identified by High-Throughput Single-Cell Sequencing of Convalescent Patients’ B Cells. Cell, 182(1), 73-84.e16.
Baum, A., Fulton, B. O., Wloga, E., Copin, R., Pascal, K. E., & Russo, V. (2020). Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science, 369(6506), 1014–1018. https://doi.org/10.1126/science.abd0831
Weinreich, D. M., Sivapalasingam, S., Norton, T., Ali, S., Gao, H., & Bhore, R. (2021). REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19. New England Journal of Medicine, 384, 238–251.
O’Brien, M. P., Forleo-Neto, E., Musser, B. J., Isa, F., Chan, K. C., & Sarkar, N. (2021). Subcutaneous REGEN-COV antibody combination to prevent Covid-19. The New England Journal of Medicine, 385(13), 1184–1195. https://doi.org/10.1056/NEJMoa2109682
Isa, F., Forleo-Neto, E., Meyer, J., Zheng, W., Rasmussen, S., Armas, D., ... for the COVID-19 Multi-dose Trial Team. (2022). Repeat subcutaneous administration of casirivimab and imdevimab in adults is well-tolerated and prevents the occurrence of COVID-19. International Journal of Infectious Diseases, 122, 585–592.
Jones, B. E., Brown-Augsburger, P. L., Corbett, K. S., Westendorf, K., Davies, J., & Cujec, T. P. (2021). The neutralizing antibody, LY-CoV555, protects against SARS-CoV-2 infection in nonhuman primates. Science Translational Medicine, 13, 1906.
Chen, P., Nirula, A., Heller, B., Gottlieb, R. L., Boscia, J., & Morris, J. (2020). SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. The New England Journal of Medicine, 384(3), 229–237. https://doi.org/10.1056/NEJMoa2029849
Group ACTIVTICOLYCS. (2020). A neutralizing monoclonal antibody for hospitalized patients with Covid-19. The New England Journal of Medicine, 384, 905–914. https://doi.org/10.1056/NEJMoa2033130
Benschop, R. J., Tuttle, J. L., Zhang, L., Poorbaugh, J., Kallewaard, N. L., Vaillancourt, P., … Marovich, M. (2022). The anti-SARS-CoV-2 monoclonal antibody bamlanivimab minimally affects the endogenous immune response to COVID-19 vaccination. Science Translational Medicine, 14, 3041.
Agarwal, A., Rochwerg, B., Lamontagne, F., Siemieniuk, R. A., Agoritsas, T., Askie, L., … Vandvik, P. O. (2020). A living WHO guideline on drugs for covid-19. BMJ, 370, m3379. https://doi.org/10.1136/bmj.m3379
Bruel, T., Hadjadj, J., Maes, P., Planas, D., Seve, A., Staropoli, I., … Schwartz, O (2022). Serum neutralization of SARS-CoV-2 Omicron sublineages BA.1 and BA.2 in patients receiving monoclonal antibodies. Nature Medicine, 28, 1297–302.
Iketani, S., Liu, L., Guo, Y., Liu, L., Chan, J. F.-W., Huang, Y., … Ho, D. D. (2022). Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature, 604, 553–556.
Wang, Q., Guo, Y., Iketani, S., Nair, M. S., Li, Z., Mohri, H., … Ho, D. D. (2022). Antibody evasion by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4 and BA.5. Nature, 608, 603–8.
Westendorf, K., Žentelis, S., Wang, L., Foster, D., Vaillancourt, P., Wiggin, M., … Barnhart, B. C. (2022). LY-CoV1404 (bebtelovimab) potently neutralizes SARS-CoV-2 variants. Cell Reports, 39, 110812.
World Health Organization. (2021) COVID-19 vaccine tracker and landscape. Available: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
Voysey, M., Clemens, S. A. C., Madhi, S. A., Weckx, L. Y., Folegatti, P. M., Aley, P. K., … Zuidewind, P. (2021). Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. The Lancet, 397, 99–111.
Lopez Bernal, J., Andrews, N., Gower, C., Robertson, C., Stowe, J., Tessier, E., … Ramsay, M. (2021). Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ, 373, n1088. https://doi.org/10.1136/bmj.n1088
Greinacher, A., Thiele, T., Warkentin, T. E., Weisser, K., Kyrle, P. A., & Eichinger, S. (2021). Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. New England Journal of Medicine, 384, 2092–2101.
Scully, M., Singh, D., Lown, R., Poles, A., Solomon, T., Levi, M., … Lester, W. (2021). Pathologic Antibodies to Platelet Factor 4 after ChAdOx1 nCoV-19 Vaccination. New England Journal of Medicine, 384, 2202–2211.
Bérezné, A., Bougon, D., Blanc-Jouvan, F., Gendron, N., Janssen, C., Muller, M., … Smadja, D. M. (2021). Deterioration of vaccine-induced immune thrombotic thrombocytopenia treated by heparin and platelet transfusion: Insight from functional cytometry and serotonin release assay. Research and Practice in Thrombosis and Haemostasis., 5, 12572.
Smadja, D. M., Yue, Q. Y., Chocron, R., Sanchez, O., & Lillo-Le, L. A. (2021). Vaccination against COVID-19: Insight from arterial and venous thrombosis occurrence using data from VigiBase. European Respiratory Journal, 58, 2100956.
See, I., Su, J. R., Lale, A., Woo, E. J., Guh, A. Y., Shimabukuro, T. T., … Broder, K. R. (2021). US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S Vaccination, March 2 to April 21, 2021. JAMA, 325(24), 2448–56.
Herrera-Comoglio, R., & Lane, S. (2022). Vaccine-Induced Immune Thrombocytopenia and Thrombosis after the Sputnik V Vaccine. New England Journal of Medicine, 387(15), 1431–1432.
Pormohammad, A., Zarei, M., Ghorbani, S., Mohammadi, M., Razizadeh, M. H., & Turner, D. L. (2021). Efficacy and safety of COVID-19 vaccines: a systematic review and meta-analysis of randomized clinical trials. Vaccines., 9, 467.
Jackson, L. A., Anderson, E. J., Rouphael, N. G., Roberts, P. C., Makhene, M., Coler, R. N., … Beigel, J. H. (2020). An mRNA Vaccine against SARS-CoV-2 — Preliminary Report. New England Journal of Medicine, 383, 1920–1931.
Anderson, E. J., Rouphael, N. G., Widge, A. T., Jackson, L. A., Roberts, P. C., Makhene, M., … mRNA-1273 Study Group. (2020). Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. The New England Journal of Medicine, 383(25), 2427–2438. https://doi.org/10.1056/NEJMoa2028436
Walsh, E. E., Frenck, R. W., Falsey, A. R., Kitchin, N., Absalon, J., Gurtman, A., … Gruber, W. C. (2020). Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. New England Journal of Medicine, 383, 2439–2450.
Mulligan, M. J., Lyke, K. E., Kitchin, N., Absalon, J., Gurtman, A., Lockhart, S., … Jansen, K. U. (2020). Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature, 586, 589–593.
Chu, L., McPhee, R., Huang, W., Bennett, H., Pajon, R., Nestorova, B., & Leav, B. (2021). A preliminary report of a randomized controlled phase 2 trial of the safety and immunogenicity of mRNA-1273 SARS-CoV-2 vaccine. Vaccine., 39, 2791–2799.
Dagan, N., Barda, N., Kepten, E., Miron, O., Perchik, S., Katz, M. A., … Balicer, R. D. (2021). BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. New England Journal of Medicine, 384, 1412–1423.
Woldemeskel, B. A., Garliss, C. C., & Blankson, J. N. (2021). SARS-CoV-2 mRNA vaccines induce broad CD4+ T cell responses that recognize SARS-CoV-2 variants and HCoV-NL63. The Journal of Clinical Investigation, 131, 149335.
Sahin, U., Muik, A., Derhovanessian, E., Vogler, I., Kranz, L. M., Vormehr, M., … Türeci, Ö. (2020). COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature, 586, 594–599.
Hou, Y. J., Chiba, S., Halfmann, P., Ehre, C., Kuroda, M., & Dinnon, K. H. (2020). SARS-CoV-2 D614G variant exhibits efficient replication ex vivo and transmission in vivo. Science, 370(6523), 1464–1468. https://doi.org/10.1126/science.abe8499
Plante, J. A., Liu, Y., Liu, J., Xia, H., Johnson, B. A., & Lokugamage, K. G. (2020). Spike mutation D614G alters SARS-CoV-2 fitness. Nature, 592(7852), 116–121. https://doi.org/10.1038/s41586-020-2895-3
Gu, H., Chen, Q., Yang, G., He, L., Fan, H., Deng, Y.-Q., … Zhou, Y. (2020). Adaptation of SARS-CoV-2 in BALB/c mice for testing vaccine efficacy. Science, 369(6511), 1603-1607. https://doi.org/10.1126/science.abc4730
Bal, A., Destras, G., Gaymard, A., Stefic, K., Marlet, J., Eymieux, S., … the COVID-Diagnosis HCL Study Group.(2020). Two-step strategy for the identification of SARS-CoV-2 variant of concern 202012/01 and other variants with spike deletion H69–V70. Eurosurveillance, 26(3):2100008. doi: 10.2807/1560-7917.ES.2021.26.3.2100008.
Davies, N. G., Abbott, S., Barnard, R. C., Jarvis, C. I., Kucharski, A. J., Munday, J. D., … Edmunds, W. J. (2021). Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science, 372(6538), eabg3055. https://doi.org/10.1126/science.abg3055
Horby, P. (2021). NERVTAG paper on COVID-19 variant of concern B.1.1.7: NERVTAG note on B.1.1.7 severity. Available: https://www.gov.uk/government/publications/nervtag-update-note-on-b117-severity-11-february-2021
Rathnasinghe, R., Jangra, S., Ye, C., Cupic, A., Singh, G., Martínez-Romero, C., … Schotsaert, M. (2022). Characterization of SARS-CoV-2 Spike mutations important for infection of mice and escape from human immune sera. Nature Communications, 13(1), 3921. https://doi.org/10.1038/s41467-022-30763-0
Ho, D., Wang, P., Liu, L., Iketani, S., Luo, Y., Guo, Y., … Huang, Y. (2021). Increased resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7 to antibody neutralization. (preprint). https://doi.org/10.21203/rs.3.rs-155394/v1
Bergwerk, M., Gonen, T., Lustig, Y., Amit, S., Lipsitch, M., Cohen, C., … Regev-Yochay, G. (2021). Covid-19 breakthrough infections in vaccinated health care workers. The New England Journal of Medicine, 385, 1474–1484. https://doi.org/10.1056/NEJMoa2109072
Chen, R. E., Zhang, X., Case, J. B., Winkler, E. S., Liu, Y., VanBlargan, L. A., … Diamond, M. S. (2021). Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies. Nature Medicine, 27(4), 717–726. https://doi.org/10.1038/s41591-021-01294-w
Wu, K., Werner, A. P., Koch, M., Choi, A., Narayanan, E., Stewart-Jones, G. B. E., … Edwards, D. K. (2021). Serum Neutralizing Activity Elicited by mRNA-1273 Vaccine. The New England Journal of Medicine, 384(15), 1468–1470. https://doi.org/10.1056/NEJMc2102179
Kuzmina, A., Khalaila, Y., Voloshin, O., Keren-Naus, A., Boehm-Cohen, L., Raviv, Y., … Taube, R. (2021). SARS-CoV-2 spike variants exhibit differential infectivity and neutralization resistance to convalescent or post-vaccination sera. Cell Host & Microbe, 29(4), 522–528.e2. https://doi.org/10.1016/j.chom.2021.03.008
Tada, T., Dcosta, B. M., Zhou, H., Vaill, A., Kazmierski, W., & Landau, N. R. (2021). Decreased neutralization of SARS-CoV-2 global variants by therapeutic anti-spike protein monoclonal antibodies. bioRxiv: The Preprint Server for Biology, 2021.02.18.431897. https://doi.org/10.1101/2021.02.18.431897. Preprint
Madhi, S. A., Baillie, V., Cutland, C. L., Voysey, M., Koen, A. L., Fairlie, L., … Izu, A. (2021). Efficacy of the ChAdOx1 nCoV-19 Covid-19 Vaccine against the B.1.351 Variant. The New England Journal of Medicine, 384(20):1885–1898. https://doi.org/10.1056/NEJMoa2102214
Edara, V.-V., Lai, L., Sahoo, M. K., Floyd, K., Sibai, M., Solis, D., … Suthar, M. S. (2021). Infection and vaccine-induced neutralizing antibody responses to the SARS-CoV-2 B.1.617.1 variant. bioRxiv: The Preprint Server for Biology, 2021.05.09.443299. https://doi.org/10.1101/2021.05.09.443299
Liu, J., Liu, Y., Xia, H., Zou, J., Weaver, S. C., Swanson, K. A., … Shi, P.-Y. (2021) BNT162b2-elicited neutralization of B.1.617 and other SARS-CoV-2 variants. Nature, 596(7871), 273–275. https://doi.org/10.1038/s41586-021-03693-y
Lopez Bernal, J., Andrews, N., Gower, C., Gallagher, E., Simmons, R., Thelwall, S., … Ramsay, M. (2021). Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. The New England Journal of Medicine, 385(7), 585–594. https://doi.org/10.1056/NEJMoa2108891
Vasileiou, E., Simpson, C. R., Shi, T., Kerr, S., Agrawal, U., Akbari, A., … Sheikh, A. (2021). Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: A national prospective cohort study. The Lancet., 397, 1646–1657.
Motozono, C., Toyoda, M., Zahradnik, J., Saito, A., Nasser, H., Tan, T. S., … Sato, K.(2021). SARS-CoV-2 spike L452R variant evades cellular immunity and increases infectivity. Cell Host & Microbe, 29(1124–36), 11.
Liu, J., Chandrashekar, A., Sellers, D., Barrett, J., Jacob-Dolan, C., Lifton, M., … Barouch, D. H. (2022). Vaccines elicit highly conserved cellular immunity to SARS-CoV-2 Omicron. Nature, 603, 493–496.
Syed, A. M., Ciling, A., Taha, T. Y., Chen, I. P., Khalid, M. M., & Sreekumar, B. (2022). Omicron mutations enhance infectivity and reduce antibody neutralization of SARS-CoV-2 virus-like particles. Proceedings of the National Academy of Sciences, 119(31), 2200592119.
Chen, J., Wang, R., Gilby, N. B., & Wei, G. W. (2022). Omicron Variant (B.1.1.529): Infectivity, vaccine breakthrough, and antibody resistance. Journal of Chemical Information and Modeling, 62, 412–22.
Who WHO. (2022). Tracking SARS-CoV-2 variants. Available: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/
Butt, A. A., Dargham, C. S. R., P, Y., Hm, A.-K., A, A.-S., A, B. (2022). COVID-19 disease severity in persons infected with omicron BA.1 and BA.2 sublineages and association with vaccination status. JAMA Internal Medicine, 182(10), 1097–1099. https://doi.org/10.1001/jamainternmed.2022.3351
Wang, J., Choy, K. W., Lim, H. Y., Ho, P. (2022). Laboratory markers of severity across three COVID-19 outbreaks in Australia: Has Omicron and vaccinations changed disease presentation? Internal and Emergency Medicine. https://doi.org/10.1007/s11739-022-03081-y
Nyberg, T., Ferguson, N. M., Nash, S. G., Webster, H. H., Flaxman, S., Andrews, N., … Thelwall, S. (2022). Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: A cohort study. The Lancet. 399, 1303–12.
Dhawan, M., Saied, A. A., Mitra, S., Alhumaydhi, F. A., Emran, T. B., Wilairatana, P. (2022). Omicron variant (B.1.1.529) and its sublineages: What do we know so far amid the emergence of recombinant variants of SARS-CoV-2? Biomedicine & Pharmacotherapy, 154, 113522.
Obermeyer, F., Jankowiak, M., Barkas, N., Schaffner, S. F., Pyle, J. D., Yurkovetskiy, L., … Lemieux, J. E. (2022). Analysis of 6.4 million SARS-CoV-2 genomes identifies mutations associated with fitness. Science, 376, 1327–32.
Yamasoba, D., Kimura, I., Nasser, H., Morioka, Y., Nao, N., Ito, J., … Sato, K. (2022). Virological characteristics of the SARS-CoV-2 Omicron BA.2 spike. Cell, 85, 2103–15 19.
Kirsebom, F. C. M., Andrews, N., Stowe, J., Toffa, S., Sachdeva, R., Gallagher, E., … Bernal, J. L. (2022). COVID-19 vaccine effectiveness against the omicron (BA.2) variant in England. The Lancet Infectious Diseases, 22, 931–3.
Buchan, S. A., Chung, H., Brown, K. A., Austin, P. C., Fell, D. B., Gubbay, J. B., … Kwong, J. C. (2022). Estimated Effectiveness of COVID-19 Vaccines Against Omicron or Delta Symptomatic Infection and Severe Outcomes. JAMA Network Open, 5, 2232760.
Amano, M., Otsu, S., Ichikawa, Y., Higashi-Kuwata, N., Matsushita, S., Shimada, S. (2022). Restoration of neutralization activity against Omicrons BA.2 and BA.5 in older adults and individuals with risk factors following the 4th-dose of SARS-CoV-2 BNT162b2 vaccine. The Journal of Infectious Diseases, jiac393. https://doi.org/10.1093/infdis/jiac393. Online ahead of print.
Thomas, S. J., Moreira, E. D., Kitchin, N., Absalon, J., Gurtman, A., Lockhart, S., … Jansen, K. U. (2021). Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months. New England Journal of Medicine, 385, 1761–1773.
Đaković Rode, O., Bodulić, K., Zember, S., Cetinić Balent, N., Novokmet, A., Čulo, M., … Markotić, A. (2022). Decline of Anti-SARS-CoV-2 IgG antibody levels 6 months after Complete BNT162b2 vaccination in healthcare workers to levels observed following the first vaccine dose. Vaccines, 10, 153.
Feikin, D. R., Higdon, M. M., Abu-Raddad, L. J., Andrews, N., Araos, R., Goldberg, Y., … Patel, M. K. (2022). Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. The Lancet., 399, 924–944.
Cox, R. J., & Brokstad, K. A. (2020). Not just antibodies: B cells and T cells mediate immunity to COVID-19. Nature Reviews Immunology, 20, 581–582.
Laidlaw, B. J., & Ellebedy, A. H. (2022). The germinal centre B cell response to SARS-CoV-2. Nature Reviews Immunology, 22, 7–18.
Turner, J. S., Kim, W., Kalaidina, E., Goss, C. W., Rauseo, A. M., Schmitz, A. J., … Ellebedy, A. H. (2021). SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans. Nature, 595, 421–425.
Rydyznski Moderbacher, C., Ramirez, S. I., Dan, J. M., Grifoni, A., Hastie, K. M., Weiskopf, D., … Crotty, S. (2020). Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell, 183, 996–1012 19.
Breton, G., Mendoza, P., Hägglöf, T., Oliveira, T. Y., Schaefer-Babajew, D., Gaebler, C., … Nussenzweig, M. C.(2021). Persistent cellular immunity to SARS-CoV-2 infection. Journal of Experimental Medicine, 218, 20202515.
Teijaro, F. J. R., D, L. (2021). COVID-19 vaccines: Modes of immune activation and future challenges. Nature Reviews Immunology, 21, 195–7.
Pardi, N., Hogan, M. J., Porter, F. W., & Weissman, D. (2018). mRNA vaccines — a new era in vaccinology. Nature Reviews. Drug Discovery, 17, 261–279.
Sayedahmed, E. E., Elkashif, A., Alhashimi, M., Sambhara, S., & Mittal, S. K. (2020). Adenoviral Vector-Based Vaccine Platforms for Developing the Next Generation of Influenza Vaccines. Vaccines, 8, 574.
Polack, F. P., Thomas, S. J., Kitchin, N., Absalon, J., Gurtman, A., Lockhart, S., … Gruber, W. C. (2020). Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. New England Journal of Medicine, 383, 2603–2615.
White, E. M., Yang, X., Blackman, C., Feifer, R. A., Gravenstein, S., & Mor, V. (2021). Incident SARS-CoV-2 infection among mRNA-vaccinated and unvaccinated nursing home residents. New England Journal of Medicine, 385(5), 474–476. https://doi.org/10.1056/NEJMc2104849
Folegatti, P. M., Ewer, K. J., Aley, P. K., Angus, B., Becker, S., Belij-Rammerstorfer, S., … Oxford COVID Vaccine Trial Group. (2020). Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet, 396, 467–478.
Xie, J., Prats-Uribe, A., Feng, Q., Wang, Y., Gill, D., Paredes, R., Prieto-Alhambra, D. (2022). Clinical and Genetic Risk Factors for Acute Incident Venous Thromboembolism in Ambulatory Patients With COVID-19. JAMA Internal Medicine, 182(10), 1063–1070.
Kim, Y. E., Huh, K., Park, Y. J., Peck, K. R., & Jung, J. (2022). Association Between Vaccination and Acute Myocardial Infarction and Ischemic Stroke After COVID-19 Infection. JAMA, 328(9), 887–889.
Cavanaugh, A. M., Spicer, K. B., Thoroughman, D., Glick, C., Winter, K. (2021). Reduced Risk of Reinfection with SARS-CoV-2 After COVID-19 Vaccination — Kentucky, May–June 2021. MMWR. Morbidity and Mortality Weekly Report. 70.
he Oxford COVID Vaccine Trial Group, Ewer, K. J., Barrett, J. R., Belij-Rammerstorfer, S., Sharpe, H., Makinson, R., … Lambe, T. (2021) T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nature Medicine, 27(2), 270–8.
Author information
Authors and Affiliations
Contributions
SP, and DS wrote the original draft original manuscript. FH, EF, YS, HG, LZ equally contributed to the writing.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publish
Yes.
Competing Interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pons, S., Uhel, F., Frapy, E. et al. How Protective are Antibodies to SARS-CoV-2, the Main Weapon of the B-Cell Response?. Stem Cell Rev and Rep 19, 585–600 (2023). https://doi.org/10.1007/s12015-022-10477-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-022-10477-y