Abstract
Ionizing radiation (IR) is an important medical tool. Despite the effects associated with high-dose radiation during or after treatment, as well as in accidental exposures, the direct or indirect effect of low-dose IR in cells remain poorly documented. IR can affect the tissue microenvironment, including mesenchymal stem cells (MSCs), which have high regenerative and immunomodulatory capacities. This study aimed to investigate the effect of low-dose IR in association with the inflammatory stimuli of TNF-α on the immunomodulatory capacity of MSCs. MSCs were irradiated with a low-dose IR, stimulated with TNF-α, and cultivated in a bystander system with murine spleen cells. The results showed that TNF-R1 is expressed in MSCs and is not affected, even in irradiated MSCs. However, irradiated MSCs produced reduced amounts of IL-6 and increased amounts of IL-10. The levels of PGE2 and NO• in MSCs were also increased when stimulated with TNF-α. Furthermore, conditioned media from irradiated MSCs reduced the proliferation of bystander lymphocytes and reduced the metabolic activity of macrophages. In addition, conditioned media from irradiated MSCs modulated the profile of cytokines in bystander spleen cells (lymphocytes and macrophages), reducing inflammatory and increasing anti-inflammatory cytokines, also increasing Treg cells. In conclusion, low-dose IR in association with an inflammatory stimulus affects the immunomodulatory properties of MSCs. In this way, the immunosuppressive capability of MSCs can be explored for several disease treatments where IR usually part of the context of the treatment. However, a complete understanding of the mechanisms underlying these interactions need further investigation.

Graphical Abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mesenchymal stem cells (MSCs) have a mesodermal origin and play an important role in the formation and regulation of the microenvironment of tissues. They also act as a vital component in the reconstruction of injured tissues and in the regulation of the immune system during tissue repair [1, 2]. Moreover, MSCs produce soluble factors, especially cytokines and growth factors, that act in the repair and regeneration of damaged tissues, as well as modulate the cellular components of the innate and adaptive immune responses [3,4,5].
Ionizing radiation (IR) is an important medical tool. Despite numerous effects associated with high-dose radiation during or after treatment, as well as in accidental exposures, the direct or indirect effect of low-dose IR in cells remain poorly documented [6,7,8]. Data in the literature shows that radiation may cause damage to the microenvironment in tissues, even in low doses [9], and is usually associated with inflammation [10]. However, this subject is not completely elucidated. It is accepted that different cytokines, especially tumor necrosis factor alpha (TNF-α), play a pivotal role in tissue inflammation affecting the entire microenvironment of cells [11, 12].
Therefore, under stress conditions, MSCs are susceptible to environmental changes, and their immunomodulatory functions can be activated by exposure to pro-inflammatory cytokines [13, 14], especially TNF-α, which trigger its pro-inflammatory effects by binding to the type 1 TNF receptor (TNF-R1) [15, 16].
Taking into consideration that IR can affect (even in low doses) cells of the tissue microenvironment, also that MSCs are present in the microenvironment, and that these cells have high regenerative and immunoregulatory capacities that are specially regulated by inflammation, this study aimed to understand the effect of low-dose radiation in bystander cells in association with a TNF-α stimulus on some immunomodulatory functions of the MSCs to provide new approaches on the effects of IR on MSCs.
Materials and Methods
MSCs: Cell Line and Cell Irradiation
The mouse C3H/10T1/2 lineage of MSCs used in this study were acquired from ATCC ® (Clone 8, CCL-226™; American Type Culture Collection, Rockville, MD, USA). The MSCs were cultured in low glucose DMEM (Corning, Manassas, VA, USA) supplemented with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT, USA) and penicillin (U/mL) and streptomycin (100 mg/mL) (Sigma-Aldrich, Natick, MA, USA). The MSCSs were used between passages 7 and 9. The cells were grown at 37 °C in a humidified cell incubator, with an atmosphere consisting of 5% CO2. Cells were harvested and plated in 6- or 12-well culture plates and cultivated for 24 h. After that, cells were irradiated using a linear particle accelerator (LINAC) of 6 MeV (Varian, Palo Alto, CA, USA) at a dose rate of 3Gy/min. Plates were placed in a field camp of 35 × 35 cm on a 0.5 cm acrylic plate and received a single dose of 0.5Gy (the Gantry angle rotated by 180° and in a source-to-surface distance of 100 cm). After irradiation, cells were placed in the 37 °C humidified cell incubator for 24 h. The control samples were treated in the same way but were not irradiated.
TNF-R1 Expression
Flow cytometry was used to determine the expression of TNF-R1 in C3H/10T1/2 MSCs. The antibodies used in this study were from APC-TNF-RI (Biolegend Inc., San Diego, CA, USA, Cat. 113,006). The cells were cultivated as described previously and incubated with 1 μg of antibody/106 cells/mL, incubated for 20 min at 25 °C, and protected from light. After this period, cells were centrifuged at 400 x g for 3 min, the supernatant was discarded, and the cell sediment was washed twice with PBS (Sigma-Aldrich, St. Louis, MO, USA). The sediment was re-suspended in 500 μL of PBS, and the cells were acquired in a FACS Aria flow cytometer (Becton & Dickinson, San Jose, CA, USA). Fluorescence measurements were obtained with at least 1 × 104 cells. The data was analyzed using the software package FLOW JO 7.6® (Tree Star, Ashland, OR, USA). To determine the fluorescence measurements and negative controls, FMO was used as the fluorochrome control [17].
Additionally, qPCR for TNF-R1 in C3H/10T1/2 MSCs irradiated or not was performed. The total RNA was obtained using the RNeasy Mini Kit (Qiagen, Germantown, MD, USA) according to the manufacturer’s protocol, and the total RNA was reverse-transcribed into cDNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). The samples were amplified using TaqMan universal master for TNF-R1 (Mm 00441883_g1, Applied Biosystems, Foster City, CA, USA), and ACTB (Mm00607939_s1, Applied Biosystems, Foster City, CA, USA) was used as the internal control. The expression of TNF-R1 was evaluated by quantitative PCR using StepOne Plus™ (Applied Biosystems, Foster City, CA, USA). The amplification conditions were as follows: an initial 5 min at 95 °C, then 40 cycles of denaturation at 95 °C for 10 s and annealing and extension at 60 °C for 30 s. The relative quantification of TNF-R1 was conducted according to the ΔΔCt method [18].
TNF-α Stimulus
C3H/10T1/2 MSCs were cultivated as described previously. A total of 5 × 105 cells were plated in 6-well culture plates and cultivated for 24 h. Irradiation was performed for the respective groups. Then, cells from both groups (irradiated and not irradiated) were stimulated with 10 ng/mL (for each 1 × 106) TNF-α (R & D Systems, Abingdon, OXON, UK, # 410MT), a pro-inflammatory cytokine that acts directly in the inflammatory response. The dosage of TNF-α was previously standardized in our laboratory, and this concentration had the potential to amplify the inflammatory response and activate the innate immune response without resulting in a high percentage of cell death [19, 20].
Viability and Apoptosis
C3H/10T1/2 MSCs were cultured, irradiated and stimulated with TNF-α as described previously. Then, cells were harvested in PBS (Sigma-Aldrich, St. Louis, MO, USA) and centrifuged; the pellet was resuspended with 50 μL of annexin buffer and incubated with 3 μL of annexin-V (Becton & Dickinson, San Diego, CA, USA, # BD 556419) and 5 μL of propidium iodide (PI) (Becton & Dickinson, San Diego, CA, USA, # BD 556463) for 20 min, protected from light. After incubation, cells were centrifuged and resuspended in 200 μL of annexin buffer for data acquisition using a FACS Aria flow cytometer. Data analysis was performed using FLOW JO 7.6® software (Tree Star, Ahland, OR, USA).
Cell Cycle and Proliferation
To assess the effect of 0.5Gy on the proliferation of C3H/10T1/2 MSCs, cells were cultivated as described previously, and after harvesting, cells were fixed with 70% ethanol for 20 min on ice. After centrifugation, the pellet of cells was resuspended with 4 mg/mL ribonuclease A (US Biological, Salem, MA, USA, # R2011) and 4 μL of PI for 45 min at 37 °C, protected from light. Data acquisition and analysis by flow cytometry were performed as described above. Cell cycle status was assessed by quantifying the percentage of histogram regions corresponding to G1/G0 and S/G2/M phases.
Senescence Evaluation by β-Galactosidase (β-Gal)
C3H/10T1/2 MSCs were cultured and treated as described previously, except the number of cells plated for this assay was 2 × 104 in order to allow to distinguish every cell in the plate, and after 24 h of irradiation, cells were stained for senescence-associated β-gal activity with the use of a cell senescence histochemical staining kit (Cell Signaling Technology, Danvers, MA, USA, # 9860) according to the manufacturer’s protocol. The quantification was performed by inverted microscopy (Nikon TMS), using amplification of 20x (dry objective lens, numerical aperture: 0.40). All the fields were evaluated for the quantification of senescent cells according to the characteristics described by Schmitt [21]: size higher than the average, increased quantity of granules, undefined borders, and vacuolized cytoplasm.
MSCs: Cytokines and Nitric Oxide Production
C3H/10T1/2 MSCs were cultivated as described previously. Cells were harvested and the supernatant was collected to measure the production of interleukin 6 (IL-6), interleukin 10 (IL-10) and prostaglandin E2 (PGE2) by ELISA kits (R&D Systems, Minneapolis, MN, USA and for PGE2 Cayman Chemical, Ann Arbor, MI, USA). Additionally, nitric oxide (NO•) measurement was performed using a chemiluminescence analyzer (NOA™ 280 Sievers, Boulder, CO, USA) as described by Archer [22]. Moreover, the supernatant was collected to be used in the evaluation of the immunomodulatory potential on macrophages (Raw 264.7) as described below.
Evaluation of MSCs on Immunomodulatory Properties of Spleen Cells in a Bystander System: Cytokines and Cellular Population Characterization
Spleens from C57BL/10 mice were used as a source of normal immune cells composed basically by lymphocytes, especially T cells. Three-month-old mice were used in the current study, which was approved by the Institutional Animal Care and Use Committee of the Faculty of Pharmaceutical Sciences at the University of São Paulo (CEUA/465). The spleens were removed and gently dissociated using needles and tweezers in Petri dishes containing 5 mL low glucose DMEM (Corning, Manassas VA, USA) supplemented with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT, USA) and penicillin (U/mL) and streptomycin (100 mg/mL) (Sigma-Aldrich, St. Louis, MO, USA). The intracapsular content was submitted to two washes with hypertonic NaCl and hypotonic buffers for hemolysis. A total of 2 × 106 mononuclear cells were placed in the transwell inserts (0.45 μM porosity) inside the wells containing C3H/10T1/2 MSCs that were plated and already irradiated (as previously described), establishing the bystander system.
Cells were then stimulated with TNF-α (as previously described), and 24 h after stimulation, both the cells and supernatant were collected. The proliferation index of spleen cells was evaluated by flow cytometry using the PKH26 Red Fluorescent Cell Linker Kit (Sigma-Aldrich, St. Louis, MO, USA) [23]. Additionally, spleen cell populations were characterized as described below, and the supernatants were used to measure the production of IL-6, TNF-α, IL-10 and transforming growth factor beta (TGF-β) by cytometric bead array (Becton & Dickinson, San Diego, CA, USA) following the manufacturer’s instructions.
Furthermore, spleen cell populations were characterized by flow cytometry. The cells were quantified for T cell populations [CD3+ (PE), CD3+ (PE)/CD4+ (APC) and CD3+ (PE)/CD8+ (PECy-7)] and T suppressor cells [(CD4+ (PerCP) and CD25+ (APC)], as well as for natural killer (NK) cells [(NK1.1+ (PE), CD49+ (APC)] and B lymphocytes [B220+ (FITC)]. The macrophages were also quantified [Gr-1− (PECy-7), CD11b+ (FITC), F4/80+ (APC), and MHC-II+ (PE)].
The antibodies used were purchased from BD Biosciences, and the cells were acquired in a FACS Aria flow cytometer (Becton & Dickinson, San Jose, CA, USA) and analyzed using the software package FLOW JO 7.6® (Tree Star, Ashland, OR, USA). To determine the fluorescence measurements and negative controls, FMO was used as the fluorochrome control [17].
Evaluation of Conditioned Media of MSCs on Immunomodulatory Properties of Macrophages
Macrophages (mouse leukemic monocyte macrophage cell line Raw 264.7) were cultured in high glucose DMEM (Corning, Manassas VA, USA) supplemented with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT, USA) and penicillin (U/mL) and streptomycin (100 mg/mL) (Sigma-Aldrich, St. Louis, MO, USA) and 2 mM L-glutamine (Sigma-Aldrich, St. Louis, MO, USA) and maintained at 37 °C and 5% CO2 in a humidified atmosphere. When 90% confluent, macrophages were trypsinized and resuspended in medium from C3H/10T1/2 MSCs irradiated or not and cultivated with TNF-α stimulus and seeded in 96-well plates at a density of 5 × 105 cells per mL and cultured for 24 h. Macrophages were cultured in DMEM without conditioned medium and used as control cultures. After this period, the production of interleukin 1 beta (IL1-β), IL-6, IL-10 and interleukin 12 (IL-12) was determined by ELISA, and the proliferation rate evaluated by MTT assay [24].
Additionally, the expression of nuclear factor kappa B (NFκB), signal transducers and activators of transcription 1 (STAT-1) and signal transducers and activators of transcription 3 (STAT-3) were evaluated by Western blot. Macrophages were cultured as previously described and were subsequently lysed with RIPA® buffer (Pierce, Rockford, IL, USA) containing protease and phosphatase inhibitors (0.5 mM PMSF, 50 mM NaF, 10 μg/mL leupeptin, and 10 μg/mL aprotinin (Sigma-Aldrich, St. Louis, MO, USA). Protein quantification was performed based on the Bradford method, and a commercial kit (BCATM Protein Assay Kit® Pierce, Rockford, IL, USA) was used for this aim. Subsequently, sodium dodecyl sulfate polyacrylamide gel electrophoresis (10%) was performed using 20 μg of protein sample, followed by a polyvinylidene fluoride membrane (PVDF®, Amersham Biosciences, Pittsburg, PA, USA) transfer. A molecular weight standard (BioRad, Philadelphia, PA, USA) was used to compare separated molecular weight fractions. The primary antibodies from Santa Cruz Biotechnology (Santa Cruz, CA, USA) [anti-NFκB (cat no. sc-372), anti-phosphorylated NFκB (Ser 311, cat no. sc-33,039), anti-STAT-1 (cat no. sc-346), anti-phosphorylated STAT-1 (cat no. sc-135,648), anti-STAT-3 (cat no. sc-482) and anti-phosphorylated STAT-3 (cat no sc-8001-R)] were diluted in TBS-Tween buffer to 1:1000 and incubated overnight. Finally, membranes were incubated for 1 h with anti-IgG rabbit biotin-conjugated secondary antibody (Cell Signaling Technology, Danvers, MA, USA) diluted to 1:1000 in TBS-Tween buffer. Immunoreactive bands were visualized using the ECL detection system® (Amersham Biosciences, Pittsburg, USA), and images were captured using ImageQuantTM 400® version 1.0.0 (Amersham Biosciences, Pittsburg, PA, USA). For standardization and quantification, images were analyzed using the ImageQuant TL® program (Amersham Biosciences, Pittsburg, PA, USA). Results were normalized to the intensity of β-actin (Sigma-Aldrich, St. Louis, MO, USA), which was diluted at 3:10000 in TBS-Tween buffer.
Statistical Analysis
All data were firstly evaluated by the normality test. For data analysis of multiple comparisons among groups, two-way analysis of variance and Bonferroni’s post hoc test were performed. For comparison between two groups, sets were analyzed using Student’s t test, and the adopted level of significance was p < 0.05. Values were expressed as mean ± standard deviation of the mean. Statistical analyses were performed using GraphPad Prism® software (GraphPad Software Inc., La Jolla, CA, USA).
Results
Determination of TNF-R1 Expression on MSCs after Irradiation
Immunophenotyping shows, as can be seen in Fig. 1a and b, that the percentage of TNF-R1 expression did not differ between MSCs irradiated and not irradiated. The mRNA expression was in agreement with the flow cytometry where no difference was observed between the groups (Fig. 1c).
Expression of TNF-R1 by flow cytometry in MSCs. (a) Histograms of the flow cytometry analyses. (b) Percentage of TNF-R1 expression in MSCs irradiated or not irradiated. (c) Expression of TNF-R1 mRNA in MSCs irradiated or not irradiated. The results are expressed as the mean ± SD of six independent experiments
Effects of 0.5Gy of IR Combined with TNF-α Did Not Affect Viability, Apoptosis, Senescence or Cell Cycle of MSCs
The viability of all groups tested did not show differences among them, showing more than 95% of viable cells associated with less than 3% of apoptotic cells. Additionally, senescence did not show differences among the groups, showing about 0.05% of senescent cells in all groups tested. The cell cycle phases were also not different among the studied groups, showing about 60% and 40% of cells in the G0/G1 and S/G2/M phases, respectively (data not shown).
Effects of 0.5Gy of IR Combined with TNF-α on the Production of Cytokines by MSCs
The dosage of soluble factors produced by MSCs showed that these cells are affected by low-dose radiation, TNF-α, or by the combination of both. First, we evaluated the production of IL-6,which is a cytokine with both anti- and pro-inflammatory activities depending on the signaling mode of its activation as well as the target cells. Our results showed that IL-6 production by irradiated MSCs not stimulated with TNF-α decreased, but when these cells were stimulated with TNF-α, they produced higher amounts of IL-6 independent of the radiation (Fig. 2a).
Regarding IL-10 production, it was higher in cells irradiated and not stimulated with TNF-α when compared to cells not stimulated and not irradiated (Fig. 2b). Additionally, MSCs not irradiated but stimulated with TNF-α produced higher levels of IL-10 in comparison to cells not stimulated and not irradiated. Although when cells not irradiated were compared with the irradiated ones, with both being stimulated with TNF-α, no difference in the IL-10 levels were found (Fig. 2b).
Furthermore, PGE2, which is an anti-inflammatory mediator, showed the highest levels when MSCs were irradiated and stimulated with TNF-α. In addition, cells irradiated and stimulated with TNF-α also showed higher production of PGE2 when compared to cells not irradiated and stimulated with TNF-α (Fig. 2c). NO• production, which is also an immunomodulatory compound, was higher in cells stimulated with TNF-α in both groups; however, we could not show statistical significance (only a tendency) (Fig. 2d). These data suggest the existence of an alternative mechanism of irradiated MSCs to control inflammation induced by exogenous TNF-α stimulation.
Immunomodulatory Action of Soluble Factors Produced by MSCs: Action on Lymphocytes
The effects that conditioned media have on the immunomodulation of immune cells are widely demonstrated in the literature [2, 3, 20]. On the other hand, the impact of low-dose radiation on MSCs and how this can affect their immunomodulatory properties is poorly studied in the literature. So, in order to evaluate these aspects, murine spleen cells composed basically of lymphocytes were cultured in a bystander system with MSCs (irradiated or not irradiated) stimulated with TNF-α (referred to as conditioned media).
Firstly, we evaluated the proliferation rate of spleen cells, and as is shown in Fig. 3a, the conditioned media from irradiated MSCs reduced the proliferation rate of spleen cells. Concerning cytokine production, irradiated MSCs in the conditioned media were able to reduce the production of IL-6 (Fig. 3b), TNF-α (Fig. 3d) and TGF-β (Fig. 3e), in contrast with increased production of IL-10 (Fig. 3c).
Effects of MSC conditioned media on (a) lymphocyte proliferation and production of (b) IL-6, (c) IL-10, (d) TNF-α and (e) TGF-β by spleen cells from the bystander system. The results are expressed as the mean ± SD of six independent experiments. Significant differences between the treatment groups are illustrated by *(p ≤ 0.05) and **(p ≤ 0.01)
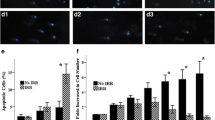
The percentage of cells were analyzed by flow cytometry following the gates strategies described in Figs. 4a–g. Regarding the spleen cell profile, the percentage of CD3+, as well as CD19+, cells did not change, regardless of treatment (data not shown). They also showed no significant differences in the percentage of cells double positive for CD3 and CD8, as well as for NK cells (data not shown). However, the percentage of cells CD4+/CD25+ increased in the irradiated MSCs in conditioned media in comparison to non-irradiated MSCs in conditioned media (Fig. 4h). Additionally, the percentage of macrophages (GR1−, F4/80+, CD11b+, MHC-II+) decreased in the bystander model of spleens and irradiated MSCs with conditioned media in comparison to the other group (Fig. 4i).
Representative dot plots of flow cytometry data acquisition. (a) Size and complexity of spleen cells (FSC x SSC); (b) B and T lymphocyte populations: B220+ cells in R1 and CD3+ cells in R2; (c) T lymphocytes: CD3+CD8+CD4− cells in Q1 and CD3+CD4+CD8− cells in Q3; (d) Suppressor T cells: CD4+CD25+ in Q2; (e) Natural killer cells: NK1.1+CD49+ in Q2; (f) Gr-1− F4/80+ cell population selected (g) Macrophages: Gr-1− F4/80+ CD11b+ MHC-II+ in Q2 . Effect of MSC conditioned media on the modulation of the percentage of (h) macrophages and (i) T suppressor cells. The results are expressed as the mean ± SD of six independent experiments. Significant differences between the treatment groups are illustrated by *(p ≤ 0.05)
Immunomodulatory Action of Soluble Factors Produced by MSCs: Action on Macrophages
To further detail the modulation of macrophages by MSCs, the metabolic activity of macrophages was evaluated after being cultured with different conditioned media from MSCs. The results showed that conditioned media from irradiated MSCs stimulated with TNF-α reduced the metabolic activity of macrophages when compared to conditioned media from non-irradiated MSCs (Fig. 5a). Additionally, the results showed that conditioned media from irradiated MSCs stimulated with TNF-α acted on macrophages by reducing the production of IL-1β (Fig. 5b) and IL-6 (Fig. 5c), but without statistical differences in the produced levels of IL-10 (Fig. 5d) and IL-12 (Fig. 5e).
Effect of MSC conditioned media on Raw 264.7. (a) Metabolic activity evaluated by MTT assay and production of (b) IL-1β, (c) IL-6, (d) IL-10 and (e) IL-12. The results are expressed as the mean ± SD of six independent experiments. Significant differences between the treatment groups are illustrated by *(p ≤ 0.05)
Moreover, the analysis of transcription factors did not show changes in the expression of NFκB and STAT-1 between the studied groups (Fig. 6a and b, respectively). However, when STAT-3 expression was analyzed, differences were observed. The ratio between phosphorylated and total STAT-3 was significantly higher for macrophages cultured in conditioned media from irradiated MSCs stimulated with TNF-α when compared to the other studied group (Fig. 6c).
Effect of MSC conditioned media Raw 264.7 expression of (a) p-NFκB/NFκB ratio, (b) p-STAT-1/STAT-1 ratio and (c) p-STAT-3/STAT-3 ratio. Results of Western blot analysis of three independent experiments. The results are expressed as the mean ± SD of three independent experiments. Significant differences between the treatment groups are illustrated by *(p ≤ 0.05)
Discussion
It is well known that MSCs are multipotent cells, being part of the microenvironment of tissues. They have immunomodulatory function [3, 4, 25, 26] and may have the ability to home into injured tissues, assisting with regeneration [27,28,29].
In this context, IR, even in low dose, can modulate the microenvironment of both directly irradiated and bystander cells (which comprises the unirradiated cells that neighbor irradiated cells) [30], as consequence of oxidative stress and inflammation [7, 8, 11]. However, it is not clear how radiation associated with an inflammatory condition can modulate the MSCs.
In this study, MSCs were irradiated and stimulated with TNF-α, which is a pleiotropic pro-inflammatory cytokine [15, 16]. Firstly, we showed that MSCs expressed TNF-R1, which is recognized as the TNF-α receptor responsible for mediating the pro-inflammatory effects [16]. Additionally, we also showed that 0.5Gy radiation did not affect the expression of this receptor in MSCs. Another proposition was to understand, in part, how low-dose radiation in an inflammatory state triggered by stimulation with TNF-α could act on some of the immunomodulatory properties of MSCs.
The literature reports that the survival rate of MSCs was not affected by single low-dose irradiation, mainly due to the activation of DNA repair mechanisms [7, 31]. These data are in accordance with our results that showed irradiation with 0.5Gy associated or not with TNF-α did not affect the survival, cell cycle phases or the percentages of apoptotic and senescent MSCs.
The immunomodulatory activities of MSCs have been demonstrated in studies performed in vitro and in vivo, showing the cytoprotective and reparative effects of MSCs [3, 4, 32, 33]. The exact mechanism by which MSCs regulate the immune system is not fully understood, but it has been suggested to occur via secretion of soluble factors that are dependent on the microenvironment, such as the presence of TNF-α, IL1-β or IFN-γ [3, 4, 34].
Among these soluble factors produced by MSCs, it is highlighted that IL-10, NO• and PGE2 have roles in mediating the immunosuppressive effects of MSCs, in addition to IL-6, which inhibits the differentiation of monocytes into antigen presenting dendritic cells [3, 4, 35]. In our study, we showed that pre-activation of MSCs by TNF-α significantly increased the concentrations of IL-6, IL-10, PGE2 and NO•. Additionally, in MSCs irradiated and stimulated with TNF-α, these factors were also increased, especially the production of PGE2, which is an important soluble factor able to act in macrophages to increase IL-10 production.
In order to test the immunomodulatory properties of MSCs upon radiation, MSCs were stimulated with TNF-α in a bystander cell culture system with spleen cells (a source of lymphocytes, especially T cells). Our data showed that the irradiated MSCs were able to reduce the proliferation rate of bystander spleen cells in a more effective way when compared to MSCs not irradiated. Thus, interestingly, both IL-6 and TNF-α production by spleen cells was decreased in contrast to increased production of IL-10 and TGF-β.
Both IL-10 and TGF-β are anti-inflammatory cytokines with a number of context-dependent effects on immune cells, controlling excessive inflammatory responses. In other words, IL-10 can attenuate pro-inflammatory signals by inhibiting pro-inflammatory cytokines, and TGF-β can inhibit T cell proliferation and stimulates the generation of CD4+/CD25+ cells [36, 37].
The complexity of the immunomodulatory mechanism is represented by the involvement of several soluble factors, which were shown to act on both innate and adaptive immune cells. In association with changes in dendritic cell functions, they can act by suppressing T cell proliferation [3, 4, 32, 33], by suppressing the proliferation of CD4+ and CD8+ T cell populations, and by mediating a shift from the Th1 driven response to the anti-inflammatory Th2 response, reducing the expression of MHC-class II as well as affecting the production of cytokines by these cells [32, 33, 38].
In this way, as described above, besides the changes in the production of soluble factors produced by spleen cells (as well as a decrease in the spleen’s proliferation rate) when cultivated upon the media of irradiated and stimulated MSCs, a reduction in the percentage of macrophages was also observed, in addition to an increase in the percentage of CD4+/CD25+ cells. However, the percentages of CD8+, B, and NK cells were not affected.
It is known that macrophages can regulate lymphocyte activation and proliferation, and they are essential in the activation process of T and B lymphocytes by antigens and allogenic cells [39, 40]. In contrast, CD4+/CD25+ regulatory T cells (Tregs), which comprise 5–10% of the circulating CD4+ T cell population, suppress immune responses. They play a pivotal role in preventing organ-specific autoimmune disease and in the induction of tolerance after allogenic organ transplantation [39,40,41]. The ability of MSCs to convert conventional T cells to Tregs has been previously observed [42, 43], and in this current work, it was shown that radiation in association with TNF-α stimulation led to an increase in the immunosuppressive capacity of MSCs. We believe that such capability might be explored as a promising tool in regenerative medical research.
Additionally, macrophages perform many functions in the immune system, with a wide secretion ability, including enzymes, plasma proteins, reactive oxygen and nitrogen species, prostaglandins, and cytokines [39, 40]. Some responses and intracellular pathways are activated during the inflammatory process, and the production of most cytokines and chemokines is regulated primarily at the transcription level. A scenario in which the participation of key transcription factors, STAT-1, STAT-3 and NFκB, is well known [39, 40, 44].
In macrophages, increased activation of the STAT-1 pathway leads to improvement of the killing process of phagocytized microorganisms, mainly by the high production of reactive oxygen and nitrogen species [45,46,47]. Furthermore, STAT-3 induces the expression of genes that have anti-inflammatory functions. Therefore, the anti-inflammatory cytokine IL-10 inhibits expression of molecules that propagate inflammation in a manner that depends on the transcription factor STAT-3 [48]. On the other hand, NFκB is a prominent transcription factor involved in the immune/inflammatory response, being the most potent activated pro-inflammatory transcription factor, able to induce genes that control the encoding of inflammatory cytokines and chemokines [44, 49].
In the current work, although no difference in the expression of STAT-1 and NFκB were observed in macrophages (Raw 264.7), when these cells were cultivated with the conditioned media supernatant of irradiated MSCs, an increased level of p-STAT-3/STAT-3 ratio expression was observed. Moreover, changes in the cytokine profile of macrophages were also observed. It is well known that MSCs are able to induce macrophages to acquire an anti-inflammatory M2 phenotype, suppressing the production of inflammatory cytokines [3,4,5, 50]. The results showed that the conditioned media supernatant of irradiated MSCs reduced the metabolic activity of these macrophages and also decreased the production of IL-1β and IL-6, but did not affect the production of IL-10.
Besides extending the knowledge of the interactions of MSCs with cells of the immune system, our data showed an important immunomodulatory capability of MSCs in response to IR, both in steady state and upon stimulation with TNF-α. The immunosuppression induced by MSCs can be at the backstage of many different pathological processes, and consequently, its respective capability can be explored for several disease treatments, especially the oncological ones, where IR usually part of the context of the treatment. A complete understanding of the mechanisms underlying these interactions are, however, yet to be clarified.
References
Stefani, F. R., Eberstål, S., Vergani, S., Kristiansen, T. A., & Bengzon, J. (2018). Low-dose irradiated mesenchymal stromal cells break tumor defensive properties in vivo. International Journal of Cancer, 143(9), 2200–2212. https://doi.org/10.1002/ijc.31599.
Harrell, C. R., Jovicic, N., Djonov, V., & Volarevic, V. (2020). Therapeutic use of Mesenchymal stem cell-derived Exosomes: From basic science to clinics. Pharmaceutics, 12(5), E474. https://doi.org/10.3390/pharmaceutics12050474.
Kyurkchiev, D., Bochev, I., Ivanova-Todorova, E., Mourdjeva, M., Oreshkova, T., Belemezova, K., & Kyurkchiev, S. (2014). Secretion of immunoregulatory cytokines by mesenchymal stem cells. World Journal Stem Cells, 6, 552–570. https://doi.org/10.4252/wjsc.v6.i5.552.
De Miguel, M. P., Fuentes-Julián, S., & Blázquez-Martínez, A. (2012). Immunosuppressive properties of mesenchymal stem cells: Advances and applications. Current Molecular Medicine, 12, 574–591. https://doi.org/10.2174/156652412800619950.
Wang, Y., Chen, X., Cao, W., & Shi, Y. (2014). Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nature Immunology, 15, 1009–1016. https://doi.org/10.1038/ni.3002.
Prise, K. M., & O’Sullivan, J. M. (2009). Radiation-induced bystander signalling in cancer therapy. Nature Reviews Cancer, 9(5), 351–360. https://doi.org/10.1038/nrc2603.
Wakeford, R., & Tawn, E. J. (2010). The meaning of low dose and low dose-rate. Journal of Radiological Protection, 30(1), 1–3. https://doi.org/10.1088/0952-4746/30/1/E02.
Arnold, K. M., Flynn, N. J., Raben, A., Romak, L., Yu, Y., Dicker, A. P., Mourtada, F., & Sims-Mourtada, J. (2018). The impact of radiation on the tumor microenvironment: Effect of dose and fractionation schedules. Cancer Growth Metastasis, 11, 1179064418761639. https://doi.org/10.1177/1179064418761639.
Short, S. C., Bourne, S., Martindale, C., Woodcock, M., & Jackson, S. P. (2005). DNA damage responses at low radiation doses. Radiation Research, 164(3), 292–302. https://doi.org/10.1667/rr3421.1.
Schröder, S., Kriesen, S., Paape, D., Hildebrandt, G., & Manda, K. (2018). Modulation of inflammatory reactions by low-dose ionizing radiation: Cytokine release of murine endothelial cells is dependent on culture conditions. Journal of Immunology Research, 2856518, 1–13. https://doi.org/10.1155/2018/2856518.
Barcellos-Hoff, M. H., Park, C., & Wright, E. G. (2005). Radiation and the microenvironment – Tumorigenesis and therapy. Nature Reviews Cancer, 5(11), 867–875. https://doi.org/10.1038/nrc1735.
Tsai, K. K. C., Chuang, E. Y., Little, J. B., & Yuan, Z. M. (2005). Cellular mechanisms for low-dose ionizing radiation-induced perturbation of the breast tissue microenvironment. Cancer Research, 65(15), 6734–6744. https://doi.org/10.1158/0008-5472.CAN-05-0703.
Xu, T., Zhang, Y., Chang, P., Gong, S., Shao, L., & Dong, L. (2018). Mesenchymal stem cell-based therapy for radiation-induced lung injury. Stem Cell Research Therapy, 9(1), 18. https://doi.org/10.1186/s13287-018-0776-6.
Yan, L., Zheng, D., & Xu, R. H. (2018). Critical role of tumor necrosis factor signaling in mesenchymal stem cell-base therapy for autoimmune and inflammatory diseases. Frontiers Immunology, 9, 1658. https://doi.org/10.3389/fimmu.2018.01658.
Crisotomo, P. R., Wang, Y., Markel, T. A., Wang, M., Lahm, T., & Meldrum, D. R. (2008). Human mesenchymal stem cells stimulated by TNF-α, LPS, or hypoxia produce growth factors by an NFκB- but not JNK-dependent mechanism. American Journal Physiology-Cell Physiology, 294(3), C675–C682. https://doi.org/10.1152/ajpcell.00437.2007.
Hehlgans, T., & Pfeffer, K. (2005). The intriguing biology of the tumour necrosis factor/tumour necrosis factor receptor superfamily: Players, rules and the games. Immunology, 115(1), 1–20. https://doi.org/10.1111/j.1365-2567.2005.02143.x.
Roederer, M. (2001). Spectral compensation for flow cytometry: Visualization artifacts, limitations, and caveats. Cytometry, 45(3), 194–205. https://doi.org/10.1002/1097-0320(20011101)45:3<194::aid-cyto1163>3.0.co;2-c.
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods, 25, 402–408. https://doi.org/10.1006/meth.2001.1262.
Lima, F. S., Romero, A. B. R., Hastreiter, A., Nogueira-Pedro, A., Makiyama, E., Colli, C., & Fock, R. A. (2018). An insight into the role of magnesium in the Immunomodulatory properties of Mesenchymal stem cells. The Journal of Nutritional Biochemistry, 55, 200–208. https://doi.org/10.1016/j.jnutbio.2018.02.006.
Oliveira, D. C., Hastreiter, A. A., Mello, A. S., Beltran, J. S. O., Santos, E. W. C. O., Borelli, P., & Fock, R. A. (2014). The effects of protein malnutrition on the TNF-RI and NF-κB expression via the TNF-α signaling pathway. Cytokine, 69(2), 218–225. https://doi.org/10.1016/j.cyto.2014.06.004.
Schmitt, C. A. (2007). Cellular senescence and Cancer treatment. Biochimica et Biophysica Acta, 1775(1), 5–20. https://doi.org/10.1016/j.bbcan.2006.08.005.
Archer, S. (1993). Measurement of nitric oxide in biological models. FASEB Journal, 7(2), 349–360. https://doi.org/10.1096/fasebj.7.2.8440411.
Tario, J. D., Muirhead, K. A., Pan, D., Munson, M. E., & Wallace, P. K. (2011). Tracking immune cell proliferation and cytotoxic potential using flow Cytometry. Methods Molecular Biology, 699, 119–164. https://doi.org/10.1007/978-1-61737-950-5_7.
Mosmann, T. (1983). Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Journal of Immunology Methods, 65(1–2), 55–63. https://doi.org/10.1016/0022-1759(83)90303-4.
Di Nicola, M., Carlo-Stella, C., Magni, M., Milanesi, M., Longoni, P. D., Matteucci, P., Grisanti, S., & Gianni, A. M. (2002). Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood, 99(10), 3838–3843. https://doi.org/10.1182/blood.v99.10.3838.
Abdi, R., Fiorina, P., Adra, C. N., Atkinson, M., & Sayegh, M. H. (2008). Immunomodulation by mesenchymal stem cells : A potential therapeutic strategy for type 1 diabetes. Diabetes, 57(7), 1759–1767. https://doi.org/10.2337/db08-0180.
Shen, F. H., Visger, J. M., Balian, G., Hurwitz, S. R., & Diduch, D. R. Systemically Administered Mesenchymal Stromal Cells Transduced With Insulin-Like Growth factor-I Localize to a Fracture Site and Potentiate Healing. Journal of Orthopaedic Trauma, 16(9), 651–659. https://doi.org/10.1097/00005131-200210000-00007.
Neuss, S., Becher, E., Wöltje, M., Tietze, L., & Jahnen-Dechent, W. (2004). Functional expression of HGF and HGF receptor/c-met in adult human mesenchymal stem cells suggests a role in cell mobilization, tissue repair, and wound healing. Stem Cells, 22(3), 405–414. https://doi.org/10.1634/stemcells.22-3-405.
Satoh, H., Kishi, K., Tanaka, T., Kubota, Y., Nakajima, T., Akasaka, Y., & Ishii, T. (2004). Transplanted mesenchymal stem cells are effective for skin regeneration in acute cutaneous wounds. Cell Transplantation, 13(4), 405–412. https://doi.org/10.3727/000000004783983765.
Yang, H., Asaad, N., & Held, K. D. (2005). Medium-mediated intercellular communication is involved in bystander responses of X-ray-irradiated normal human fibroblasts. Oncogene, 24(12), 2096–2103. https://doi.org/10.1038/sj.onc.1208439.
Sugrue, T., Lowndes, N. F., & Ceredig, R. (2013). Mesenchymal stromal cells: Radio-resistant members of the bone marrow. Immunology Cell Biology, 91(1), 5–11. https://doi.org/10.1038/icb.2012.61.
Ankrum, J. A., Ong, J. F., & Karp, J. M. (2014). Mesenchymal stem cells: immune evasive, not immune privileged. Nature Biotechnology, 32(3), 252–260. https://doi.org/10.1038/nbt.2816 Epub 2014 Feb 23.
Wang, Y., Chen, X., Cao, W., & Shi, Y. (2014). Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nature Immunology, 15(11), 1009–1016. https://doi.org/10.1038/ni.3002.
English, K. (2013). Mechanisms of mesenchymal stromal cell immunomodulation. Immunology & Cell Biology, 91(1), 19–26. https://doi.org/10.1038/icb.2012.56.
Uccelli, A., Moretta, L., & Pistoia, V. (2008). Mesenchymal stem cells in health and disease. Nature Reviews Immunology., 8(9), 726–736. https://doi.org/10.1038/nri2395.
Vieira, P. L., Christensen, J. R., Minaee, S., O'Neill, E. J., Barrat, F. J., Boonstra, A., Barthlott, T., Stockinger, B., Wraith, D. C., & O’Garra, A. IL-10-secreting Regulatory T Cells Do Not Express Foxp3 but Have Comparable Regulatory Function to Naturally Occurring CD4+CD25+ Regulatory T Cells. The Journal of Immunology, 172(10), 5986–5993. https://doi.org/10.4049/jimmunol.172.10.5986.
O'Garra, A., Vieira, P. L., Vieira, P., & Goldfeld, A. E. (2004). IL-10-producing and naturally occurring CD4+ Tregs: Limiting collateral damage. The Journal of Clinical Investigation, 114(10), 1372–1378. https://doi.org/10.1172/JCI23215.
Consentius, C., Akyüz, L., Schmidt-Lucke, J. A., Tschöpe, C., Pinzur, L., Ofir, R., Reinke, P., Volk, H.-D., & Juelke, K. (2015). Mesenchymal stromal cells prevent Allostimulation in vivo and control checkpoints of Th1 priming: Migration of human DC to lymph nodes and NK cell activation. Stem Cells, 33(10), 3087–3099. https://doi.org/10.1002/stem.2104.
Gordon, S. (2003). Alternative activation of macrophages. Nature Reviews Immunology, 3(1), 23–35. https://doi.org/10.1038/nri978.
Melief, S. M., Schrama, E., Brugman, M. H., Tiemessen, M. M., Hoogduijn, M. J., Fibbe, W. E., & Roelofs, H. (2013). Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes toward anti-inflammatory macrophages. Stem Cells, 31(9), 1980–1991. https://doi.org/10.1002/stem.1432.
Okeke, E. B., & Uzonna, J. E. (2019). The pivotal role of regulatory T cells in the regulation of innate immune cells. Frontiers in Immunology, 10, 680e. https://doi.org/10.3389/fimmu.2019.00680.
Duffy, M. M., Ritter, T., Ceredig, R., & Griffin, M. D. (2011). Mesenchymal stem cell effects on T-cell effector pathways. Stem Cell Research & Therapy, 2(4), 34. https://doi.org/10.1186/scrt75.
Ramasamy, R., Tong, C. K., Seow, H. F., Vidyadaran, S., & Dazzi, F. (2008). The immunosuppressive effects of human bone marrow-derived mesenchymal stem cells target T cell proliferation but not its effector function. Cellular Immunology, 251(2), 131–136. https://doi.org/10.1016/j.cellimm.2008.04.009.
O'Brown, Z. K., Van Nostrand, E. L., Higgins, J. P., & Kim, S. K. (2015). The inflammatory transcription factors NFκB, STAT1 and STAT3 drive age-associated transcriptional changes in the human kidney. PLoS Genetics, 11(12), e1005734. https://doi.org/10.1371/journal.pgen.1005734.
Arora, S., Hernandez, Y., Erb-Downward, J. R., McDonald, R. A., Toews, G. B., & Huffnagle, G. B. Role of IFN-gamma in regulating T2 immunity and the development of alternatively activated macrophages during allergic bronchopulmonary mycosis. The Journal of Immunology, 174(10), 6346–6356. https://doi.org/10.4049/jimmunol.174.10.6346.
Kaplan, M. H. (2013). STAT signaling in inflammation. JAKSTAT, 2(1), e24198. https://doi.org/10.4161/jkst.24198.
Wager, C. M. L., Hole, C. R., Wozniak, K. L., Olszewski, M. A., & Wormley Jr., F. L. STAT1 Signaling Is Essential for Protection Against Cryptococcus neoformans Infection in Mice. The Journal of Immunology, 193(8), 4060–4071. https://doi.org/10.4049/jimmunol.1400318.
Gaba, A., Grivennikov, S. I., Do, M. V., Stumpo, D. J., Blackshear, P. J., & Karin, M. (2012). IL-10 mediated Tristetraprolin induction is part of feedback loop that controls macrophage STAT3 activation and cytokine production. The Journal of Immunology, 189(5), 2089–2093. https://doi.org/10.4049/jimmunol.1201126.
Zhang, T. L. L., Joo, D., & Sun, S. C. (2017). NF-κB signaling in inflammation. Signal Transduction and Targeted Therapy, 2, 17023. https://doi.org/10.1038/sigtrans.2017.23.
Abumaree, M. H., Al Jumah, M. A., Kalionis, B., Jawdat, D., Al Khaldi, A., Abomaray, F. M., Fatani, A. S., Chamley, L. W., & Knawy, B. A. (2013). Human placental mesenchymal stem cells (pMSCs) play a role as immune suppressive cells by shifting macrophage differentiation from inflammatory M1 to anti-inflammatory M2 macrophages. Stem Cell Reviews and Reports, 9(5), 620–641. https://doi.org/10.1007/s12015-013-9455-2.
Acknowledgments
This work was supported by grants from the Fundação de Amparo a Pesquisa do Estado de São Paulo – FAPESP (grant number: 2018/25813-8). Fock RA is fellow of the Conselho Nacional de Pesquisa e Tecnologia (CNPq).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Standard and Informed Consent
All procedures performed in studies involving animals were in accordance with the ethical standards of the University of São Paulo (CEUA/FCF/344).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nogueira-Pedro, A., Makiyama, E.N., Segreto, H.R.C. et al. The Role of Low-Dose Radiation in Association with TNF-α on Immunomodulatory Properties of Mesenchymal Stem Cells. Stem Cell Rev and Rep 17, 968–980 (2021). https://doi.org/10.1007/s12015-020-10084-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-020-10084-9