Abstract
In our quest to standardize our formula for a clinical trial, transforming growth factor-beta3 (TGF-β3) alone and in combination with bone morphogenetic protein-6 (BMP-6) were evaluated for their effectiveness in cartilage differentiation. Bone Marrow Stem Cells (BMSCs) and Adipose Derived Stem Cells (ADSCs) were induced to chondrogenic lineage using two different media. Native chondrocytes served as positive control. ADSCs and BMSCs proved multipotency by tri-lineage differentiations. ADSC has significantly higher growth kinetics compare to Chondrocyte only p ≤ 0.05. Using TGF-β3 alone, BMSC revealed higher expressions for hyaline cartilage genes compare to ADSCs. Chondrocyte has significantly higher early chondrogenic markers expression to ADSCs and BMSCs, while BMSCs was only higher to ADSC at chondroadherin, p ≤ 0.0001. On mature chondrogenic markers, chondrocytes were significantly higher to ADSCs and BMSCs for aggrecan, collagen IX, sry (sex determining region y)-box9, collagen II and fibromodullin; and only to ADSC for collagen XI. BMSC was higher to ADSC for aggrecan and collagen IX, p ≤ 0.0001. The combination of TGF-β3 + BMP-6 revealed increased gene expressions on both BMSCs and ADSCs for early and mature chondrogenic markers, but no significance difference. For dedifferentiation markers, ADSC was significantly higher to chondrocyte for collagen I. Glycosaminoglycan evaluations with both formulas revealed that chondrocytes were significantly higher to ADSCs and BMSCs, but none was significant to each other, p ≤ 0.0001. Combination of 10 ng TGF-β3 with 10 ng of BMP-6 enhanced chondrogenic potentials of BMSCs and ADSCs compare to TGF-β3 alone. This could be the ideal cocktail for either cell’s chondrogenic induction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The main approach of tissue engineering for musculoskeletal tissues treatment has focused on cell based therapy using multipotent stromal cells, also known as mesenchymal stem cells (MSCs). MSCs are non-haematopoietic progenitors located mainly within the stroma of bone marrow (Ruszymah 2006). Many studies have identified such cells in several other tissues such as skin, muscles, synovium and adipose tissue etc. (Wickham et al. 2003). They are phenotypically linked to the positive expression of several markers which include: CD73, CD90 and CD105; and negative expression of CD11b, CD14, CD19, CD34, CD45, CD79 and HLA-DR surface molecules (Nur Hidayah et al. 2012; Prado et al. 2011). As there is no specific marker for MSCs, other principal criteria for their identification include: adherence to the culture flask, the fibroblast-like morphology, prolonged capacity for proliferation in supportive medium and the ability to differentiate into cells of mesodermal origin (chondrocytes, adipocytes and osteocytes) (Prockop 1997; Prado et al. 2011). BMSCs isolated from the iliac crest have been reported to have multipotent characteristics (Alfaqeh et al. 2012). ADSCs, isolated from various part of the body including the infra patella fat pad have also been found to be multipotent (Ude et al. 2014). Both stem cells have been proposed to be suitable sources for chondrogenesis (Chua et al. 2005). It has also been revealed that MSCs from different tissue sources retain memory of their origin and have biological distinctions, hence MSCs derived from bone marrow have a higher affinity for osteogenic differentiation (Muraglia et al. 2000), while MSCs of synovial origin show greater tendency toward chondrogenic differentiation (Djouad et al. 2005; Prado et al. 2011). Though several experimental studies have documented the ability of mesenchymal stem cells to form articular-like cartilage, it has been difficult to standardize these formulas and translate these experimental findings into clinical practice. Various articles have suggested that these cells behave differently in different combination of growth factors and cytokines (Kim et al. 2009; Puetzer et al. 2010). In our previous work, it was reported that a chondrogenic induction cocktail comprising of TGFβ-3 favoured chondrogenesis of BMSCs more than ADSCs. There were low expressions of chondrogenic genes in ADSCs compare to BMSCs (Ude et al. 2014). Currently in effort to optimize a standard for both cells of interest, towards better chondrogenic inductions for a proposed clinical trial, we evaluated the effectiveness of using TGF-β3 alone and in a combination with BMP-6 for chondrogenesis. We hypothesize that ADSCs from infrapatellar fat pad will have greater tendency towards chondrogenic differentiations.
Materials and methods
Experimental design
Ethics approval was granted by the Universiti Kebangsaan Malaysia Animal Ethics Committee (PP/TEC/RUSZYMAH/25-NOV/342-DEC-2010-JUN-2012) and the Universiti Putra Malaysia Animal Ethics Committee (RUJ: ACUC 07R6/JULY 07-DEC 09) using the UK National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs) guidelines. A total of twelve healthy uncastrated male sheep (Siamese long tail cross), aged ~3 years and weighing ~30 kg were used. Experiments were grouped into ADSC, BMSC and Chondrocyte groups. Bone marrow and adipose tissue were harvested during the surgical operations, while the primary chondrocytes was supplied from our lab. After a recovery period of 3 weeks, animals were allowed free movement within raised slatted floor housing. Stocking density was 3 sheep in a 4 × 4 m2 pen confined environment. Isolated ADSCs and BMSCs were cultured until the third passage and 1 × 106 cells each were differentiated to chondrogenic lineage for 21 days. The differentiated ADSCs and BMSCs served as test samples, while chondrocytes served as positive controls for some parameters. Cell morphology, kinetics, histology, gene expressions and glycosaminoglycans were used for comparisons.
Harvest of adipose tissue
Adipose tissue was harvested according to a previous method with modifications (Ude et al. 2014). Briefly, the medial aspect of the vastus medialis and the joint capsule of the right knee were incised and the patella was luxated laterally to expose the knee fat pad. Approximately 10 g of adipose tissue was harvested and kept in PBS solution at +4 °C until further processing.
Harvest of bone marrow
Bone marrow was harvested according to a previous method (Al Faqeh et al. 2012). A Trocar (Cardinal Health Inc. USA) was inserted in a horizontal position at the most lateral part of the iliac crest to avoid iatrogenicity. With the aid of a 50 ml syringe (Cringe™ Malaysia) containing 1 mL of 103 IU heparin, 10 mL of bone marrow was aspirated from the right iliac crest and kept at +4 °C until further processing.
Isolation and culture of ADSCs and BMSCs
All samples were processed within 6–12 h after collection. Adipose tissue was minced to approximately 2 mm thick before digestion with an equal volume of 0.6% collagenase I (Gibco USA) in an orbital incubator (Stuart scientific UK) and filtered with 100 µm cell strainer (Orange Scientific). The filtrate was centrifuged (CR3i) at 2300 rcf for 5 min at 37 °C. Pellet was washed twice with PBS (Sigma USA) and once with Dulbecco’s Modified Eagles Medium/F12 (D-MEM/F12) +10% foetal bovine serum (basal medium) before culture. Bone marrow stem cells were isolated and processed using the Ficoll-Paque method (Ikebe et al. 2014). Following the isolation of ADSCs and BMSCs, they were cultured in 6 well plates (Corning Incorporated USA), in a GalaxyR CO2 incubator (RS Biotech). After the initial confluence at P0, they were trypsinized, and passaged to T-75 cm2 flask (Corning Incorporated USA) at a density of 5 × 105 cells per flask, while monitoring the growth kinetics.
Chondrogenic inductions
The chondrogenic media were prepared following two optimized formulas. The first, from our previous works (Alfaqeh et al. 2008, 2012; Ude et al. 2012, 2014) was prepared by the addition of TGF-β3 (Invitrogen Inc.) and other growth factors (Table 1). The second improved formula was prepared by the addition of Bone morphogenetic protein-6 (BMP-6) (Sigma USA) to the earlier cocktails (Table 2). On attaining the desired numbers, Samples were trypsinized and cultured in the prepared chondrogenic medium. Media were changed every 3–4 days and the induction period lasted for three weeks.
Cell morphology evaluation
The structural changes of the monolayer cells during induction were assessed using an inverted microscope (CK40; Olympus, Japan). The spindle, fibroblastic and mesenchymal-like morphology; the interaction of cells with the induction media and the aggregation of cells in forming cartilage matrixes were evaluated.
Quantitative RT-PCR assay
Chondrogenic gene profiling assay was done. Total RNA from the samples of primary chondrocytes; BMSCs and ADSCs before and after inductions to chondrocytes were extracted by dissolution in trizol reagent (Gibco BRL, USA). Complementary DNA was synthesized using the iscript™ (BIO-RAD) and analyzed for gene expression using the iQ™ SYBRR green super mix (BIO-RAD) on MyiQ™ single colour Real Time PCR detection system (BIO-RAD). Primers designed by (Biobasic Canada) were used to determine transcript levels in triplicate for a housekeeping gene GAPDH and twelve different genes of interest: These include early chondrogenic markers (cartilage oligomeric protein (COMP), sry (sex determining region y)-box 5 (SOX5), pro-alpha Col II and chondroadherin (CHAD)); genes for mature chondrocyte (agreccan (Agg), Col IX, SOX9, Col II alpha 1, Col XI and fibromodullin (FMOD)); and dedifferentiation markers, Col I and Col X. Data were analyzed by calculating the fold difference of the differentiated cells compared to undifferentiated cells after normalized to their own GAPDH value.
GAG analysis of induced cells
Cells were trypsinized and washed with 1X PBS to remove the culture medium; then homogenized and stored overnight at −20 °C. After two freeze–thaw cycles were performed to break the cell membranes, the homogenates were centrifuged for 5 min at 5000×g. Supernatants were removed immediately for assay or stored at −20 °C. The microtiter plate provided with the kit has been pre-coated with antibody specific to GAG. Standards or samples are then added to the appropriate microtiter plate wells with a biotin-conjugated polyclonal antibody preparation specific for GAG and Avidin conjugated to Horseradish peroxidase (HRP). This is then added to each microplate well and incubated. The standards, controls or samples were triplicated and average readings for each were subtracted from the average zero standard of the optical density. Colour change was measured spectrophotometrically at a wavelength of 450 nm ± 2 nm. The concentration of GAG in the samples is then determined by comparing the O.D. of the samples to the standard curve.
Statistical analysis
Data were presented as mean ± standard deviation (SD) of sample size. The total population doubling time and number of population doubling from (P0-P2) were analysed using paired t-tests. The gene analysis was done using one-way ANOVA with Bonferroni’s post hoc multiple comparisons of selected pairs. Uncertainties were presented within 95% confidence intervals and all statistical analysis was performed using the version 17.0 of the SPSS software and the graph pad prism5.
Results
Cell morphology
The isolated cells from native cartilage, the infra patella fat pad and iliac crest showed characteristics of mesenchymal stem cells, which is attachment to the culture flask and expansion under culture medium. Both cells had different morphological appearance in early cultures, but turned into spindle mesenchymal morphology from passage 2 (Fig. 1a).
Morphology and growth kinetics analysis of cells a Inverted phase contrast images of Chondrocytes, ADSCs and BMSCs in P2 showing similar spindle morphology of mesenchymal stem cells as they progressed in culture. Scale = 70 um. b Showed the graph of Population doubling time that were calculated from passage1 and 2; and the number of population doubling from passage1–2. ADSCs had significantly higher proliferation rate and number of cell doubling compare to Chondrocytes only. There was no significance difference with BMSC; depicting similar rate of replication, p ≤ 0.0001
Growth profile
After the initial P0 cultures, cells were seeded at low density of 5000 cell/cm2 in 75 cm2 culture flask. ADSCs reached confluence faster than BMSCs and Chondrocytes at P1 to P2. The population doubling time (PDT) for chondrocyte was 38.2 ± 6.4; ADSCs was 31.4 ± 4.9 h and BMSCs was 34.2 ± 0.7 h. ADSCs had higher score in population doubling time compare to the three cell types. On the number of replication per cell during culture duration, estimated as number of population doubling (NPD). Chondrocytes had 9.4 ± 1.4, ADSC had 13.6 ± 4.9 and BMSC had 10.6 ± 0.7 (Fig. 1b). ADSCs had significantly higher replication time and number compare to Chondrocytes only in both evaluations, depicting a similar rate of replication with BMSCs, p ≤ 0.05.
Multipotency evaluation and morphology during induction
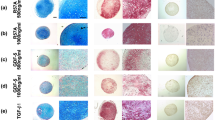
As one of the means of estimating stem cell characteristics, both cell cultures underwent adipogenic, osteogenic and chondrogenic differentiations. Under the same adipogenic culture condition, both cells revealed the abilities of adipogenesis. Lipid droplets were noticed within the cells in a cluster of shiny oily appearances. Oil red staining was used to confirm the lipid depositions (Fig. 2a). The same goes for osteogenic culture conditions, ADSCs and BMSCs showed cluster arrangements of cells, typical of calcium deposition in osteoblasts differentiation, with more on ADSCs. Alizarin Red stain was used to confirm the presence of calcium deposition and both cells were positive to the dye (Fig. 2b). On the chondrogenic evaluations, the initial and the present optimized formula were used for the evaluation. Both cells showed signs of cell aggregation and matrix deposition. With TGF-β3 alone, it was more on BMSC (Fig. 3a), but with TGF-β3 + BMP-6, it improved both ADSCs and BMSCs cultures (Fig. 3b). With the improved formula, from the 5th day, both cells showed signs of aggregation and matrix deposition. As the induction progressed to 10th day, aggregations turned into pockets of cell nodules with matrix sheets condensing from the 15th day. At day 20, the aggregation of cells turned into solid whitish mass. This is typical of early chondrogenesis process. Toluidine blue stained positive for the extracellular matrix and proteoglycans formation on both cells (Fig. 4).
Inverted phase contrast images of ADSCs and BMSCs during multipotency evaluation. a Adipogenic induction to prove multipotency. Lipid droplets were noticed within the cells in a cluster of shiny oily appearances (red arrows). Oil red staining (white arrows) were used to confirm the lipid depositions as seen in early adipogenesis. Scale = 70 μm. b During Osteogenic inductions. Both samples showed a mineralised cluster arrangements of cells (red arrow), typical of early calcium deposition seen in osteoblasts differentiation. Alizarin Red stain was used to confirm the presence of calcium deposition; Scale = 70 μm. (Color figure online)
Gross photographic and inverted phase contrast images of ADSCs and BMSCs during multipotency evaluation. a Using our initial chondrogenic formula (TGF-β3 alone), both cells showed signs of cell aggregation and matrix deposition. Toluidine blue staining proved the presence of proteoglycan in the extracellular matrix formation. Scale = 2 mm; 70 μm. b Using our optimised chondrogenic formula (TGF-β3 + BMP-6); both cells showed signs of cell aggregation and matrix deposition, with more enhancements on ADSCs. Toluidine blue stained positive for the extracellular matrix and proteoglycans formation on both cells. Scale = 2 mm; 35 μm
Gross photographic and inverted phase contrast images of BMSCs and ADSCs morphology during chondrogenic inductions using our present optimised formula (TGF-β3 + BMP-6). The trend was the same on both cell types, with the formation of aggregation (5th day), condensing sheet (10th day), multiple folds (15th day) and the strong whitish structure of cartilage by the 20th day. More enhancements were shown on ADSCs. 70 μm; 2 mm
Gene expression
Inductions with TGF-β3 alone revealed that BMSCs had higher gene expression for hyaline cartilage compare to ADSCs. Primary chondrocyte, which served as the positive control had higher gene expressions than both samples. For early chondrogenic markers: COMP, SOX5, pro-alpha Col II and CHAD; ADSCs, BMSCs and primary chondrocytes had (210 ± 56.57, 295 ± 109.60, 686.7 ± 49.50), (75.7 ± 19.09, 142 ± 60.81, 315 ± 67.88), (81.7 ± 24.75, 148.6 ± 35.35, 426.7 ± 63.64) and (88.3 ± 29.70, 291.3 ± 24.04, 526.6 ± 69.30) respectively. With one way ANOVA, the means were significantly difference; p ≤ 0.0001. The Bonferroni’s multiple comparison tests further revealed that primary chondrocytes were significantly higher to ADSCs and BMSCs for COMP, SOX5, pro-alpha Col II and CHAD; while BMSCs was significantly higher only to ADSCs, for CHAD (Fig. 5a). For the mature chondrocyte: Agg, Col IX, SOX9, Col II alpha 1, Col XI and FMOD; ADSCs, BMSCs and Primary chondrocytes had (216.67 ± 28.29, 382.33 ± 19.80, 551 ± 86.27), (143.33 ± 70.71, 373.33 ± 14.14, 486 ± 67.88), (136.67 ± 14.14, 207 ± 28.28, 474.33 ± 26.16), (213.33 ± 147.79, 220.67 ± 71.42, 620 ± 83.44), (113.33 ± 21.21, 139 ± 57.98, 253.33 ± 70.71) and (143.33 ± 14.14, 148 ± 35.35, 430.33 ± 21.92) respectively. With one way ANOVA, the means were significantly difference at p ≤ 0.0001. The Bonferroni’s multiple comparison tests showed that primary chondrocytes were significantly higher to ADSCs and BMSCs at Agg, SOX9, Col II and FMOD; and significantly higher to ADSC only at Col IX. BMSC was significantly higher to ADSC at Agg and Col IX, while none was significant at Col XI (Fig. 5b). For the negative and dedifferentiation markers: Col I and Col X; ADSCs, BMSCs and Primary chondrocytes had (553 ± 17.67, 582 ± 131.52, 313.67 ± 127.99 and 126.27 ± 53.03, 153.33 ± 35.35, 79.67 ± 28.28) respectively. One way ANOVA, showed the means were significantly difference at p ≤ 0.0004; while the Bonferroni’s multiple comparison tests, revealed no significant difference between the groups (Fig. 5c).
Graph of the quantitative RT-PCR analysis with TGF-β3 alone. Inductions revealed that BMSCs had higher gene expression for hyaline cartilage compare to ADSCs. Primary chondrocyte, as the positive control had higher gene expressions than both. a For early chondrogenic markers: COMP, SOX5, pro-alpha Col II and CHAD; the primary chondrocytes were significantly higher to ADSCs and BMSCs for COMP, SOX5, pro-alpha Col II and CHAD; while BMSCs was significantly higher only to ADSCs, for CHAD; p ≤ 0.0001.b For the mature chondrocyte: Agg, Col IX, SOX9, Col II alpha 1, Col XI and FMOD; chondrocytes were significantly higher to ADSCs and BMSCs at Agg, SOX9, Col II and FMOD; and significantly higher to ADSC only at Col IX. BMSC was significantly higher to ADSC at Agg and Col IX; p ≤ 0.0001. c The negative and dedifferentiation markers: Col I and Col X; there was no significant difference between the groups
With TGF-β3 + BMP-6, the analysis revealed increased hyaline cartilage gene expressions in both BMSCs and ADSCs. The expressions of the primary chondrocyte above, was also used as positive control to both samples. For early chondrogenic markers: COMP, SOX5, pro-alpha Col II and CHAD; ADSCs, BMSCs and Primary chondrocytes had 546.67 ± 56.57, 566.67 ± 98.10, 686.67 ± 49.50, 258.33 ± 14.14, 253.33 ± 14.14, 315 ± 67.88, 289.33 ± 50.91, 326.67 ± 35.36, 426.67 ± 63.64, 393.33 ± 56.57, 373.33 ± 35.36, 526.67 ± 69.30 respectively. With one way ANOVA, the means were significantly difference at p ≤ 0.0001. The Bonferroni’s multiple comparison tests showed that there was no difference among the groups (Fig. 6a). With the markers for mature chondrocyte: Agg, Col IX, SOX9, Col II alpha 1, Col XI and fibromodullin; ADSCs, BMSCs and Primary chondrocytes had (522 ± 63.64, 520 ± 63.64, 551 ± 86.27), (420 ± 70.71, 432 ± 141.42, 486 ± 67.88), (390 ± 141.42, 408.67 ± 72.13, 474.33 ± 26.16), (423 ± 71.42, 468.33 ± 83.44, 620 ± 24.04), (180 ± 84.85, 179.33 ± 55.15, 253.33 ± 70.71), (253.33 ± 56.57, 260 ± 42.43, 430.33 ± 21.92) respectively. With one way ANOVA, the means were significantly difference at p ≤ 0.0003. The Bonferroni’s multiple comparison tests showed that there was no difference among the groups (Fig. 6b). Then for the negative and dedifferentiation markers: Col I and Col X; ADSCs, BMSCs and Primary chondrocytes had (600 ± 141.42, 557.67 ± 70.71, 297.17 ± 127.99, 182.67 ± 56.57, 186.67 ± 14.14, 80 ± 28.28 respectively. With one way ANOVA, the means were significantly difference at p ≤ 0.0005. The Bonferroni’s multiple comparison tests showed that only ADSC was significantly higher to primary chondrocytes at Col I (Fig. 6c).
Graph of the quantitative RT-PCR analysis with TGF-β3 plus BMP-6. This revealed increased hyaline cartilage gene expressions on both BMSCs and ADSCs. The primary chondrocyte was also used as positive control to both samples. a For early chondrogenic markers: COMP, SOX5, pro-alpha Col II and CHAD; there was no difference among the groups. b With the markers for mature chondrocyte: Agg, Col IX, SOX9, Col II alpha 1, Col XI and fibromodullin; there was also no difference among the groups. c Then for the negative and dedifferentiation markers: Col I and Col X; the tests revealed that only ADSC was significantly higher to primary chondrocytes at Col I; p ≤ 0.0005
GAG analysis
Data from the chondrogenic inductions with TGF-β3 alone revealed that Chondrocytes, induced ADSCs and induced BMSCS had (20.24 ± 4.56, 7.93 ± 1.10, 10.83 ± 3.41) sGAG respectively. Induced BMSCs had higher glycosaminoglycan content compare to ADSCs; while the primary chondrocyte had higher sGAG content than both samples. With one way ANOVA, the means were significantly different at p ≤ 0.0001. The Bonferroni’s multiple comparison tests showed that primary chondrocytes were significantly higher to ADSCs and BMSCs; while there was no difference between the two test groups (Fig. 7a). From the inductions with TGF-β3 + BMP-6, Chondrocytes, induced ADSCs and induced BMSCs had (20.24 ± 4.56, 15.40 ± 2.08, 16.29 ± 1.55) sGAG respectively. BMSCs were slightly higher than ADSCs in quantity of GAG; while the primary chondrocyte retained higher sGAG content than both. One way ANOVA revealed that the means were significantly difference at p ≤ 0.0001. The Bonferroni’s multiple comparison tests showed that primary chondrocytes were significantly higher to ADSCs and BMSCs, while there was no difference between the two test groups (Fig. 7b).
Graph of the sGAG quantification of cell sample after induction with the two media. a With TGF-β3 alone, it revealed that Induced BMSCs had higher glycosaminoglycan content compare to ADSCs; while the primary chondrocyte had higher content than both samples. Primary chondrocytes were significantly higher to ADSCs and BMSCs; while there was no difference between the two test groups; p ≤ 0.0001. b From the inductions with TGF-β3 + BMP-6, BMSCs were slightly higher than ADSCs in quantity of GAG; while the primary chondrocyte retained higher sGAG compare to both. Primary chondrocytes were significantly higher to ADSCs and BMSCs, while there was no difference between the two test groups; p ≤ 0.0001
Discussion
It was reported that no standard method has been delineated for the induction of mesenchymal stem cells to articular cartilage (Saw et al. 2010). The abundance, undifferentiated native cells in various tissues has long been emphasized as the major tools for tissue engineering and regenerative medicine (Ruszymah et al. 2007). This study compared the chondrogenic differentiation capacity of ADSCs from infra patella fat pad and BMSCs form iliac crest under the same conditions in 2D culture flask. Ideally, more standard method of inducing chondrogenesis should not only include the chondrogenic differentiation media, but also pellet/matrix composition, oxygen tension, and appropriate mechanical stimulus. One of the major determining factors among these seems to be the optimal combination of growth factors, cytokines and chemicals. Soluble inductive growth factors known to enhance cells to chondrogenesis was our focus, being that slight changes in concentrations can alter the end product of an induction process. It is known that some different tissues have common embryological origin; thus some growth factors and cytokines can play a part in multiple differentiation process especially those sharing similar signalling pathway (Puetzer et al. 2010). Knowing the appropriate combinations to get to the desired goal is pertinent. The report that adipose tissue had more cells than bone marrow was in agreement with earlier studies which stated that they attracted interests due to ease of isolation and relative abundance of cells as compared to BMSCs (Aust et al. 2004; Puetzer et al. 2010; Ude et al. 2014). It has also been reported that ADSCs achieved higher passage number compare to BMSCs before senescence (Izadpanah et al. 2006; Rider et al. 2008). In the previous work, our chondrogenic medium comprised of TGFβ-3 with other chemicals, which enhanced the chondrogenic induction of BMSCs (Alfaqeh et al. 2008, 2012; Ude et al. 2012). With the plethora of ADSCs usage in tissue engineering and regenerative medicine; we saw the need to explore the effective media to induce both BMSCs and ADSCs to chondrogenesis for future treatment of osteoarthritis. With TGFβ-3 alone, BMSCs had higher expressions on all the twelve genes investigated compare to ADSCs. Collagen II which is the most abundant hyaline cartilage oligomeric matrix protein and aggrecan, the predominant proteoglycan (Mehlhorn et al. 2007) were highly expressed. This was reflected on the nature of cartilages formed and the regeneration on our earlier treated sheep (Ude et al. 2014). It was revealed that ADSCs have reduced or absent transforming growth factor β (TGF-β) receptor ALK-5 (Hennig et al. 2007), and cell surface marker vascular cell adhesion molecule 1 (CD106) (Brian et al. 2010) thus, under chondrogenic differentiations, typically utilizing TGF-β and dexamethasone; BMSCs will have enhanced potential for compare to ADSCs (Rider et al. 2008, Adila et al. 2012). However, other evidences suggested that ADSCs can be induced towards chondrogenic lineage more effectively by a high dose of BMP-6 than by TGF-β or other cocktails (Brian et al. 2010). Hennig et al. demonstrated that addition of BMP-6 to a TGF-β culture resulted in robust chondrogenesis of ADSCs similar to BMSCs with TGF-β (Hennig et al. 2007). Another report suggested that higher concentrations, up to fivefold of TGF-β and IGF for ADSCs is required to overcome the chondrogenic differences with BMSCs (Im et al. 2005; Kim et al. 2009; Sha’ban et al. 2012). With these reports in view, we optimised the present chondrogenic formula with the addition of BMP-6; keeping in mind a concentration that could be optimal to both cells of interest. From the present results, chondrogenesis of ADSCs were greatly enhanced by the addition of BMP-6. In addition, BMSCs were also enhanced to better expression compare to TGF-β3 alone. ADSCs had better expression of SOX5, CHAD, Agg, and Col XI. Even with these enhancements, both cells still fall short of the expressions recorded for the primary chondrocytes with either of the media. We attributed this to the superiority of the native over the engineered cells/tissues.
In an effort to see if the differentiation was towards hyaline cartilage, we tested for the expression of Col I and Col X. On the contrary collagen I, which is considered a negative marker for hyaline cartilage and collagen X, which is a hypertrophic cartilage marker were also highly expressed on both cells more than primary chondrocyte; with higher expression on BMSCs with TGF-β3 alone and Col X on ADSCs with TGF-β3 + BMP-6. This was considered a limitation to this study owing that mesenchymal stem cell induced better to hyaline cartilage in a 3D model or pellet system (Ruszymah et al. 2005, 2007). Reports have also shown that induced cells regained their hyaline phenotype when implanted in vivo environment (Munirah et al. 2005).
The results of the sGAG for both cells reflected similar trend with the gene expressions. GAG depositions were more on BMSC with both medium. Though there was an increase in both cells sGAG quantification with TGF-β3 + BMP-6, but they were still lower than the primary chondrocytes which is superior as native.
Conclusion
It was found that induction media containing TGF-β3 alone and TGF-β3 with BMP-6 have chondrogenic potentials. TGF-β3 alone favoured the chondrogenesis of BMSC, while the combination of BMP-6 increased chondrogenic gene expressions on both cells, with greater enhancement on ADSCs. A combination of 10 ng TGF-β3 with 10 ng of BMP-6, could be the ideal cocktail for either cell’s chondrogenesis.
References
Adila AH, Ruszymah BHI, Aminuddin BS, Somasumdaram S, Chua KH (2012) Characterization of human adipose-derived stem cells and expression of chondrogenic genes during induction of cartilage differentiation. Clinics 67(2):99–106
Al Faqeh H, NorHamdan BMY, Chen HC, Aminuddin BS, Ruszymah BHI (2012) The potential of intra-articular injection of chondrogenic-induced bone marrow stem cells to retard the progression of osteoarthritis in a sheep model. Exp Gerontol 47(2012):458–464
Alfaqeh H, Chua KH, Aminuddin BS, Ruszymah BHI (2008) Effects of different media on the in vivo chondrogenesis of sheep bone marrow stem cells; histological assessment. MJM 63(A):119–120
Aust L, Devlin B, Foster SJ, Halvorsen YD, Hicok K, du Laney T, Sen A (2004) Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy 6(1):7–14
Brian OD, Christopher RR, Donald PL, Arnold IC, Guilak F (2010) Chondrogenesis of adult stem cells from adipose tissue and bone marrow: induction by growth factors and cartilage-derived matrix. Tissue Eng Part A 16(2):523–533
Chua KH, Aminuddin BS, Fuzina NH, Ruszymah BHI (2005) Insulin-transferrin-selenium prevent human chondrocyte dedifferentiation and promote the formation of high quality tissue engineered human hyaline cartilage. Euro Cells Mater 9:58–67
Djouad F, Bony C, Häupl T, Uzé G, Lahlou N, Louis-Plence P, Apparailly F, Canovas F, Réme T, Sany J, Jorgensen C, Noël D (2005) Transcriptional profiles discriminate bone marrow-derived and synovium-derived mesenchymal cells. Arthritis Res Ther 7:1304–1315
Hennig T, Lorenz H, Thiel A, Goetzke K, Dickhut A, Geiger F (2007) Reduced chondrogenic potential of adipose tissue derived stromal cells correlates with an altered TGF beta receptor and BMP profile and is overcome by BMP-6. J Cell Physiol 211(3):682–691
Ikebe C, Suzuki K (2014) Mesenchymal stem cells for regenerative therapy: optimization of cell preparation protocols. BioMed Res Int ID 951512: 11
Im GI, Shin YW, Lee KB (2005) Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells? Osteoarthr Cartil OARS Osteoarthr Res Soc 13(10):845–853
Izadpanah R, Trygg C, Patel B, Kriedt C, Dufour J, Gimble JM, Bunnell BA (2006) Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem 99(5):1285–1297
Kim HJ, Im GI (2009) Chondrogenic differentiation of adipose tissue-derived mesenchymal stem cells: greater doses of growth factor are necessary. J Orthop Res 27(5):612–619
Mehlhorn AT, Niemeyer P, Kaschte K, Muller L, Finkenzeller G, Hartl D, Sudkamp NP, Schmal H (2007) Differential effects of BMP-2 and TGF-beta 1 on chondrogenic differentiation of adipose derived stem cells. Cell Prolif 40(6):809–823
Munirah S, Aminuddin BS, Samsudin OC, Chua KH, Fuzina NH (2005) The re-expression of Collagen II, Aggrecan and SOX9 in tissue engineered human articular cartilage. Tissue Eng Regen Med 2(2005):347–355
Muraglia A, Cancedda R, Quarto R (2000) Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci 13:1161–1166
Nur Hidayah H, Ahmad Fadzli S, Ng MH, Ohnmar H, Ruszymah BHI, Naicher AS, Shalimar A (2012) Neural-differentiated mesenchymal stem cells incoporated into muscle stuffed vein scaffold forms a stable living nerve conduit. J Orthop Res 30(10):1674–1681
Prado SMD, Isaac, FB, Francisco, JB (2011) Cell therapy and tissular engeenering to regenerate articular cartilage; biomedical engineering, trends. Res Technol9: 194–216. Dr. Sylwia Olsztynska (Ed.), ISBN: 978-953-307-514- 3, InTechProckop, 1997
Puetzer JL, Petitte JN, Loboa EG (2010) Comparative review of growth factors for induction of three-dimensional in vitro chondrogenesis in human mesenchymal stem cells isolated from bone marrow and adipose tissue. Tissue Eng Part B Rev 16(4):435–444
Rider DA, Dombrowski C, Sawyer AA, Ng GH, Leong D, Hutmacher DW (2008) Autocrine fibroblast growth factor 2 increases the multipotentiality of human adipose-derived mesenchymal stem cells. Stem Cells (Dayton) 26(6):1598–1608
Ruszymah BHI (2006) Tissue engineering initiatives in Malaysia. Tissue Eng Regen Med 3(1):34–38
Ruszymah BHI, Chua KH, Mazlyzam AL, Fuzina NH, Aminuddin BS (2005) Formation of in vivo tissue engineered human hyaline cartilage in the shape of a trachea with internal support. Int J Pediatr Otolaryngol 69(11):1489–1495
Ruszymah BHI, Chua KH, Farah Wahida I, Fuzina H, Aminuddin BS (2007) The expansion potential of human nasal septum chondrocytes for the formation of engineered cartilage. ScienceAsia 33(2):141–149
Saw KY, Adam A, Shahrin M, Tay YG, Kunaseegaran R, Caroline SYJ (2010) Articular cartilage regeneration with autologous peripheral blood progenitor cells and hyaluronic acid after arthroscopic subchondral drilling: a report of 5 cases with histology. J Arthro 27(4):493–506
Sha’ban M, Samsudin OC, NorHamdan MY, Aminuddin BS, Ruszymah BHI (2012) Sox-9 transient transfection enhances chondrogenic osteoarthritic in vitro: preliminary analysis. Tissue Eng Regen Med 8(1):32–41
Ude CC, Shamsul BS, Ng MH, Chen HC, Aminuddin BS (2012) Bone marrow and adipose stem cells can be tracked with PKH26 until post staining passage 6 invitro and invivo. Tissue Cell 44(2012):156–163
Ude CC, Sulaiman SB, Min-Hwei N et al (2014) Cartilage regeneration by chondrogenic induced adult stem cells in osteoarthritic sheep model. PloS One 9(6):9
Wickham MQ, Erickson GR, Gimble JM, Vail TP, Guilak F (2003) Multipotent stromal cells derived from the infrapatellar fat pad of the knee. Clin Orthop Relat Res 412:196–212
Acknowledgements
The authors wish to acknowledge the sponsorship by the Research University Grant/UKM-AP2011-26, DPP-2013-084 from the Ministry of Higher Education of Malaysia and DIP-2012-08.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors wish to declare that there was no conflict of interest during the study.
Rights and permissions
About this article
Cite this article
Ude, C.C., Chen, H.C., Norhamdan, M.Y. et al. The evaluation of cartilage differentiations using transforming growth factor beta3 alone and with combination of bone morphogenetic protein-6 on adult stem cells. Cell Tissue Bank 18, 355–367 (2017). https://doi.org/10.1007/s10561-017-9638-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-017-9638-1