Abstract
Exosomes, nano-sized cell-derived vesicles, have been employed as non-synthetic carriers of various pharmaceutics in numerous studies. As higher expression levels of miR-142-3p and miR-150 in breast cancer stem cells (BCSCs) are associated with their clonogenic and tumorigenic capabilities, the present study aims to exploit the mesenchymal stem cells-derived exosomes (MSCs-Exo) to deliver LNA-antimiR-142-3p into MCF7-derived cancer stem-like cells to suppress expression levels of miR-142-3p and miR-150 in order to reduce clonogenicity and tumorigenicity. Our results indicated that the MSCs-Exo can efficiently deliver the LNA-antimiR-142-3p to breast cancer stem-like cells to reduce the miR-142-3p and miR-150 expression levels. Furthermore, the inhibition of the oncomiRs with the delivery of LNA-antimiR-142-3p resulted in a significant reduction of clone-formation and tumor-initiating abilities of the MCF7-derived cancer stem-like cells. In conclusion, we showed that MSCs-derived exosomes could be used as a feasible nanovehicles to deliver RNA-based therapeutics into BCSCs to improve the cancer treatment.
Highlights
-
Exosomes secreted by bone marrow-derived mesenchymal stem cells efficiently transfer the LNA-antimiR-142-3p to breast cancer stem cells.
-
Exosomes-mediated delivery of LNA-antimiR-142-3p to the breast cancer stem cells leads to downregulation of miR-142-3p and miR-150 and the overexpression of target genes.
-
Delivery of LNA-antimiR-142-3p by the exosomes reduces the colony formation capability of breast cancer stem cells in vitro.
-
Inhibition of miR-142-3p and miR-150 by the LNA-antimiR-142-3p loaded exosomes reduces the tumorigenicity of breast cancer stem cells in vivo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exosomes, a distinct class of extracellular vesicles, are defined as small membrane-enclosed vesicles with a diameter of approximately between 30 and 150 nm. The naïve nanovesicles are generated by inward budding of the membrane of late endosomes and released into the extracellular milieu by fusion of the peripheral membrane of intracellular multivesicular bodies (MVB) with the plasma membrane [1]. Exosomes are secreted by all types of cells in culture and they are present in various biological fluids. Isolated exosomes are usually characterized based upon their size, morphology, flotation density and the presence of surface marker proteins [2]. Several studies have shown that exosomes play key roles in various physiological processes such as homeostasis and cell survival by excluding unwanted substances, and they carry out their roles by efficiently exchange various genetic and biochemical molecules such as mRNA, non-coding regulatory RNAs, membranous and cytoplasmic proteins and lipids between different cells or tissues [3]. Exosomes with different membranous protein markers, depending on the cell of origin, have the different circulating pattern in the whole body. Once reaching to their recipient cells, exosomes uptake can be mediated by two main pathways, endocytosis or fusion, for delivering their contents into the cytosol of recipient cells [4]. To date, exosomes have been employed as carriers of various pharmaceutical molecules in numerous studies due to their unique structure and physicochemical features including potential targeting abilities, efficient cellular entry, intrinsic capacity to overpass biological barriers, high stability in blood circulation due to their slightly negative charge, more compatibility with the immune system and lower toxicity compared to synthetic nanocarriers under in vivo conditions [5].
Cancer stem cells (CSCs) are defined as a small population of cancer cells within a tumor that are responsible for regrowth of tumors, metastasis, and the resistance of cancers to chemotherapy and radiotherapy and thus relapse [6]. Breast cancer stem cells (BCSCs) were originally identified as a distinct population with surface markers of CD44+, CD24−/low, and ALDH+ that are enriched within many human breast tumors and were characterized for the ability to drive tumor formation in a mouse xenograft model [7]. Emerging evidence reported that many cancer cell lines have ability to form spheroids that extensively exhibit stem-like properties, such as the capability to form tumors in vivo and CD44 and ALDH positivity, revealing the potential of studying BCSCs via an easy and available method by in vitro culture of established cancer cell lines [8]. Thus, for the purposes of this study, we used a mammosphere culture assay to isolate BCSCs from MCF7 breast cancer cell line.
Comprehensive analyses of the expression profile of various miRNAs revealed that many miRNAs are differentially expressed in BCSCs, providing potential targets to eradicate the cells in breast cancers [9]. It has been recently reported that upregulation of miR-142-3p and miR-150 expression levels in breast cancer stem cells compared to non-tumorigenic cancer cells can be associated with tumorigenicity and clonogenicity of BCSCs [10]. So, reducing the amount of miR-142 by using complementary miRNA inhibitors in breast cancer stem cells could slow the growth rate of breast tissue. In the field of RNA interference (iRNA)-based therapy, several studies have been carried out to find the appropriate carriers for the transmission of iRNA-based therapeutics to cope with the limitations encountered in the transfer of these molecules [11]. In recent years, exosomes have been introduced as a new promising nano-carriers to the transmission of diverse bio-macromolecules including miRNA inhibitor oligonucleotides [12]. To the best of our knowledge, there are very few studies that use the mesenchymal stem cells-derived exosomes to transfer the iRNA-based therapeutics to breast cancer stem cells to reduce clonogenicity and tumorigenicity. Given the available evidence, we decided in this study to harness mesenchymal stem cell-derived exosomes (MSCs-Exo) to functional delivery of locked nucleic acid (LNA) modified antimiR-142-3p molecules into MCF7-derived breast cancer stem cells. Therefore, this report could be a basic study for the use of MSCs-derived exosomes to target breast cancer stem cells. The efficiency of the MSCs-Exo for the transfer of the LNA-antimiR-142-3p to breast cancer stem cells was confirmed by a significant reduction of miR-142-3p and miR-150 expression levels using Real-Time PCR method. Furthermore, inhibition of miR-142-3p by LNA-antimiR-142-3p via MSCs-Exo delivery led to a significant reduction of colony-formation and tumor-initiating capabilities of breast cancer stem-like cells. Our results provide evidence that exosomes derived from mesenchymal stem cells can be harnessed in gene therapy to introduce exogenous LNA-antimiR-142-3p to breast cancer stem-like cells in order to functionally decrease miR-142-3p and miR-150 and reduce the tumorigenicity of the cancer stem-like cells.
Material and Methods
Animal Studies
Tumorigenicity assay was performed by subcutaneous injection of single-cell suspensions from treated mammospheres in 6 to 8 week-old female BALB/c SCID mice. The BALB/c SCID mice have purchased from the animal laboratory of Imam Khomeini Hospital and maintained under standard conditions according to the guidelines of the animal care committee of the cancer institute. The BALB/c SCID mice were kept under optimized hygienic conditions in an individually ventilated cage system and were fed with autoclaved commercial diet and water ad libitum. All animal experiments and procedures were carried out under the approval of the Institutional Ethical Committee and Research Advisory Committee of Mashhad University of Medical Sciences (Education Office, dated May 13, 2015; proposal code 931289), based on the Specific National Ethical Guidelines for Biomedical Research issued by the Research and Technology Deputy of Ministry of Health and Medical Education (MOHME) of Iran that was issued in 2005.
Cells and Cell Culture
MCF7 and MCF10A cell lines were obtained from American type culture collection center (ATCC). MCF10A cells were cultured in DMEM/F12 Ham’s Mixture supplemented with 5% horse serum (Gibco, USA), hydrocortisone 0.5 mg/ml (Sigma-Aldrich, USA), insulin 10 μg/ml (Sigma-Aldrich, USA), recombinant human EGF 20 ng/ml (Sigma-Aldrich, USA), cholera toxin 100 ng/ml (Sigma-Aldrich, USA), 100 units/ml penicillin and 100 units/ml streptomycin (Sigma-Aldrich, USA). MCF7 cells were cultured in complete DMEM containing 10% fetal bovine serum (FBS) (Gibco, USA), 100 units/ml penicillin and 100 units/ml streptomycin at 37 °C in humidified air with 5% CO2.
Isolation and Characterization of Mesenchymal Stem Cells
The bone marrow obtained from the femurs and tibias of 6 to 8 week-old BALB/c mice were used to isolate the primary mesenchymal stem cells. After flushing the bone marrow out of the bone cavity, we then washed the extracted cells with PBS and suspended them in α-MEM medium with 10% FBS. The bone marrow cells (1 × 107/ml) were placed in a 75-cm2 cell culture flask and incubated at 37 °C with 5% CO2. After 3 days spindle-shaped cells appeared and non-adherent cells were removed, and fresh culture medium was added. Bone marrow-derived MSCs at passage 3 were phenotyped using CD29, CD44, CD90, CD105, CD34 and CD45 by a FACS Calibur cytometry (Becton Dickinson, San Jose, CA). In order to evaluate the differentiation capability of MSCs, the cells (2 × 103/cm2) were seeded into six-chamber slides. Osteogenic differentiation medium (α-MEM containing dexamethasone 1 × 10−8 M, β-glycerophosphate 10 mM, ascorbic 0.3 mM and 10% FBS) and adipogenic induction medium (α-MEM containing 1 μM dexamethasone, 10 μg/mL recombinant insulin, 0.5 mM 3-isobutyl-1-methyl-xanthine, and 10% FBS) were added to the chamber slides. In vitro osteocyte differentiation of MSCs was evaluated 3 weeks later by Alizarin red staining and adipocyte differentiation of MSCs was then examined using Oil Red O stain.
Preparation Conditioned Medium and Exosomes Isolation
The MSCs at passage 3 or higher were used for preparing a conditioned medium (CM). The exosomes were isolated from CM using Exoquick reagent according to the International Society of Extracellular Vesicles (ISEV) recommendations. In brief, 150 ml CM were collected from 150 mm plates (n = 10) each containing 13 million MSCs after 48 h culture in complete medium containing exo-depleted FBS (Gibco, USA). Cell debris was removed by centrifuging at 300×g for 5 min and then 2000×g for 20 min and 16,000×g for 1 h. The cleared CM was passed through a 0.22 μm filter and concentrated using 100 kDa molecular weight Amicon Ultra-15 Centrifugal Filter (Merck Millipore, United States). The filtered supernatants were incubated with the appropriate volume of Exoquick precipitation solution according to the manufacturers’ instructions (System Biosciences, California) for 16 h at 4 °C, centrifuged for 30 min at 1500×g to pellet exosomes. Some studies have indicated that there are no significant differences in exosomes population isolated by the Exoquick protocol compared with ultracentrifugation methods [13, 14].
Characterization of MSCs-Derived Exosomes
To identify exosomal marker using western blot, exosome proteins or whole cell were lysed in reducing sample buffer [0.25 M Tris HCl (pH 6.8), 40% glycerol, 8% SDS, 5% 1-mercaptoethanol and 0.04% bromophenol blue] and boiled for 10 min at 95 °C. Resolved proteins were then transferred to nitrocellulose membranes and incubated separately with CD9, CD81, CD63, and calnexin specific primary antibodies (cell guidance systems, UK) at the supplier’s recommended dilutions overnight at 4 °C. After subsequent washing, the membranes were further incubated with horseradish peroxidase-coupled secondary antibodies (Abcam, Cambridge, UK). Bound Proteins were visualized using the ECL prime Western blotting detection system (GE Healthcare). Size distribution of purified exosomes was evaluated using dynamic light scattering (DLS). Briefly, about 20 μL exosome sample was diluted in 1 mL PBS and shaken at 4 °C for 20 min prior to DLS measurement. DLS measurements were conducted at 25 °C using Nano Zetasizer (Malvern Instruments Ltd., UK). Morphological assessment of the isolated exosomes was performed using transmission electron microscopy (TEM) (Philips CM30 electron microscopy) at 80 kV. For this, the exosomes preparation was fixed for 1 h in 4% paraformaldehyde and washed once with PBS. Then, the pellets were fixed in 2.5% glutaraldehyde, loaded on Formvar−/−carbon-coated EM grids. The grids were blocked with 5% bovine serum albumin for 10 min. The blocked grids were incubated with anti-CD63 antibody (Cell guidance system, UK) overnight at 4 °C, washed six times in 0.1% BSA and then incubated with a recommended dilution of a 10 nm-gold-coupled secondary antibody (Abcam, Cambridge, UK) for 1 h at room temperature. The grids were then postfixed in 1% glutaraldehyde and contrasted successively in 2% methylcellulose/0.4% uranyl acetate (pH 4.0).
Mammosphere Preparation
Single-cell suspension was prepared from MCF7 cell monolayer and was cultured in complete Mammocult™ Human Medium Kit (Mammocult Basal Medium+Mammocult proliferation Supplement+heparin+hydrocortisone) according to the manufacturer’s instructions (Stem cell technologies) at a density of 40,000 cells/cm2 in ultralow attachment culture plate (Corning CoStar). For first generation culturing, fresh medium was half-replaced every 3 days over 7–10 days. For secondary and tertiary cultures, mammospheres were collected, washed, trypsinized, mechanically dispersed and equal number of cells were replated in mammosphere media. The number of mammospheres with solid or hollow morphology that were larger than 60 μm in size were counted under a Nikon microscope.
Flow Cytometric Analysis of General Cancer Stem Cell Markers Expression
Single-cell suspension was obtained from monolayer culture or mammosphere culture, washed with PBS, and incubated on ice for 30 min with a labeled monoclonal antibody specific for the more common cancer stem cells markers including CD44 (CD44-FITC) or CD24 (CD24-PE) (Stem cell technologies). In negative control experiments, cells were incubated with fluorescently labeled isotype-matched preimmune IgG instead (Abcam, Cambridge, UK). Cells were washed, then analyzed by a FACS Calibur cytometry (Becton Dickinson, San Jose, CA).
Aldefluor Assay
Aldefluor assay, a common assay to identify cells with high aldehyde dehydrogenase (ALDH) activity, was done according to the manufacturer’s guidelines (Stem cell technologies, Canada). Single cells obtaining from mammospheres or corresponding adherent cells were incubated in Aldefluor assay buffer containing an ALDH substrate, bodipy-aminoacetaldehyde (1 μmol/L per 106 cells), for 40 min at 37 °C. As a negative control, a fraction of cells from each sample was incubated under an identical condition in the presence of the ALDH inhibitor diethylaminobenzaldehyde. Flow cytometry was used to measure ALDH-positive cell population.
Cancer Stem Cell-Related Gene Expression Analysis by qRT-PCR
In order to detect the expression levels of octamer-binding transcription factor 4 (Oct4), sex-determining region Y-box2 (Sox2), Kruppel-like factor 4 (Klf4) and Nanog genes, total RNA was isolated from MCF7 or MCF7-derived mammosphere cells using RNeasy mini kit (Qiagen, Hilden, Germany) following the manufacturer’s protocol. Total RNA was converted to cDNA using first strand cDNA synthesis kit according to manufacturer’s protocol (Fermentase, Germany). cDNA was used for qPCR analysis using SYBRGreen PCR master mix (Takara, Dalian, China) on a Rotor-Gene RG-3000 real-time PCR machine (Corbett Research, United Kingdom). The primer pairs used for amplification were summarized in supplementary Table S1. The relative mRNA expression was presented as fold differences between MCF7 derived mammosphere cells and MCF7 monolayer cells. β-actin expression was used as internal control.
Sphere Differentiation
In order to differentiate the spheres into an adherent culture, cells were dissociated into single cells and plated in their original medium supplemented with FBS.
Tumorigenesis Assay
In order to evaluate the effect of the exosomes-mediated transfer of the miR-142-3p inhibitory oligonucleotides on the tumorigenic capability of sphere-forming cells, the mammosphere cells were subjected to treatments in four groups including cells treated with unloaded MSCs-Exo, MSCs-Exo loaded with LNA-antimiR-142-3p, MSCs-Exo loaded with LNA-miRNA inhibitor negative control, or PBS as a negative control for 48 h. Subsequently, dissociated cells in each group were resuspended in DMEM medium and injected in the flank region of 6-week-old female BALB/c SCID mice (n = 24; 6 mice in each group) with 1 × 106 cells. The mice were monitored daily and tumor growth was inspected by biweekly measurement of tumor diameters with a Vernier caliper. Tumor volume (TV) was calculated using the formula: TV (mm3) = d2 × D/2, where the d and D are the shortest and the longest diameter, respectively. Survival of mice was monitored for 60 days in tumor-bearing mice. A Kaplan-Meier method was used to evaluate the probability of mice survival, and the log-rank test was used to compare the fraction of surviving mice between groups (α = 0.05).
Clonogenic Cell Survival Assay
To determine the effect of exosomes-based delivery of LNA-antimiR-142-3p on the ability of mammosphere cultures to colonize, MCF7-derived mammospheres were dissociated into single cells and plated at a density of 100 cells/well in 96-well plate in 200 μl of growth medium. They were treated with grown for 7 days and then stained with 0.1% Crystal Violet in PBS for 5 min. After rinsing in water, the colonies were photographed and counted to compare the size and number of colonies.
In Vitro Visualization of Fluorescently-Labeled Exosomes
MCF7 derived mammospheres in spherical or single cell forms were cultured in 24 well plates in sufficient mammocult medium and then 5 μg of PKH67 (Sigma-Aldrich, USA)-labeled exosomes was added to each well and the cells incubated for 24 h at 37 °C with 5% CO2. After incubation, cellular uptake of PKH67-labeled exosomes was visualized using confocal laser scanning microscopy (Leica TCS SPE, Germany).
Loading Exosomes with LNA-antimiR-142-3p by Electroporation
In order to load the exosomes with MiR-142-3p miRCURY LNA miRNA Power Inhibitor (LNA-antimiR-142-3p) (Cat no. YI04100271) (Qiagen, Hilden, Germany) and miRCURY LNA miRNA Power Inhibitor Negative Control (LNA-antimiR negative control) (Cat no. YI00199006) (Qiagen, Hilden, Germany), electroporation method with the validated condition was used [15, 16]. For this purpose, the pellet of exosomes was suspended in pre-chilled EDTA (1 mM) and trehalose (25 mM) containing hypoosmolar electroporation buffer (Eppendorf Multiporator, Germany). LNA-antimiR-142-3p and LNA-antimiR negative control molecules at a final amount of 150 pmol were added to 1 μg/μL of the exosomes sample and the mixture was transferred into a cold 0.4 cm electroporation cuvette. Electroporation was performed at 0.200 kV and 100 μF with three pulses (all containers and buffers were RNase free). The sample was then incubated at room temperature for 30 min and subsequently treated with one unit of RNase H to eliminate free unincorporated antimiR molecules and the loaded exosomes re-isolated using the Exoquick protocol.
Determination of LNA-antimiR-142-3p Encapsulated in MSCs-Exo
In order to estimate the amount of LNA-antimiR-142-3p and LNA-antimiR negative control oligonucleotides in MSCs-derived exosomes, the sample preparations were centrifuged twice at 100,000×g for 1 h to precipitate the exosomes loaded with the anti-microRNA oligonucleotides. The supernatant was carefully collected and the pellet (loaded exosomes) were lysed by adding 5% Triton X100 and applying subsequently sonication. The anti-microRNA concentration was determined by measurement of absorbance at 260 nm (Abs260). The percentage of the loaded anti-microRNA oligonucleotides was calculated as follows:
RNA Isolation, mRNA and miRNA Analysis
The expression level of miRNA-142 and miRNA-150 were quantified using the ExiLENT SYBR Green master mix kit (Qiagen, Hilden, Germany), and expression level of APC and P2X7R genes were measured using the Roche master mix containing SYBR green on a Rotor-Gene RG-3000 real-time PCR machine (Corbett Research, United Kingdom). MicroRNAs quantitations were normalized to an endogenous control U6 snRNA and expression levels of target genes were normalized to β-actin gene. The miR-specific LNA enhanced primers for miR-150, miR-142-3p and primers for U6 snRNA were obtained from Qiagen (Hilden, Germany). MiRNAs and genes quantification levels were calculated using 2-∆∆Ct method.
Cell Viability Assay
The cells were seeded at a density of 104 cells/well in 96 well plate before treatment. After overnight plating, cells were treated with unloaded MSCs-Exo, MSCs-Exo loaded with LNA-antimiR-142-3p, or MSCs-Exo loaded with LNA-antimiR negative control for 48 h. The same amount of exosomes equal to 5 μg was added to each treated group. MTT (5 mg/ml) was added to each well for 4 h additional incubation. The resulting formazan was then extracted by the addition of 100 μl dimethyl sulfoxide and the optical density was measured at 540 nm.
Quantification of Apoptosis by Flow Cytometry
Cell apoptosis was measured by using an Annexin V-Fluos apoptosis detection kit (Hoffman-La Roche Ltd., Basel, Switzerland) according to the manufacturer’s protocol. In brief, MCF10A, MCF7 cell lines and MCF7-derived cancer stem-like cells in each treated groups, unloaded MSCs-Exo, MSCs-Exo loaded with LNA-antimiR-142-3p, or MSCs-Exo loaded with LNA-antimiR negative control, were harvested and washed twice with PBS. Each pellet was resuspended in 400 μl PBS at a concentration of 1 × 106 cells/ml and then 100 μl incubation buffer containing 2 μl of annexin V and 2 μl of propidium iodide was added to each experimental group cells. Treated cells as well as control cells were analyzed using a BD flow cytometer within 1 h.
Statistical Analyses
Based on data distribution, Mann-Whitney nonparametric test or one-way analyses of variance (ANOVA) were used in this study to make comparisons between different groups and the error bars are shown as Average ± standard error of the mean (SEM). All experiments were carried out three times and P values<0.05 were considered statistically significant.
Results
Characterization of Bone Marrow-Derived Mesenchymal Stem Cells and MSCs-Exo
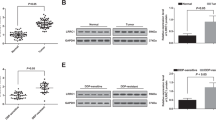
Murine BM-derived MSCs had a defined spindle-shaped fibroblastic morphology. Multipotent nature of MSCs was confirmed by assessing the differentiation potential of the isolated MSCs into osteoblasts and adipocytes by culturing in an appropriate culture medium (Fig. 1a). Analysis of phenotypic markers confirmed that isolated MSCs were positive for CD29, CD44, CD90, CD105 and negative for CD34 and CD45 (Fig. 1b). We isolated exosomes from the supernatants of the MSCs. TEM analysis verified the disc-shaped of MSCs-Exo labeled with gold nanoparticles for CD63 marker and showed that the exosomes had an average size between 30 and 150 nm (Fig. 2a). Expression of some important exosomal markers such as CD81 and CD63, and not the expression of Calnexin, an endoplasmic reticulum marker, was observed using western blot (Fig. 2b). The average size of the MSCs-derived exosomes was 103 nm according to zeta sizer results (Fig. 2c). These results indicated that MSCs and their secreted exosomes successfully isolated and they are of the required verification criteria according to previous studies. We measured the amount of the isolated exosomes from MSCs based on their protein content by using Bradford assay, according to this results 200 μg exosomes approximately were obtained from 13 × 107 mesenchymal stem cells (150 mL supernatants) after 48 h cultivation in the α-MEM medium.
Characterization of exosomal particles. a Transmission electron micrograph of the negatively stained MSCs-derived exosomes with a diameter of 30–150 nm, labeled for CD63. b Western blotting analysis of cellular and exosomal lysates using antibodies that detect marker proteins for exosomes (CD9, CD81, CD63) and endoplasmic reticulum (Calnexin). The blots are gathered at different times. c Size distribution by intensity (a) and size distribution by the number of MSCs-derived exosomes (b)
Characterization of the Cancer Stem-like Properties of MCF7-Derived Mammospheres
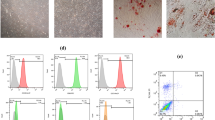
In terms of morphology, the MCF7-derived mammospheres were comprised tightly packed, coherent cells within a well-defined border, and they were observed as floating, non-adherent spheres (Fig. 3a). The average size of the tumorspheres was 150 μm, and they were formed up to three consecutive passages that represent the most important characteristic of stem cells, namely, their self-renewal. The number of tumorspheres with the diameter of 60 μm or larger was quantified in the first, second and third generation, the results indicated that efficiency of MCF7-derived tumorspheres formation was relatively high, but the number of tumorspheres decreased gradually as a result of successive passages (Fig. 3b). To investigate the differentiation ability of mammospheres into parental cells, floating tumorsphere cells were plated under differentiating condition, as it has shown in Fig. 3c, they were able to re-differentiate into adherent cells re-acquiring epithelial-like morphology once cultured in parental growth condition. Flow cytometric analysis of stem cells-related surface markers has demonstrated that most of the cancer stem cells in mammospheres showed CD44+/CD24− (85%), while the majority of the MCF7 as parental cells showed CD44−/CD24+ (78%) phenotype, meaning that MCF7-derived mammospheres have breast cancer stem cells phenotype (Fig. 3d). Analysis of ALDH1 activity in the mammospheres and corresponding parental cell line indicated that MCF7-derived mammospheres have exhibited more ALDH1 activity compared to the parental cell line (Fig. 3e). The quantification of stem cells molecular markers demonstrated that MCF7-derived mammospheres expressed significantly higher levels of the stem cell molecular markers NANOG, OCT4, KLF4, and SOX2 in comparison to the parental cells (P < 0.05) (Fig. 3f).
Characterization of cancer stem-like properties of MCF7-derived mammospheres. a MCF7-derived cancer stem-like cells morphology as tumorspheres and single cells. b Sphere-forming efficiency of spheroid cells on serial passages. c The differentiation ability of mammospheres into parental cells once culturing in adherent growth condition. d 7 days old MCF7-derived mammospheres were enriched for cells with a CD44+/CD24−/low phenotype compared with MCF7 cells. e Percentage of ALDEFLUOR-positive cells in MCF7-derived mammospheres and their parental cells analyzed by flow cytometry. f qRT-PCR analysis of expression levels of stem cell-related genes in 7 days old MCF7-derived mammospheres and parental cells. *indicative of p < 0.05
MSCs-Derived Exosomes Have the Ability to Penetrate the MCF7-Derived Cancer Stem-like Cells
One of the unique properties of exosomes compared to other nanovehicels is incorporation into target cells simply through the co-culture method. Cellular uptake of the fluorescently PKH67-labeled MSCs-derived exosomes by MCF7-derived mammospheres as spherical or single cell forms after 24 h co-culturing was visualized by confocal laser scanning microscopy (CLSM). Fluorescently-labeled MSCs-Exo were clearly observed in both spherical and single cell forms of MCF7-derived mammospheres (Fig. 4). In addition, we assessed the biodistribution and tumor penetration of MSCs-Exo in tumor-bearing mice in our previous study. Our results revealed that the intravenously injected MSCs-Exo had the ability to penetrate the tumor site 3 h post-injection due to its EPR effect, and even after clearance from other tissues, they were still present in the tumor tissue with the low systematic toxicity [16]. This Observation demonstrated that MSCs-Exo had the ability to penetrate the cancer stem cells in the in vitro culture and tumor tissues under in vivo condition.
Efficient Delivery of LNA-antimiR-142-3p Via MSCs-Exo After Incorporating into the MCF7-Derived Cancer Stem-like Cells
Thereafter, we used the MSCs-Exo to transfer the LNA-based miR-142-3p inhibitor to suppress miR-142 and investigate the functional effects on miR-150 expression and their respective target genes, APC and P2X7 receptor. Furthermore, our results indicated efficient loading of the MSCs-Exo with LNA-antimiR-142-3p (about 57%) and LNA-antimiR negative control (about 55%) oligonucleotides. Therefore, we assessed the functional effects of antimiR-142-3p and the MSCs-Exo as carriers on MCF10A, MCF7 cell lines and MCF7-derived cancer stem-like cells in 4 groups including untreated, unloaded MSCs-Exo, MSCs-Exo loaded with LNA-antimiR-142-3p, and MSCs-Exo loaded with LNA-antimiR negative control. In order to inhibit the miR-142 using MSCs-Exo, MSCs-derived exosomes should be free of the microRNA-142 molecules. In our previous study, we demonstrated by RT-PCR that MSCs-derived exosomes didn’t contain the miR-142 and miR-150 [16]. Quantitative real-time PCR results demonstrated that MSCs-Exo can efficiently transfer the antimiR-142-3p to the MCF7-derived cancer stem-like cells indicated by a statistically significant decrease in miR-142-3p levels and a subsequent statistically significant increase in APC mRNA levels in the cells treated with MSCs-Exo loaded with antimiR-142-3p compared to the other groups (p < 0.05) (Fig. 5a). Additionally, exosomes mediated miR-142-3p inhibition resulted in a significant decrease in miR-150 levels and a significant increase in P2X7 receptor mRNA levels compared to the control groups (p < 0.05) (Fig. 5b). According to previous studies, miR-142-3p isn’t significantly expressed in non-tumorigenic cancer cells. Our results also indicated that MSCs-Exo mediated delivery of LNA-antimiR-142-3p to the MCF7 and MCF10A cells, as parental and normal control cells, respectively, didn’t lead to significant differences in the expression levels of miR-142-3p, miR-150, APC, and P2X7R genes between different groups (Fig. 5a, b). Our results confirm the previous studies that the miR-142-3p is distinctly expressed in tumorigenic cancer stem cells.
Delivery of LNA-antimiR-142-3p to the MCF7-derived cancer stem-like cells via exosomes. a qRT-PCR analysis of relative expression of mir-142-3p (a) and APC mRNA (b) levels in MCF7, MCF10A, and cancer stem-like cells, normalized to U6 snRNA or β-actin mRNA. c qRT-PCR analysis of relative expression of mir-150 (a) and P2X7R mRNA (b) levels in MCF7, MCF10A, and cancer stem-like cells. Data are presented as mean ± SEM (n = 3) (*p < 0.05)
MSCs-Derived Exosomes Had Minimal Cytotoxicity in Cancer Stem-like Cells
Cytotoxicity effect of the unloaded MSCs-Exo, LNA-antimiR-142-3p loaded MSCs-Exo and LNA-antimiR negative control loaded MSCs-Exo were subsequently investigated on MCF7-derived cancer stem-like cells, their parental cells, and MCF10A as a normal breast cell line by MTT assay after 48 h. Cell viability and proliferation of the cancer stem-like cells, as well as MCF7 treated with MSCs-Exo, were moderately increased compared to the untreated cells (Not significant). On the other hand, cell viability of MCF7-derived cancer stem-like cells treated with LNA-antimiR-142-3p loaded MSCs-Exo was reduced compared to the cells treated with MSCs-Exo and LNA-antimiR negative control groups (p < 0.05), probability due to the transfer of LNA-antimiR-142-3p to the recipient cells, but there was no significant difference in cellular viability between MCF10A cells treated with LNA-antimiR-142-3p loaded MSCs-Exo and untreated cells. It is noteworthy that delivery of LNA-antimiR-142-3p via MSCs-Exo, in contrast to MCF7-derived cancer stem-like cells, had no significant cytotoxicity effect on MCF7 cells, as shown in Fig. 6.
Evaluating cytotoxicity effect of exosome-based LNA-antimiR-142-3p delivery on MCF10A, MCF7, and MCF7-derived cancer stem-like cells. After 48 h of co-culture, unloaded exosomes and LNA-antimir negative control loaded exosomes showed nonsignificant toxicity versus untreated cells, while LNA-antimiR-142-3p loaded exosomes showed significant cytotoxicity in MCF7-derived cancer stem-like cells (*p < 0.05). There were no significant differences between different treatment groups of parental cells and MCF10A cells. The results are presented as mean ± SEM (n = 3)
Delivery of LNA-antimiR-142-3p Molecules Using MSCs-Derived Exosomes Induce Apoptosis in Cancer Stem-like Cells
To further investigation of functional role of LNA-antimiR-142-3p in MCF7-derived cancer stem-like cells, the effect of LNA-antimiR-142-3p delivery via MSCs-Exo was assessed on apoptosis of MCF7-derived cancer stem-like cells, their parental cells, and MCF10A as a normal breast cell line after 48 h treatment with the unloaded MSCs-Exo, LNA-antimiR-142-3p loaded MSCs-Exo, and LNA-antimiR negative control loaded MSCs-Exo. As presented in Fig. 7, MSCs-derived exosomes had no significant effects on apoptosis of MCF7-derived cancer stem-like cells compared to the untreated cells. But, apoptosis percentage of the cells was significantly increased following treatment with LNA-antimiR-142-3p loaded MSCs-Exo compared to the unloaded MSCs-Exo or LNA-antimiR negative control loaded MSCs-Exo (p < 0.05). In contrast, MSCs-Exo mediated delivery of LNA-antimiR-142-3p to MCF7 and MCF10A, as parental and normal control cells, respectively, did not significantly increase the amount of apoptosis as compared to the other groups.
The effect of the LNA-antimiR-142-3p loaded exosomes assessment on cell apoptosis. (a and b) Breast cancer stem-like cells, their parental cells, and MCF10A cells were co-cultured with different MSCs-Exo preparations for 48 h followed by flow cytometry analysis of apoptotic cells (*p < 0.05). The data represent three independent experiments and are expressed as mean ± SEM
LNA-antimiR-142-3p Reduces Tumorigenic and Colony Formation Capabilities of MCF7-Derived Cancer Stem-like Cells
To examine the effect of functional delivery of LNA-antimiR-142-3p via MCSs-derived exosomes on the tumorigenic capacity of MCF7-derived cancer stem-like cells, the mammosphere cells in each treatment groups including unloaded MSCs-Exo, MSCs-Exo loaded with LNA-antimiR-142-3p, MSCs-Exo loaded with LNA-miRNA inhibitor negative control, or untreated cells were injected subcutaneously into BALB/c SCID mice. As shown in Fig. 8a, the tumorigenic capacity of cancer stem-like cells was significantly decreased in vivo by downregulation of miR-142 and miR-150 via exosome-mediated delivery of LNA-antimiR-142-3p compared to the other groups (p < 0.05). In other words, the size of the tumor that arose from the cancer stem-like cells treated with LNA-antimiR-142-3p was significantly less than the size of the tumor that formed by the other groups. However, there were no significant differences between the volume of the tumors formed by breast cancer stem-like cells treated with unloaded MSCs-Exo, MSCs-Exo loaded with LNA-miRNA inhibitor negative control, and untreated breast cancer stem-like cells. In addition, survival analyses showed that the mice injected with LNA-antimiR-142-3p loaded exosomes-treated cancer stem-like cells had a significantly longer survival (mean survival [ms], 38 days) than the mice injected with unloaded exosomes-treated cells (ms, 19.5 days) or control mice including both injected with LNA-antimiR negative control-treated cells (ms, 22 days) and injected with PBS-treated cells (ms, 19 days) (Fig. 8a).
Functional delivery of MSC-Exo mediated LNA-antimiR-142-3p into MCF7-derived cancer stem-like cells suppresses breast cancer stem cells tumorigenicity both in vitro and in vivo. a Average tumor volumes (mm3) of different treatment groups after inoculation (n = 24; 6 mice in each group) (a) Survival analyses of the mice injected with the breast cancer stem-like cells treated with unloaded exosomes, LNA-antimiR-142-3p loaded MSCs-Exo, LNA-antimiR negative control loaded MSCs-Exo, or PBS (b). b Representative micrograph and colony numbers in the anchorage-independent growth assay. Each bar represents the mean of three independent experiments (*P < 0.05)
Figure 8b has shown the functional effect of MSCs-Exo mediated transfer of LNA-antimiR-142-3p on the colony formation property of MCF7-derived cancer stem-like cells in the form of three treated groups plus one control group. Delivery of LNA-antimiR-142-3p via MSCs-Exo significantly reduced the number of cancer stem-like cells colonies compared to the other control groups (p < 0.05), indicating that inhibition of miR-142-3p and miR-150 resulted in significant suppression of the proliferation rate of the cancer stem-like cells.
Evaluation of miR-142-3p, miR-150, and Their Targets Expression in Tumor Tissues
To evaluate the functional effect of the exosomes-mediated delivery of LNA-antimiR-142-3p in tumor tissues, all BALB/c SCID mice in each group (n = 24; 6 mice in each group) were sacrificed and tumor tissues were removed for RNA extraction and qRT-PCR analyses. We observed a significant decrease in miR-142-3p level in tumor tissues grown from BCSCs treated with LNA-antimiR-142-3p loaded exosomes in comparison to three other groups as indicated (p < 0.05). Next, to evaluate the functional effect of antimiR-142-3p on target miRNA and target genes, we measured the expression levels of miR-150, APC and P2X7R genes by real-time PCR assay. As expected, reduction of miR-142-3p led to a significant reduction of the miR-150 level (p < 0.05) and a significant increase of target genes, APC and P2X7R (p < 0.05) (Fig. 9).
Reduction of miR-142-3p, miR-150 and its effect on target genes by exosomes-based delivery of LNA-antimiR-142-3p in tumor tissues. a qRT-PCR analysis showed significant reduction of miR-142-3p and miR-150 expression levels in tumor tissues grown from BCSCs treated with MSCs-Exo loaded with LNA-antimiR-142-3p compared to three other groups, normalized to U6 snRNA. b APC and P2X7R mRNA expression were significantly increased in tumor tissues grown from BCSCs treated with MSCs-Exo loaded with LNA-antimiR-142-3p compared to three other groups, normalized to β-actin mRNA. 24 BALB/c SCID mice were divided into 4 groups and the BCSCs treated with unloaded exosomes, LNA-antimiR-142-3p loaded MSCs-Exo, LNA-antimiR negative control loaded MSCs-Exo, or PBS were injected subcutaneously into the mice in each group. Data are presented as mean ± SEM, calculated from three independent experiments (*P < 0.05)
Discussion
In the present study, we exploited the MSCs-derived exosomes as a feasible nanovehicles to effectively transfer the LNA modified anti-microRNA-142-3p to MCF7-derived cancer stem-like cells to reduce the tumorigenic and clonogenic capabilities of breast cancer stem cells. Since cancer stem cells are responsible for the recurrence of tumors, many studies have been focused on eradicating these cells for the treatment of cancer. So far, several experimental methods have been used to characterize and identify BCSCs [17]. It has been recently reported that cancer stem-like cells isolation by sphere culture of established cancer cell lines is a readily available and straight-forward method for analyzing tumorigenesis and carcinogenesis properties of cancer stem cells [18]. So, we decided in this study to use MCF7 breast cancer cell line to isolate CSCs-like cells for tumorigenic and clonogenic studies. Our results indicated that MCF7-derived cancer stem-like cells, according to previous studies, had prominent stem cells characteristics, including mammospheres generation in non-adherent culture condition, stem cells markers expression, CD44+/CD24− phenotype, ALDH1 activity, and tumorigenicity in vivo (Figs. 3 and 8). Cancer stem cells with distinct molecular markers and cellular features compared to non-tumorigenic cells may provide new insight into developing more effective therapy in breast cancer. Extensive research have shown that cancer stem cells differentially express a different subset of microRNAs that could be used as potential candidates to identify and target the cells [9]. The deregulated miRNAs may act as oncomirs or tumor suppressive miRNAs that have been linked to different aspects of the breast cancer stem cells features [19,20,21,22]. Several researchers in the 2009 work, compared the microRNAs expression profile in BCSCs with other cancer cells within the same tumor. They found two hyperactive microRNAs, miR-142 and miR-150, in human breast cancer stem cells [9, 23]. Isobe et al. indicated that miR-142-3p expression is upregulated in breast cancer stem cells that efficiently target and silence Adenomatous Polyposis Coli (APC) to activate Wnt/β-catenin signaling and thereby enhances miR-150 expression which is also upregulated in breast cancer stem cells [10]. MiR-150 over-expression promotes growth, clonogenicity and reduces apoptosis in breast cancer stem cells by targeting the pro-apoptotic purinergic P2X7 receptor gene [24]. Upregulation of miR-142-3p as well as miR-150 enhance tumorigenicity of breast cancer stem cells and cause excessive organoid formation in vivo [25]. So, Inhibition of miR-142-3p by miRNA inhibitor oligonucleotides can suppress the tumorigenicity and clonogenicity of BCSCs to slow the growth of breast tumors. One of the critical issues in the transfer of the RNA-based therapeutics in gene therapy studies is the use of suitable carriers under in vivo conditions. There are several drawbacks for using the conventional gene therapy vehicles in clinical practice, such as immunogenicity, cytotoxicity, low probability of integration and low efficiency in specific patients [26, 27]. In recent years, harnessing exosomes as nanocarriers in nanomedicine applications is of great interest to many scientists [28]. Several types of research have been found different classes of microRNAs in exosomes which can be delivered from the cell of origin to recipient cells to exert their functional role. These observations have led scientists to use exosomes as nanocarriers to deliver siRNAs, miRNAs and chemotherapeutic agents [29,30,31,32]. Since then, exosomes isolated from various types of cells have been used as nanocarriers to transfer different kinds of therapeutic agents in the treatment and diagnosis of cancer or other types of diseases [33,34,35,36]. One of the important sources of exosomes that are particularly considered in clinical applications is mesenchymal stem cells. Mesenchymal stem cells due to their unique characteristics such as self-renewal, tissue repair ability, immune properties modulation, and differentiation capacity, have been used in cell-based therapies for variety clinical conditions [37]. There are increasing evidence that MSCs exert their therapeutic effects via secretion of soluble paracrine factors and exosomes. In fact, secreted exosomes by MSCs can act as extracellular messengers to deliver special cargo from MSCs to recipient cells. It is believed that the functional effects of MSCs-derived exosomes are likely similar to the effects mediated by MSCs itself. So, the exosomes can be used as cell-free carriers, which may not pose the risk of tumorigenesis, unlike MSCs [38]. In this study, we isolated exosomes from bone marrow-derived mesenchymal stem cells, characterized by analyzing the phenotypic molecular markers and differentiation capacity, using a standard method (Fig. 1). The exosomes derived from MSCs possessed common and typical features of cell-derived nanovesicles identified by transmission electron microscopy, western blotting, and size distribution determination (Fig. 2).
According to our experiment, MSCs-Exo efficiently loaded with LNA-antimiR-142-3p using electroporation method and quantitative real-time PCR results indicated that the loaded exosomes can deliver the LNA-antimiR molecules to the MCF7-derived cancer stem-like cells, causing inhibition of miRNA-142-3p, and functionally led to a statistically significant increase of APC gene level. On the other hand, miRNA-142-3p inhibition led to a significant decrease of miRNA-150 expression level. Since P2X7R is a putative target of miRNA-150 and its expression is inversely correlated with miRNA-150 level, successful delivery of LNA-antimiR-142-3p to the MCF7-derived cancer stem-like cells led to significant increase of P2X7R mRNA level (Fig. 5). In agreement with our findings, there are a few studies have previously used the mesenchymal stem cells-derived exosomes as nanovehicles for the delivery of iRNA-based oligonucleotides in stem cell-based anti-cancer therapy research [31, 39]. For example, Munoz and his colleagues harnessed the mesenchymal stem cell-derived exosomes for the delivery of antimiR-9 to glioblastoma cells to confer chemosensitivity and increase the cell death [31].
Functional effects of exosomal delivery of LNA-antimiR-142-3p in MCF7-derived cancer stem-like cells was further investigated by clonogenicity and tumorigenicity assessment. Our results showed that inhibition of miR-142-3p and subsequently miR-150 using LNA-antimiR-142-3p delivered by MSCs-derived exosomes significantly alleviated proliferation and colony formation ability of MCF7-derived cancer stem-like cells. On the other hand, transfer of LNA-antimiR-142-3p to cancer stem-like cells using MSCs-Exo significantly reduced tumor-initiating capability of the cells compared to the control groups under in vivo conditions (Fig. 8). There are only few studies that have used the MSCs-derived exosomes to transfer the anti-cancer agents to inhibit the tumor growth. For example, Katakowski and his colleagues showed that the MSCs exosomes could be used as a vehicle for delivery of miR-146b to significantly reduced glioma xenograft growth [40]. Conducting such studies to target the cancer stem cells by harnessing the exosomes can provide a potential new tool for eradicating cancer stem cells in anti-cancer therapies.
Conclusion
Together, our data suggest that mesenchymal stem cell-derived exosomes as naïve nanovesicles can be more reliable, more efficient, and safer than common synthetic vehicles in therapeutic and diagnostic applications. Efficient uptake of the exosomes by the breast cancer stem cells was obviously confirmed using microscopy in our in vitro studies. Notably, our quantitative PCR results indicated that MSCs-derived exosomes mediated effective delivery of LNA-antimiR-142-3p molecules into MCF7-derived mammospheres, which led to a reduction in expression level of miR-142-3 and miR-150, and subsequently upregulation of their target genes, APC and P2X7R, respectively. Considering that aberrant expression of miR-142-3p and miR-150 in breast cancer stem cells is associated with increased clonogenic and tumorigenic capabilities of the cells, inhibition of the oncomiRs with the delivery of LNA-antimiR-142-3p resulted in a significant reduction of tumorigenicity and clonogenicity. Our findings provide proof of concept for using MSCs-derived exosomes as an effective nano-carriers for delivery of RNA-based therapeutics in nanomedicine applications, especially in cancer therapies. Further studies should be conducted to design specific engineered exosomes to target BCSCs-specific markers for use in development of targeted agents.
References
Andaloussi, S. E., Mäger, I., Breakefield, X. O., & Wood, M. J. (2013). Extracellular vesicles: Biology and emerging therapeutic opportunities. Nature Reviews Drug Discovery, 12, 347.
Vlassov, A. V., Magdaleno, S., Setterquist, R., & Conrad, R. (2012). Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochimica et Biophysica Acta (BBA)-General Subjects, 1820, 940–948.
Morishita, M., Takahashi, Y., Nishikawa, M., & Takakura, Y. (2017). Pharmacokinetics of exosomes—An important factor for elucidating the biological roles of exosomes and for the development of exosome-based therapeutics. Journal of Pharmaceutical Sciences, 106, 2265–2269.
Colombo, M., Raposo, G., & Théry, C. (2014). Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annual Review of Cell and Developmental Biology, 30, 255–289.
Johnsen, K. B., Gudbergsson, J. M., Skov, M. N., Pilgaard, L., Moos, T., & Duroux, M. (2014). A comprehensive overview of exosomes as drug delivery vehicles—Endogenous nanocarriers for targeted cancer therapy. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer, 1846, 75–87.
Wicha, M. S., Liu, S., & Dontu, G. (2006). Cancer stem cells: An old idea—A paradigm shift. Cancer Research, 66, 1883–1890.
Al-Hajj, M., Wicha, M. S., Benito-Hernandez, A., Morrison, S. J., & Clarke, M. F. (2003). Prospective identification of tumorigenic breast cancer cells. Proceedings of the National Academy of Sciences, 100, 3983–3988.
Setoguchi, T., Taga, T., & Kondo, T. (2004). Cancer stem cells persist in many cancer cell lines. Cell Cycle, 3, 412–413.
Shimono, Y., Zabala, M., Cho, R. W., et al. (2009). Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell, 138, 592–603.
Isobe, T., Hisamori, S., Hogan, D. J., et al. (2014). miR-142 regulates the tumorigenicity of human breast cancer stem cells through the canonical WNT signaling pathway. Elife, 3, e01977.
Singh, M. S., & Peer, D. (2016). SiRNA delivery: Current trends and future perspectives. Therapeutic Delivery, 7, 51–53.
Shtam, T. A., Kovalev, R. A., Varfolomeeva, E. Y., Makarov, E. M., Kil, Y. V., & Filatov, M. V. (2013). Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Communication and Signaling, 11, 88.
Umezu, T., Ohyashiki, K., Kuroda, M., & Ohyashiki, J. (2013). Leukemia cell to endothelial cell communication via exosomal miRNAs. Oncogene, 32, 2747.
Zhu, L., Qu, X.-H., Sun, Y.-L., Qian, Y.-M., & Zhao, X.-H. (2014). Novel method for extracting exosomes of hepatocellular carcinoma cells. World Journal Of Gastroenterology: WJG, 20, 6651.
Momen-Heravi, F., Bala, S., Bukong, T., & Szabo, G. (2014). Exosome-mediated delivery of functionally active miRNA-155 inhibitor to macrophages. Nanomedicine: Nanotechnology, Biology and Medicine, 10, 1517–1527.
Naseri, Z., Oskuee, R. K., Jaafari, M. R., & Moghadam, M. F. (2018). Exosome-mediated delivery of functionally active mirna-142-3p inhibitor reduces tumorigenicity of breast cancer in vitro and in vivo. International Journal of Nanomedicine, 13, 7727.
Duan, J.-j., Qiu, W., Xu, S.-l., et al. (2013). Strategies for isolating and enriching cancer stem cells: Well begun is half done. Stem Cells and Development, 22, 2221–2239.
Charafe-Jauffret, E., Ginestier, C., Iovino, F., Wicinski, J., Cervera, N., Finetti, P., Hur, M. H., Diebel, M. E., Monville, F., Dutcher, J., Brown, M., Viens, P., Xerri, L., Bertucci, F., Stassi, G., Dontu, G., Birnbaum, D., & Wicha, M. S. (2009). Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Research, 69, 1302–1313.
Cairo, S., Wang, Y., de Reyniès, A., et al. (2010). Stem cell-like micro-RNA signature driven by Myc in aggressive liver cancer. Proceedings of the National Academy of Sciences, 107, 20471–20476.
Hwang-Verslues, W., Chang, P., Wei, P., et al. (2011). miR-495 is upregulated by E12/E47 in breast cancer stem cells, and promotes oncogenesis and hypoxia resistance via downregulation of E-cadherin and REDD1. Oncogene, 30, 2463.
Wang, Y., Yu, Y., Tsuyada, A., et al. (2011). Transforming growth factor-β regulates the sphere-initiating stem cell-like feature in breast cancer through miRNA-181 and ATM. Oncogene, 30, 1470.
Zhou, A., Diao, L., Xu, H., et al. (2012). β-Catenin/LEF1 transactivates the microRNA-371-373 cluster that modulates the Wnt/β-catenin-signaling pathway. Oncogene, 31, 2968.
Wellner, U., Schubert, J., Burk, U. C., et al. (2009). The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nature Cell Biology, 11, 1487.
Huang, S., Chen, Y., Wu, W., et al. (2013). miR-150 promotes human breast cancer growth and malignant behavior by targeting the pro-apoptotic purinergic P2X7 receptor. PloS One, 8, e80707.
Hu, W., Ye, Y., Zhang, W., Wang, J., Chen, A., & Guo, F. (2013). miR-142-3p promotes osteoblast differentiation by modulating Wnt signaling. Molecular Medicine Reports, 7, 689–693.
Nayerossadat, N., Maedeh, T., & Ali, P. A. (2012). Viral and nonviral delivery systems for gene delivery. Advanced Biomedical Research, 1, 27.
Scholz, C., & Wagner, E. (2012). Therapeutic plasmid DNA versus siRNA delivery: Common and different tasks for synthetic carriers. Journal of Controlled Release, 161, 554–565.
Lakhal, S., & Wood, M. J. (2011). Exosome nanotechnology: An emerging paradigm shift in drug delivery: Exploitation of exosome nanovesicles for systemic in vivo delivery of RNAi heralds new horizons for drug delivery across biological barriers. Bioessays, 33, 737–741.
Alvarez-Erviti, L., Seow, Y., Yin, H., Betts, C., Lakhal, S., & Wood, M. J. (2011). Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nature Biotechnology, 29, 341–345.
Lässer, C., Eldh, M., & Lötvall, J. (2012). Isolation and characterization of RNA-containing exosomes. JoVE (Journal of Visualized Experiments), e3037.
Munoz, J. L., Bliss, S. A., Greco, S. J., Ramkissoon, S. H., Ligon, K. L., & Rameshwar, P. (2013). Delivery of functional anti-miR-9 by mesenchymal stem cell–derived exosomes to glioblastoma multiforme cells conferred chemosensitivity. Molecular Therapy-Nucleic Acids, 2, e126.
Ohno, S.-i., Takanashi, M., Sudo, K., et al. (2013). Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Molecular Therapy, 21, 185–191.
Kim, S. H., Bianco, N. R., Shufesky, W. J., Morelli, A. E., & Robbins, P. D. (2007). Effective treatment of inflammatory disease models with exosomes derived from dendritic cells genetically modified to express IL-4. The Journal of Immunology, 179, 2242–2249.
Lee, J.-K., Park, S.-R., Jung, B.-K., et al. (2013). Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS One, 8, e84256.
McLellan, A. (2009). Exosome release by primary B cells. Critical Reviews™ in Immunology, 29, 203–217.
Takahashi, Y., Nishikawa, M., Shinotsuka, H., Matsui, Y., Ohara, S., Imai, T., & Takakura, Y. (2013). Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. Journal of Biotechnology, 165, 77–84.
Greco, S. J., & Rameshwar, P. (2012). Mesenchymal stem cells in drug/gene delivery: Implications for cell therapy. Therapeutic Delivery, 3, 997–1004.
Sundaram, B., Herbert, F. J., & Kumar, S. (2017). Human mesenchymal stem cell (hMSC)-derived exosomes/exosome mimetics as a potential novel therapeutic tool for regenerative medicine. Regenerative Medicine: Laboratory to Clinic: Springer, 81–97.
Sharif, S., Ghahremani, M., & Soleimani, M. (2018). Delivery of exogenous miR-124 to glioblastoma multiform cells by Wharton’s jelly mesenchymal stem cells decreases cell proliferation and migration, and confers chemosensitivity. Stem Cell Reviews and Reports, 14, 236–246.
Katakowski, M., Buller, B., Zheng, X., Lu, Y., Rogers, T., Osobamiro, O., Shu, W., Jiang, F., & Chopp, M. (2013). Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Letters, 335, 201–204.
Acknowledgments
We are grateful to our colleagues and PhD students, for help and support during this work, in the Department of Medical Biotechnology, Faculty of Medical Sciences, Tarbiat Modares University, Tehran. We also thank Mrs. Karen Fascioli for editing the manuscript.
Funding
This research was funded by Mashhad University of Medical Sciences and Tarbiat Modares University, Tehran (NO.931289); and a grant from cancer research center of cancer institute of Iran (Shams cancer charity, Grant No: 37604–202–01-97). The funding sources played no decisive role in collection, analysis, the interpretation of the data, as well as decision to submit for publication.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 15.3 kb)
Rights and permissions
About this article
Cite this article
naseri, Z., Oskuee, R.K., forouzandeh-moghadam, M. et al. Delivery of LNA-antimiR-142-3p by Mesenchymal Stem Cells-Derived Exosomes to Breast Cancer Stem Cells Reduces Tumorigenicity. Stem Cell Rev and Rep 16, 541–556 (2020). https://doi.org/10.1007/s12015-019-09944-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-019-09944-w