Abstract
Lung fibrosis is a dysregulated repair process caused by excessive deposition of extracellular matrix that can severely affect respiratory function. Macrophages are a group of immune cells that have multiple functions and can perform a variety of roles. Lung fibrosis develops with the involvement of pro-inflammatory and pro-fibrotic factors secreted by macrophages. The balance between M1 and M2 macrophages has been proposed to play a role in determining the trend and severity of lung fibrosis. New avenues and concepts for preventing and treating lung fibrosis have emerged in recent years through research on mitochondria, Gab proteins, and exosomes. The main topic of this essay is the impact that mitochondria, Gab proteins, and exosomes have on macrophage polarization. In addition, the potential of these factors as targets to enhance lung fibrosis is also explored. We have also collated the functions and mechanisms of signaling pathways associated with the regulation of macrophage polarization such as Notch, TGF-β/Smad, JAK-STAT and cGAS-STING. The goal of this article is to explain the potential benefits of focusing on macrophage polarization as a way to relieve lung fibrosis. We aspire to provide valuable insights that could lead to enhancements in the treatment of this condition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
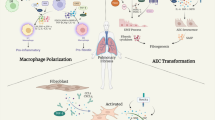
Lung fibrosis is a chronic progressive mesenchymal lung disease caused by a variety of irritants. The primary abnormalities observed are the accumulation of an excessive amount of Extracellular Matrix (ECM) and damage to the lung parenchyma. Playing a critical role in the immune response, macrophages are integral components of innate immunity, tissue repair and remodeling by engulfing exogenous substances, removing apoptotic cells and necrotic tissue, and secreting cytokines. Macrophages are heterogeneous, differing significantly in origin, morphology, structure and function [1]. The lungs contain macrophages that can be classified based on where they distribute. Alveolar Macrophages (AMs) are situated within the alveoli, while Interstitial Macrophages (IMs) are nestled within the parenchymal tissue of the lungs [2]. In the steady state, both can be replaced by self-renewal of bone marrow monocyte-derived macrophages [3]. The involvement of resident alveolar, interstitial and monocyte-derived macrophages in the development of lung fibrosis is currently being extensively studied. It is becoming increasingly clear that the way macrophages are polarized - which is regulated by various factors such as cytokines, chemokines, and transcription factors - is closely linked with the development and advancement of numerous inflammatory lung diseases. These diseases include Acute Lung Injury (ALI), Acute Respiratory Distress Syndrome (ARDS), COVID-19 (which can cause acute respiratory distress syndrome), allergic asthma, Chronic Obstructive Pulmonary Disease (COPD), and Idiopathic Lung fibrosis (IPF) [4,5,6,7,8]. IPF is a long-term respiratory condition that has a high fatality rate in cases of lung fibrosis. The disease is marked by the gradual development of scar tissue within the lungs, which results in fibrosis and difficulty breathing [9]. The specific mechanisms by which macrophages are involved in pulmonary fibrosis have not been fully elucidated, but extensive research now suggests that increased expression of monocytes and monocyte-derived macrophages detected in samples from patients with IPF leads to the development of IPF [10, 11]. The two drugs that have been approved as treatments for IPF are Nintedanib and pirfenidone, most patients on long-term use of these two drugs have serious adverse effects and significant phase symptoms. Lung fibrosis is a late stage of ALI/ARDS and COVID-19-induced lung fibrosis is also thought to be a sequel to ARDS [12], caused by fibroblast proliferation and excessive collagen deposition [13]. It has been shown that macrophage type M2 can manipulate fibroblast differentiation and proliferation into myofibroblasts and promote ECM deposition [14], so improving the progression of fibrosis in ALI/ARDS is anticipated through the control of macrophage polarization. Current studies of pulmonary IMs suggest that they may be of mixed origin, arising from both yolk sacs and monocytes in the bone marrow., with the ability to phagocytose and clear foreign bodies entering the interstitial lung [15]. Numerous research studies have indicated a noteworthy rise in IMs detected in a fibrosis model induced by Bleomycin (BLM), indicating that IMs might have a critical function in the development of lung fibrosis [16]. There are two subpopulations of interstitial macrophages: Lyve1hi MHCIIlo interstitial macrophages are located near blood vessels, while Lyve1lo MHCIIhi interstitial macrophages are seen in the alveolar interstitium. Chakarov et al. showed that depletion of Lyve1+++hi MHCIIlo IMs increases leukocyte infiltration and worsens fibrosis [17]. The number of Lyve1lo MHCIIhi IMs increases as lung fibrosis progresses; however, the specific role of IMs in lung fibrosis has not been elucidated. Further investigation is required to understand the role of IMs in the development of lung fibrosis, as there are few surface markers of IMs in lungs that are in their steady-state or are experiencing fibrosis, and there is a lack of specific intervention systems targeted towards IMs.
Macrophages have Potential Targets in Lung Fibrosis
Macrophage Polarization
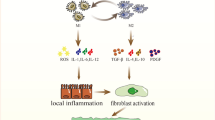
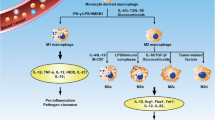
The type of macrophage activation is influenced by various local environmental factors. These polarization states mainly include the classical activation phenotype (M1) as well as the alternative activation phenotype (M2) [18]. During the early inflammatory phase, M1-type macrophages play a dominant role, whereas the proportion of M2-type macrophages increases during the pulmonary fibrosis phase [19]. Multiple studies have demonstrated that fibrotic remodeling in various internal organs, such as the heart, kidney, liver, gastrointestinal tract, and lungs, is also linked with polarized macrophage M2 [20, 21]. M1 is induced by Lipopolysaccharide (LPS), Interferon (IFN)-γ and Tumor Necrosis Factor (TNF), and secretes various cytokines such as Interleukin 6 (IL-6), IL-1β, IL-12, IL-23, CCL2, also known as monocytes chemokin 1 (MCP1) and Tumor Necrosis Factor α (TNF-α) to promote inflammatory responses [22, 23]. M1 cells exhibit elevated expression of Cluster of Differentiation 80 (CD80), Toll-like receptor 4(TLR4), Major Compatibility Complex II(MHCII), and Cluster of Differentiation 86 (CD86) [24]. LPS from Gram-negative bacteria is one of the most potent stimulators of innate immune activation known, and it induces M1 macrophage polarization [25]. Studies have shown that COPD patients have increased levels of the protein Inducible Nitric Oxide Synthase (iNOS) in their AMs. These elevated levels of iNOS can lead to the production of Reactive Oxygen Species (ROS) and Nitric Oxide (NO). Furthermore, the cytokines produced by M1 macrophages may also contribute to the development of COPD [26]. IL-4 and IL-13, IL-10, glucocorticoids are the primary drivers behind the production of M2 macrophages [27]. These macrophages can be classified into four categories, namely M2a, M2b, M2c, and M2d [28]. There is a lack of distinct surface markers that can differentiate M2 macrophages’ various subpopulations. Currently, M2 polarization is marked by heightened expression of various surface proteins, such as Cluster of Differentiation 206 (CD206) (aka the mannose receptor) [29], a protein ‘found in the inflammatory zone’ (Fizz1) [30], Arginase-1, and the family protein chitinase enzyme-like Ym1/2. In contrast, low levels of MHCII, CD86, and iNOS2 are characteristic of this polarization zone. A certain research revealed that the absence of Fizz1 hindered the development of lung fibrosis induced by BLM by suppressing the activation of pulmonary fibroblasts and the attraction of monocytes to the lungs [31]. During the induction of lung fibrosis by BLM, Li et al. demonstrated that the polarization of M2 macrophages was synergistically induced by IL-33 and IL-13. Furthermore, treating with anti-IL-33 antibodies reduced both lung inflammation and fibrosis induced by BLM [32]. Moreover, these macrophages that display polarization have the ability to change, as they can transform into M0 macrophages by depolarization or adopt the opposite phenotype (M1 to M2) through repolarization and vice versa, demonstrating their plasticity. The presence of different types of cytokines in a specific microenvironment determines the outcome [33].
The pathogenesis of IPF is complex and involves numerous network systems and signaling molecules involved in its regulation. In recent years there has been a shift in the focus of research on the pathology of IPF, which was previously defined as an inflammatory disease and is now considered to be epithelial-driven. Epithelial-mesenchymal transition (EMT) is increasingly recognised as a very important mechanism in the formation of interstitial fibrosis in the lung. EMT is a process by which epithelial cells with polarity are transformed into fibroblasts or myofibroblasts of mesenchymal phenotype in response to various factors and is involved in the formation of fibrosis and the irreversible development of IPF after interstitial lung injury [34]. Abnormal differentiation of M2-type macrophages is a direct factor in causing abnormal repair of lung tissue and even pulmonary fibrosis. Macrophages in patients with pulmonary fibrosis secrete increased M2 macrophage markers, which correlate negatively with lung function [35]. Suarez et al. found that M2 macrophage activation and secretion of cytokines prompted an epithelial-mesenchymal-like phenotypic transition in tumor cells [36]. Although the functions and mechanisms by which M1 and M2 macrophages exacerbate or attenuate pulmonary fibrosis are not fully understood, targeting M2-associated cytokines to intervene in the progression of pulmonary fibrosis by reducing M2 transformation is a key step. Therefore, further studies are necessary to clarify the specific roles of macrophage polarization and develop effective treatments for lung fibrosis.
Mitochondria
As one of the important sites of energy metabolism in the body, mitochondria have been found to be closely linked to the polarization process of macrophages [37]. In recent years, it has been found that macrophages change from resting state to M1 type mainly through the glycolytic pathway to obtain energy to support anabolism, and this process generates large amounts of NO and ROS, and ROS can promote the inflammatory response of macrophages by activating factors such as Nuclear factor-kappa B (NF-κB) [38]. According to the study conducted by He and colleagues, lung fibrosis triggers the activation of Cu, Zn peroxide dismutase (also known as Cu, Zn-SOD) by macrophages. This leads to the production of H2O2, which in turn, stimulates the transcription factors Signal Transducer and Activator of Transcription (STAT6). This entire process drives the polarization of macrophages, resulting in the formation of the M2 phenotype [39]. In case there is a malfunction in the mitochondria, the body undergoes oxidative stress, leading to the production of excessive amounts of ROS and a decrease in antioxidants like catalase, superoxide dismutase and glutathione. This, in turn, damages the ECM, resulting in the creation of extracellular tension, the initiation of cytokine and growth factor activation, thereby leading to the onset of inflammation [40]. The development of lung fibrosis is closely tied to both mitochondrial oxidative stress and mitochondrial turnover within alveolar macrophages. Despite this link, the specific molecular processes that control mitochondrial dynamics have not yet been fully elucidated [41]. Gu et al. demonstrated mitochondrial Ca2+ influx mediated by Mitochondrial Calcium Mono transporters (MCUs), as well as the production of ROS and ATP, regulating macrophage replacement activation for a profibrotic phenotype [42]. The implication here is that the polarization of macrophage M2 that contributes to the pathogenesis of lung fibrosis is controlled by mitochondrial stress, and that targeting MCU could be a potential therapy for the treatment of lung fibrosis. Although the specific mechanism by which mitochondria act on macrophages and cause lung fibrosis is less well understood. However, pathological processes such as mitochondrial dysfunction and excessive production of mitochondrial Reactive Oxygen Species(mtROS), may play a role in the pathological process of lung fibrosis. Targeting mitochondria may help improve antifibrotic therapy.
Gab Protein
Gab family proteins are an important class of anchor proteins in cells, and mammalian Gab protein families mainly include Gabl, Gab2, Gab3. Multiple signaling pathways involving Gab protein are closely linked to lung diseases, and Gab1 and Gab2 (Gab1/2) are widely expressed in macrophages [43], which can regulate lung fibrosis. GAB1 has been reported to be involved in Epidermal Growth Factor (EGF)-mediated profibrotic MAPK/ERK signal in [44]. Guo et al. detected elevated Ga1/2 levels in alveolar macrophages after BLM-induced lung fibrosis, showing typical CD206 positivity and significant increases in M2 markers (Arg1, FIZZ1, and Ym1), indicating that alveolar macrophages are transitioning to M2 type during this process [45]. In subsequent experiments, they also found that Gabl promotes the activation of the P13K/AKT signaling pathway to regulate M2 polarization, and Gab2 promotes M2 polarization by activating the JAKl/STAT6 signaling pathway. In addition, knockdown GAB1 has been found to effectively inhibit TNF-α-induced inflammatory gene expression and alleviate fibrosis [46]. Therefore, although the study is not clear how the above biomolecules are upregulated during the disease. However, Gab1, Gab2, miR-21, miR-155 and miR-142-3p may be new targeted therapeutic sites for pulmonary fibrosis, capable of inhibiting M2-type polarization.
Exosomes
Exosomes are vesicles secreted by cells to the outside of the cell through exocytosis, and are mediators that can participate in important processes such as intercellular communication and immune regulation [47]. Research has indicated that exosomes contain different components, including proteins and microRNA (miRNA), which may have an impact on the functional state of the cells that receive them. Of all the types of RNA found in exosomes, miRNA appears to make up the largest share [48]. miRNAs are a class of small endogenous non-coding RNAs. They typically contain between 18 and 24 nucleotides in their sequence. miRNA may mediate paracrine and endocrine communication between different tissues, thereby regulating gene expression and remotely regulating cellular function [49]. Studies have demonstrated that miRNAs play a crucial role in regulating transcription and contributing to the inflammatory response within the lungs. Additionally, they are involved in the process of macrophage polarization [50, 51]. In their study on Radiation-Induced Lung Fibrosis (RILF), Duru et al. examined the regulation of M2-dominant macrophage polarization by miRNAs. They identified miR-21 and miR-155 as pro-fibrotic while let-7i, miR-107, miR-126, miR-140, and miR-511 were characterized as anti-fibrotic [52]. When Mesenchymal Stem Cells (MSCs) are exposed to low oxygen levels, their extracellular vesicles exhibit raised levels of miR-21-5p. This microRNA has been found to encourage the progression of lung cancer by hindering apoptosis while simultaneously facilitating M2 polarization in macrophages [53]. The application of MSC exosomes or miR-21-5p activators results in a decrease in both pulmonary edema and dysfunction. It also decreases the M1 polarization of alveolar macrophages, as well as the secretion of various cytokines including IL-8, IL-1, IL-6, IL-17, and TNF-α [54]. Studies have revealed that miR-155 can be activated in response to inflammation in macrophages, exerting a pro-inflammatory influence. Additionally, research has shown that miR-155 promotes fibrosis mediated by cross-signaling between macrophages and fibroblasts. This signaling leads to the stimulation of collagen synthesis, controlled by the TGF1 pathway [55]. Guiot et al. used macrophage-derived miR-142-3p-rich exosomes to inhibit TGF-βR1, resulting in antifibrotic properties [56]. The findings above indicate that miR-21-5p could possibly control changes related to apoptosis and anti-apoptosis in lung disorders, implying that miR-21, miR-155 and miR-142-3p could serve as potential targets for treating lung fibrosis in the future.
Advances in Macrophage-related Signaling Pathways and Lung Fibrosis
Notch Signaling Pathway
The Notch signaling pathway has often evolved to be highly conserved. They are independent signaling modes that rely on protein shear to regulate intercellular interactions. The regulation of cell proliferation and apoptosis, as well as maintaining the internal environment’s homeostasis, are important functions that the Notch signaling pathway assumes during the body’s development process [57]. The Notch signaling pathway is mainly composed of Notch receptors, ligands and intracellular effector molecules. The Notch receptor is a type I transmembrane protein encoded by four different genes (Notch1, 2, 3, 4), all consist of extracellular subunits, transmembrane subunits and intracellular subunits, with both the extracellular and intracellular regions being highly conserved. When the signal is not activated, the Notch receptor is located on the cell membrane. DSL family proteins, also referred to as Notch ligands, comprise of a group of five ligands which include Jagged1 (JAG1), JAG2, delta-like1 (DLL1), DLL3 and DLL4. Studies have indicated that the Notch signaling pathway plays a role in controlling the activation and functioning of macrophages [58], when activated, the Notch signaling pathway regulates monocyte macrophages, polarizing them towards pro-inflammatory M1-type macrophage [59]. Some clinical data have found Notch to be highly expressed in lung specimens from patients with IPF and COPD [60]. And elevated Notch expression was found in an animal model of bleomycin-induced pulmonary fibrosis [61]. The current study found that the Notch signaling pathway promotes inflammatory responses mainly through synergy with the NF-κB signaling pathway [62]. In their study, Shin and colleagues demonstrated that Notch is involved in preserving the activity of NF-κB through direct interaction with p50 / c-Rel within the nucleus. In order to investigate this further, researchers used a gamma-secretase inhibitor (GSI) to halt the production and transfer of active Notch1 into the nucleus. This resulted in a decrease of NF-κB activity, and lowered the amount of nuclear input and sustained nuclear activity of NF-κB, ultimately leading to decreased activation of downstream molecules and a reduced ability of NF-κB to inhibit macrophage activation [63]. Palaga et al. treated the Notch receptor with GSI, this treatment significantly modulated the expression of TNF-α, IL-6 and IL-10, with a significant reduction in nitric oxide production and a decrease in NF-κB p50 translocation into the nucleus. The implication here is that the activation of the Notch signaling pathway is caused by the stimulation of macrophages through the TLR signaling cascade. This, in turn, regulates the expression of genes involved in the pro-inflammatory response through the activation of NF-κB [64]. Notch signaling can also synergistically regulate macrophage activation, collaborating with multiple inflammatory signaling pathways such as MAPK, Akt and TGF-β/Smad, and indirectly or directly enhances the expression of factors such as TNF-α and TGF-β to regulate EMT [65]. For example, it has been shown that Notch factor activates TGF-β in rat thylakoid cells at high blood glucose concentrations thereby regulating EMT [66]. In addition, Notch can induce the expression of TGF-β family members and the phosphorylation of Smad3, an important mediator of TGFβ signaling. Vera et al. treated the lungs with bleomycin, they found higher volumes of healthy lung tissue and improved lung function in the absence of Notch3 [67]. The research conducted by Wasnick and colleagues provided evidence that Notch1 signaling has a pivotal role in regulating the transformation of type II alveolar epithelial cells (AEC2) in cases of IPF. Their findings revealed that this signaling pathway stimulates the proliferation of alveolar epithelial cells and plays a significant role in the development of fibrous proliferation. However, In IPF-derived precision-cut lung sections, when the Notch signal is suppressed, was able to improve the surfactant treatment of AEC2 and reversed the fibrosis [68]. Notch-mediated macrophage recruitment and activation may be a common regulatory mechanism for fibril formation in a variety of organs, as has been demonstrated in studies of renal fibrosis [69], liver fibrosis [70] and myocardial fibrosis [71]. Therefore, blocking the Notch signaling pathway of macrophage polarization inhibits the inflammatory response and regulates lung tissue injury, which may have the potential to improve lung fibrosis and promote lung function recovery Fig. 1.
TGF-β/Smad Signaling Pathway
The TGF-/Smad pathway is essential for various physiological processes such as inflammation, wound healing and fibrosis. It is also an important trigger for ECM deposition and EMT processes in tissue fibrosis [72]. TGF-β includes TGF-β1, TGF-β2 and TGF-β3 [73], and the process of developing lung fibrosis activates these three growth factors. Smad protein is an important downstream signaling molecule within the TGF-β pathway, including Smad2, Smad3, Smad4 and Smad7. In recent years, it has been found that TGF-β1/Smad regulates lung fibrosis [74]. TGF-1 plays a crucial role in the progression of inflammation and pro-fibrosis. It stimulates the excretion and liberation of IL-1, TNF-α and various other cytokines by the inflammatory cells, contributing to multi-dimensional inflammatory responses. Under pathological conditions of fibrosis, both Smad2 and Smad3 expression is upregulated, while Smad7 is downregulated [75]. One study found that in a transgenic mouse model overexpressing TGF-β1, lung fibrosis induced by bleomycin can be inhibited by limiting the accumulation of alveolar macrophages [76]. After exposing mice to x-ray radiation, Ying et al. administered pirfenidone (PFD) and discovered that it effectively eliminated TGF-β1 in M2 macrophages by inhibiting the activation of TGF-β1/Smad3. Consequently, treatment with PFD demonstrated a significant improvement in collagen deposition and reduced the onset and severity of RILF [77]. According to these studies, the process of cell polarization is closely regulated by the TGF-1/Smad signaling pathway. The extent to which the TGF-β/Smad signaling pathway is involved in mediating macrophage polarization during the development of lung fibrosis is not known, but targeting the TGF-β signaling pathway may provide a new way of thinking in the fight against lung fibrosis.
The Notch pathway is responsible for the development of lung fibrosis by affecting the polarization of macrophages, both directly and indirectly. The pathway transmits signals through binding of receptors and ligands on adjacent cells, resulting in the release of NICD, which activates NF-κB in the nucleus. These substances regulate the polarization of macrophages, driving them towards either M1 or M2 types. As a result, fibroblast proliferation and differentiation are stimulated, and extracellular matrix components that are involved in the fibrotic process in the lungs are produced
JAK-STAT Signaling Pathway
JAKs are a group of protein kinases that are found within cells. The family of JAKs includes JAK1, JAK2, JAK3, and TYK2, which have all been identified so far [78]. STAT is a target protein downstream of JAKs and is distributed in the cytoplasm. There are seven members in the STATs family, which include STAT1, STAT2, STAT3, STAT4, STAT5a/b, and STAT6 [79]. When cytokines like IL4 and IL13 with anti-inflammatory characteristics or cytokines like IL6 with pro-inflammatory characteristics attach to receptors on the surface of cell membranes, it leads to the coming together of receptor molecules in pairs and advances the combination of JAKs. This results in the activation of STATs by JAK, which are then transferred from the cytoplasm to the nucleus in order to control gene expression [80]. The initiation of macrophage inflammation regulation may be significantly influenced by the JAK-STAT signaling pathway. Studies indicate that this pathway triggers signaling and transcriptional activation mechanisms that encourage the polarization of macrophages towards the M1 type, which is known to promote pro-inflammatory effects. This M1 polarization is linked to an increase in a crucial marker called CD86 and an elevated secretion of pro-inflammatory cytokines such as IL6 and TNF-α [81]. STAT1 is known to promote macrophage M1 polarization, while STAT6 promotes macrophage M2 polarization [82]. Ding et al. found that phycocyanin D inhibited STAT1 activation and blocked its nuclear translocation, it also activates STAT6 and enhances STAT6 nuclear translocation, to achieve repolarization of M1 macrophages to M2 type [83]. Numerous previous studies support that STAT3 phosphorylation is essential for the differentiation of macrophages into the M2 phenotype [84]. The exact mechanism between the STAT3 signaling pathway and macrophage polarization is unclear, but it has been shown that promoting STAT3 phosphorylation promotes the release of IL-4 and IL-10, which are important cytokines in promoting the polarization of M2 type macrophages. In contrast, inhibition of STAT3 phosphorylation decreases the proportion of M2 macrophages [85]. Researchers discovered increased levels of phosphorylated STAT-3 while examining lung biopsies taken from patients with IPF and mice with BLM-induced lung fibrosis [86]. Furthermore, the activation of STAT-3-mediated signaling [87] by IL-6 can result in BLM-induced lung fibrosis in mice, indicating that targeting downstream molecules associated with IL-6, such as STAT-3, may be an effective approach to enhance the treatment of lung fibrosis.
The cGAS-STING Signaling Pathway
The cGAS-STING pathway has emerged as a key mediator in inflammation, tumors and autoimmune diseases. The cGAS-STING signaling pathway mediates interferon when the cGAS receptor can bind to DNA, allowing STING to be activated and then bind to NF-κB, inducing the production of downstream signals including IFN regulatory factor 3 (IRF3) and promoting the expression of IFN genes [88]. Domizio et al, by analyzing lung samples from COVID-19 patients, found that macrophages near areas of endothelial cell injury mediated a STING-dependent type I IFN signature. And cGAS-STING activity was significantly increased with increasing levels of pathogenic IFN-I in the COVID-19 mouse model [89]. It has also been found that X-box binding protein 1 (XBP1) deficiency reduces STING/NLRP3 activation and promotes liver fibrosis by promoting BNIP3-mediated mitochondrial autophagy activation in macrophages [90]. BLM may induce lung fibrosis through activation of Sting signaling [91], and Wu et al. found that STING agonists promoted reprogramming of the tumor-associated macrophage M2 phenotype to the M1 phenotype [92]. Activation of the STING signaling pathway has the potential to drive macrophages towards M1-type polarization, it Promotes progression of pre-inflammatory lung disease and exacerbates late fibrosis. However, the specific mechanism by which the STING-TBK1-IRF3 pathway regulates the polarization of M1-type macrophages is not yet clear, Its role in the process of lung fibrosis has also not been clarified. Silica-induced lung injury has been demonstrated in animal studies in vivo, where silica particles can activate STING and induce type I IFN responses through extracellular self-dual-stranded DNA release from macrophages [93]. By focusing on the STING signaling pathway, it is possible to alter the macrophage phenotype and shift it from the pro-inflammatory M1 type to the anti-inflammatory M2 type. This, in turn, can lead to a reduction in silica-induced lung inflammation, which helps to alleviate the fibrotic process.
Conclusion
Various stages of lung fibrosis involve the differentiation of lung macrophages into distinct phenotypes. M1 macrophages are crucial for initiating the inflammatory response, while M2 macrophages aid in reducing inflammation and promoting healing. However, in cases where the phenotypic transformation is abnormal, excessive secretion of chemokines and matrix metalloproteinases by M2 macrophages contribute to the pathological remodeling of lung fibrosis. In recent years, numerous studies have identified new targets for intervening in the abnormal activation of macrophages that may help slow the development of lung fibrosis. For example, mitochondrial oxidative stress associated with M2 polarization in the pathogenesis of lung fibrosis. Knockdown of Gab1/2 inhibits M2 polarization and alleviates lung fibrosis to some extent. As well as multiple miRNAs have been shown to mediate macrophage polarization to promote lung fibrosis. The transition between macrophage phenotypes can be directly/synergistically regulated by multiple signaling pathways such as Notch, TGF-β/Smad, JAK-STAT and cGAS-STING. The specific mechanisms by which signaling pathways regulate the function or phenotype of macrophage subpopulations in vivo remain to be investigated. It is therefore essential to investigate the specific mechanisms of macrophage polarization in lung fibrosis. Investing in how the switch between macrophage phenotypes can be effectively regulated may help to provide new ideas for research in the fight against lung fibrosis.
Abbreviations
- ECM:
-
Extracellular Matrix
- AMs:
-
Alveolar Macrophages
- IMs:
-
Interstitial Macrophages
- ALI:
-
Acute Lung Injury
- ARDS:
-
Acute Respiratory Distress Syndrome
- COPD:
-
Chronic Obstructive Pulmonary Disease
- IPF:
-
Idiopathic Pulmonary Fibrosis
- BLM:
-
Bleomycin
- LPS:
-
Lipopolysaccharide
- IFN:
-
Interferon
- TNF:
-
Tumor Necrosis Factor
- IL-6:
-
Interleukin 6
- MCP1:
-
CCL2, also known as monocytes chemokin 1
- TNF-α:
-
Tumor Necrosis Factor α
- CD80:
-
Cluster of Differentiation 80
- TLR4:
-
Toll-like receptor 4
- MHCII:
-
Major Compatibility Complex II
- CD86:
-
Cluster of Differentiation 86
- iNOS:
-
Inducible Nitric Oxide Synthase
- ROS:
-
Reactive oxygen species
- NO:
-
Nitric Oxide
- CD206:
-
Cluster of Differentiation 206
- NF-κB:
-
Nuclear factor-kappa B
- STAT6:
-
Signal Transducer and Activator of Transcription
- MCUs:
-
Mitochondrial Calcium Mono
- mtROS:
-
mitochondrial Reactive Oxygen Species
- EGF:
-
Epidermal Growth Factor
- miRNA:
-
microRNA
- RILF:
-
Radiation-Induced Lung Fibrosis
- MSCs:
-
Mesenchymal Stem Cells
- JAG1:
-
Jagged1
- DLL1:
-
delta-like1
- GSI:
-
gamma-secretase inhibitor
- AEC2:
-
Alveolar epithelial cells
- PFD:
-
Pirfenidone
- IRF3:
-
IFN regulatory factor 3
- EMT:
-
Epithelial-Mesenchymal Transition
- XBP1:
-
X-box binding protein 1
References
Russell, R. E. K., Thorley, A., Culpitt, S. V., Dodd, S., Donnelly, L. E. & Demattos, C. et al. (2002). Alveolar macrophage-mediated elastolysis: roles of matrix metalloproteinases, cysteine, and serine proteases. The American Journal of Physiology-Lung Cellular and Molecular Physiology, 283, L867–L873.
Guilliams, M., De Kleer, I., Henri, S., Post, S., Vanhoutte, L. & De Prijck, S. et al. (2013). Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. Journal of Experimental Medicine, 210, 1977–1992.
Schyns, J., Bai, Q., Ruscitti, C., Radermecker, C., De Schepper, S. & Chakarov, S. et al. (2019). Non-classical tissue monocytes and two functionally distinct populations of interstitial macrophages populate the mouse lung. Nature Communications, 10, 3964.
He, S., Xie, L., Lu, J., & Sun, S. (2017). Characteristics and potential role of M2 macrophages in COPD. International Journal of Chronic Obstructive Pulmonary Disease, 12, 3029 Dove Press.
Saradna, A., Do, D. C., Kumar, S., Fu, Q.-L., & Gao, P. (2018). Macrophage polarization and allergic asthma. Translational Research, 191, 1–14.
Chen, X., Tang, J., Shuai, W., Meng, J., Feng, J. & Han, Z. (2020). Macrophage polarization and its role in the pathogenesis of acute lung injury/acute respiratory distress syndrome. Inflammation Research, 69, 883–895.
Liu, Y.-C., Zou, X.-B., Chai, Y.-F. & Yao, Y.-M. (2014). Macrophage polarization in inflammatory diseases. International Journal of Biological Sciences, 10, 520–529. Lake Haven: Ivyspring Int Publ.
Booz, G. W., Altara, R., Eid, A. H., Wehbe, Z., Fares, S. & Zaraket, H. et al. (2020). Macrophage responses associated with COVID-19: A pharmacological perspective. European Journal of Pharmacology, 887, 173547.
Barratt S. L., Creamer A., Hayton C., Chaudhuri N. Idiopathic Lung fibrosis (IPF): An Overview. Journal of Clinical Medicine [Internet]. Multidisciplinary Digital Publishing Institute (MDPI); 2018 [cited 2023 Apr 22];7. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6111543/.
Paludan, S. R. & Mogensen, T. H. (2022). Innate immunological pathways in COVID-19 pathogenesis. Science Immunology, 7(67), eabm5505. https://doi.org/10.1126/sciimmunol.Abm5505.
Selvarajah, B., Azuelos, I., Anastasiou, D. & Chambers, R. C. (2021). Fibrometabolism-Anemerging therapeutic frontier in pulmonary fibrosis. Science Signaling, 14(697), eaay1027. https://doi.org/10.1126/scisignal.aay1027.
Rumende, C. M., Susanto, E. C., & Sitorus, T. P. (2021). The management of lung fibrosis in COVID-19. Acta Medica Indonesiana, 53, 233.
Arora, S., Dev, K., Agarwal, B., Das, P., & Syed, M. A. (2018). Macrophages: Their role, activation and polarization in pulmonary diseases. Immunobiology, 223, 383–396.
Chen, J., Zhou, R., Liang, Y., Fu, X., Wang, D. & Wang, C. (2019). Blockade of lncRNA-ASLNCS5088–enriched exosome generation in M2 macrophages by GW4869 dampens the effect of M2 macrophages on orchestrating fibroblast activation. The FASEB Journal, 33, 12200–12212.
Gibbings, S. L., Thomas, S. M., Atif, S. M., McCubbrey, A. L., Desch, A. N. & Danhorn, T. et al. (2017). Three unique interstitial macrophages in the murine lung at steady state. American Journal of Respiratory Cell and Molecular Biology, 57, 66–76.
Sennello, J. A., Misharin, A. V., Flozak, A. S., Berdnikovs, S., Cheresh, P. & Varga, J. et al. (2017). Lrp5/β-catenin signaling controls lung macrophage differentiation and inhibits resolution of fibrosis. American Journal of Respiratory Cell and Molecular Biology, 56, 191–201.
Chakarov, S., Lim, H. Y., Tan, L., Lim, S. Y., See, P., & Lum, J., et al. (2019). Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science, 363, eaau0964 American Association for the Advancement of Science.
Yunna, C., Mengru, H., Lei, W., & Weidong, C. (2020). Macrophage M1/M2 polarization. European Journal of Pharmacology, 877, 173090.
Yuan, H., et al. (2023). Crystalline silica-induced proinflammatory interstitial macrophage recruitment through notch3 signaling promotes the pathogenesis of silicosis. Environmental Science & Technology, 57(39), 14502–14514. https://doi.org/10.1021/acs.est.3c03980.
Phan, T. H. G., Paliogiannis, P., Nasrallah, G. K., Giordo, R., Eid, A. H. & Fois, A. G. et al. (2021). Emerging cellular and molecular determinants of idiopathic lung fibrosis. Cellular and Molecular Life Sciences, 78, 2031–2057.
Sica, A. & Mantovani, A. (2012). Macrophage plasticity and polarization: In vivo veritas. Journal of Clinical Investigation, 122, 787–795.
Russell, D. G., Huang, L., & VanderVen, B. C. (2019). Immunometabolism at the interface between macrophages and pathogens. Nature Reviews Immunology, 19, 291 NIH Public Access.
Bronte, V. & Zanovello, P. (2005). Regulation of immune responses by L-arginine metabolism. Nature Reviews Immunology, 5, 641–654. Nature Publishing Group.
Murray P. J. Macrophage Polarization. In: Julius D., editor. Annual Review of Physiology, Vol 79 [Internet]. Palo Alto: Annual Reviews; 2017 [cited 2023 Apr 23]. p. 541–66. Available from: https://www.webofscience.com/wos/alldb/full-record/WOS:000396049000024.
Osińska, I., Wołosz, D., & Domagała-Kulawik, J. (2014). Association between M1 and M2 macrophages in bronchoalveolar lavage fluid and tobacco smoking in patients with sarcoidosis. Polish Archives of Internal Medicine, 124, 359–364.
Zhang, L., Wang, Y., Wu, G., Xiong, W., Gu, W. & Wang, C.-Y. (2018). Macrophages: friend or foe in idiopathic lung fibrosis?. Respiratory Research, 19, 170
Wang, L., Zhang, S., Wu, H., Rong, X. & Guo, J. (2019). M2b macrophage polarization and its roles in diseases. Journal of Leukocyte Biology, 106, 345–358.
Colin, S., Chinetti-Gbaguidi, G., & Staels, B. (2014). Macrophage phenotypes in atherosclerosis. Immunological Reviews, 262, 153–166.
Ji, W.-J., Ma, Y.-Q., Zhou, X., Zhang, Y.-D., Lu, R.-Y., & Sun, H.-Y., et al. (2014). Temporal and spatial characterization of mononuclear phagocytes in circulating, lung alveolar and interstitial compartments in a mouse model of bleomycin-induced pulmonary injury. Journal of Immunological Methods, 403, 7–16.
Nair, M. G., Cochrane, D. W., & Allen, J. E. (2003). Macrophages in chronic type 2 inflammation have a novel phenotype characterized by the abundant expression of Ym1 and Fizz1 that can be partly replicated in vitro. Immunology Letters, 85, 173–180.
Liu, T., Yu, H., Ullenbruch, M., Jin, H., Ito, T., & Wu, Z., et al. (2014). The in vivo fibrotic role of FIZZ1 in lung fibrosis. PLoS One, 9, e88362.
Li, D., Guabiraba, R., Besnard, A.-G., Komai-Koma, M., Jabir, M. S. & Zhang, L. et al. (2014). IL-33 promotes ST2-dependent lung fibrosis by the induction of alternatively activated macrophages and innate lymphoid cells in mice. The Journal of Allergy and Clinical Immunology, 134, 1422
Tarique, A. A., Logan, J., Thomas, E., Holt, P. G., Sly, P. D. & Fantino, E. (2015). Phenotypic, functional, and plasticity features of classical and alternatively activated human macrophages. American Journal of Respiratory Cell and Molecular Biology, 53, 676–688. American Thoracic Society - AJRCMB.
Wang, K., Zu, C., Zhang, Y., Wang, X., Huan, X., Wang, L. Blocking TG2 attenuates bleomycin-induced pulmonary fibrosis in mice through inhibiting EMT. Respiratory Physiology & Neurobiology, 2020, https://doi.org/10.1016/j.resp.2020.103402.
Wang, J., Xu, L., Xiang, Z., Ren, Y., Zheng, X., Zhao, Q., Zhou, Q., Zhou, Y., Xu, L. & Wang, Y. (2020). Microcystin-LR ameliorates pulmonary fibrosis via modulating CD206(+) M2-like macrophage polarization. Cell Death and Disease, 11(2), 136
Suarez-Carmona, M., Lesage, J., Cataldo, D. & Gilles, C. (2017). EMT and inflammation: inseparable actors of cancer progression. Molecular Oncology, 11(7), 805–823.
Vats, D., Mukundan, L., Odegaard, J. I., Zhang, L., Smith, K. L. & Morel, C. R. et al. (2006). Oxidative metabolism and PGC-1β attenuate macrophage-mediated inflammation. Cell Metabolism, 4, 13–24.
Yu, W., Wang, X., Zhao, J., Liu, R., Liu, J., & Wang, Z., et al. (2020). Stat2-Drp1 mediated mitochondrial mass increase is necessary for pro-inflammatory differentiation of macrophages. Redox Biology, 37, 101761.
He, C., Ryan, A. J., Murthy, S. & Carter, A. B. (2013). Accelerated development of lung fibrosis via Cu,Zn-superoxide dismutase-induced alternative activation of macrophages. Journal of Biological Chemistry, 288, 20745–20757.
Mathai, S. K. & Schwartz, D. A. (2019). Translational research in lung fibrosis. Translational Research, 209, 1–13.
Larson-Casey, J. L., Deshane, J. S., Ryan, A. J., Thannickal, V. J., & Carter, A. B. (2016). Macrophage Akt1 kinase-mediated mitophagy modulates apoptosis resistance and lung fibrosis. Immunity, 44, 582–596.
Gu, L., Larson-Casey, J. L., & Carter, A. B. (2017). Macrophages utilize the mitochondrial calcium uniporter for profibrotic polarization. FASEB J, 31, 3072–3083.
Sármay, G., Angyal, A., Kertész, Á., Maus, M., & Medgyesi, D. (2006). The multiple function of Grb2 associated binder (Gab) adaptor/scaffolding protein in immune cell signaling. Immunology Letters, 104, 76–82.
Meng, S., Chen, Z., Munoz-Antonia, T. & Wu, J. (2005). Participation of both Gab1 and Gab2 in the activation of the ERK/MAPK pathway by epidermal growth factor. Biochemical Journal, 391, 143–151.
Guo, X., Li, T., Xu, Y., Xu, X., Zhu, Z. & Zhang, Y. et al. (2017). Increased levels of Gab1 and Gab2 adaptor proteins skew interleukin-4 (IL-4) signaling toward M2 macrophage-driven lung fibrosis in mice. Journal of Biological Chemistry, 292, 14003–14015.
Shi, X., Liu, Q., Zhao, H., Lu, J., Huang, Y., & Ma, Y., et al. (2019). Increased expression of GAB1 promotes inflammation and fibrosis in systemic sclerosis. Experimental Dermatology, 28, 1313–1320.
Wang, X., Huang, J., Chen, W., Li, G., Li, Z. & Lei, J. (2022). The updated role of exosomal proteins in the diagnosis, prognosis, and treatment of cancer. Experimental & Molecular Medicine, 54, 1390–1400.
Goldie, B. J., Dun, M. D., Lin, M., Smith, N. D., Verrills, N. M. & Dayas, C. V. et al. (2014). Activity-associated miRNA are packaged in Map1b-enriched exosomes released from depolarized neurons. Nucleic Acids Research, 42, 9195–9208.
Mori, M. A., Ludwig, R. G., Garcia-Martin, R., Brandão, B. B. & Kahn, C. R. (2019). Extracellular miRNAs: from biomarkers to mediators of physiology and disease. Cell Metabolism, 30, 656–673.
Xie, L. & Zeng, Y. (2020). Therapeutic potential of exosomes in lung fibrosis. Frontiers in Pharmacology, 11, 590972.
Kishore, A. & Petrek, M. (2021). Roles of macrophage polarization and macrophage-derived miRNAs in lung fibrosis. Frontiers in Immunology, 12, 678457.
Duru, N., Wolfson, B. & Zhou, Q. (2016). Mechanisms of the alternative activation of macrophages and non-coding RNAs in the development of radiation-induced lung fibrosis. World Journal of Biological Chemistry, 7, 231–239.
Ren, W., Hou, J., Yang, C., Wang, H., Wu, S. & Wu, Y. et al. (2019). Extracellular vesicles secreted by hypoxia pre-challenged mesenchymal stem cells promote non-small cell lung cancer cell growth and mobility as well as macrophage M2 polarization via miR-21-5p delivery. Journal of Experimental & Clinical Cancer Research, 38, 62.
Li, J., wei, Wei, L., Han, Z., & Chen, Z. (2019). Mesenchymal stromal cells-derived exosomes alleviate ischemia/reperfusion injury in mouse lung by transporting anti-apoptotic miR-21-5p. European Journal of Pharmacology, 852, 68–76.
Eissa, M. G., & Artlett, C. M. (2019). The MicroRNA miR-155 is essential in fibrosis. Noncoding RNA., 5, 23.
Guiot, J., Cambier, M., Boeckx, A., Henket, M., Nivelles, O., & Gester, F., et al. (2020). Macrophage-derived exosomes attenuate fibrosis in airway epithelial cells through delivery of antifibrotic miR-142-3p. Thorax, 75, 870–881.
Mašek, J., & Andersson, E. R. (2017). The developmental biology of genetic Notch disorders. Development, 144, 1743–1763.
Zhou, D., Huang, C., Lin, Z., Zhan, S., Kong, L., & Fang, C., et al. (2014). Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cellular Signaling, 26, 192–197.
Adams, J. M., & Jafar-Nejad, H. (2019). The roles of notch signaling in liver development and disease. Biomolecules, 9, 608.
Aoyagi-Ikeda, K., Maeno, T., Matsui, H., Ueno, M., Hara, K., Aoki, Y., Aoki, F., Shimizu, T., Doi, H., Kawai-Kowase, K., Iso, T., Suga, T., Arai, M., & Kurabayashi, M. (2011). Notch induces myofibroblast differentiation of alveolar epithelial cells via transforming growth factor-{beta}-Smad3 pathway. American Journal of Respiratory Cell and Molecular Biology, 45(1), 136–144.
Liu, T., Hu, B., Choi, Y. Y., Chung, M., Ullenbruch, M., Yu, H., Lowe, J. B. & Phan, S. H. (2009). Notch1 signaling in FIZZ1 induction of myofibroblast differentiation. The American Journal of Pathology, 174(5), 1745–1755.
Liu, X. D., Zhang, L. Y., Zhu, T. C., Zhang, R. F., Wang, S. L. & Bao, Y. (2015). Overexpression of miR-34c inhibits high glucose-induced apoptosis in podocytes by targeting Notch signaling pathways. International Journal of Clinical and Experimental Pathology, 8(5), 4525–4534.
Hu, B. & Phan, S. H. (2016). Notch in fibrosis and as a target of anti-fibrotic therapy. Pharmacological Research, 108, 57–64.
Shin, H. M., Minter, L. M., Cho, O. H., Gottipati, S., Fauq, A. H. & Golde, T. E. et al. (2006). Notch1 augments NF-κB activity by facilitating its nuclear retention. The EMBO Journal, 25, 129–138.
Palaga, T., Buranaruk, C., Rengpipat, S., Fauq, A. H., Golde, T. E., & Kaufmann, S. H. E., et al. (2008). Notch signaling is activated by TLR stimulation and regulates macrophage functions. European Journal of Immunology, 38, 174–183.
Fung, E., Tang, S.-M. T., Canner, J. P., Morishige, K., Arboleda-Velasquez, J. F., & Cardoso, A. A., et al. (2007). Delta-like 4 induces notch signaling in macrophages implications for inflammation. Circulation, 115, 2948–2956. Philadelphia: Lippincott Williams & Wilkins.
Liu, L., Gao, C., Chen, G., Li, X., Li, J., Wan, Q. & Xu, Y. (2013). Notch signaling molecules activate TGF-beta in rat mesangial cells under high glucose conditions. Journal of Diabetes Research, 2013, 979702
Vera, L., Garcia-Olloqui, P., Petri, E., Viñado, A. C., Valera, P. S. & Blasco-Iturri, Z. et al. (2021). Notch3 deficiency attenuates lung fibrosis and impedes lung-function decline. American Journal of Respiratory Cell and Molecular Biology, 64, 465–476.
Wasnick, R., Korfei, M., Piskulak, K., Henneke, I., Wilhelm, J., Mahavadi, P., et al. Notch1 induces defective epithelial surfactant processing and lung fibrosis. American Journal of Respiratory and Critical Care Medicine [Internet]. American Thoracic Society; 2023 [cited 2023 Apr 23]; Available from: https://www.atsjournals.org/doi/10.1164/rccm.202105-1284OC?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed.
Ma, T., Li, X., Zhu, Y., Yu, S., Liu, T., & Zhang, X., et al. (2022). Excessive activation of notch signaling in macrophages promote kidney inflammation, fibrosis, and necroptosis. Front Immunol, 13, 835879.
Zheng, S., Zhang, P., Chen, Y., Zheng, S., Zheng, L., & Weng, Z. (2016). Inhibition of notch signaling attenuates schistosomiasis hepatic fibrosis via blocking macrophage M2 polarization. PLoS One, 11, e0166808.
Li, Z., Nie, M., Yu, L., Tao, D., Wang, Q. & He, Y. et al. (2022). Blockade of the notch signaling pathway promotes M2 macrophage polarization to suppress cardiac fibrosis remodeling in mice with myocardial infarction. Frontiers in Cardiovascular Medicine, 8, 639476.
Salton, F., Volpe, M. C., & Confalonieri, M. (2019). Epithelial–mesenchymal transition in the pathogenesis of idiopathic lung fibrosis. Medicina (Kaunas), 55, 83.
Meng, X., Nikolic-Paterson, D. J. & Lan, H. Y. (2016). TGF-β: the master regulator of fibrosis. Nature Reviews Nephrology, 12, 325–338. Nature Publishing Group.
Zhang, L., Cheng, X., Gao, Y., Zhang, C., Bao, J., & Guan, H., et al. (2016). Curcumin inhibits metastasis in human papillary thyroid carcinoma BCPAP cells via down-regulation of the TGF-β/Smad2/3 signaling pathway. Experimental Cell Research, 341, 157–165.
Chen, B., Huang, S., Su, Y., Wu, Y.-J., Hanna, A. & Brickshawana, A. et al. (2019). Macrophage Smad3 protects the infarcted heart, stimulating phagocytosis and regulating inflammation. Circulation Research, 125, 55–70.
Ying, H., Fang, M., Hang, Q. Q., Chen, Y., Qian, X. & Chen, M. (2021). Pirfenidone modulates macrophage polarization and ameliorates radiation‐induced lung fibrosis by inhibiting the TGF‐β1/Smad3 pathway. Journal of Cellular and Molecular Medicine, 25, 8662–8675.
Bousoik, E. & Montazeri Aliabadi, H. (2018). “Do We Know Jack” about JAK? a closer look at JAK/STAT signaling pathway. Frontiers in Oncology, 8, 287.
Seif, F., Khoshmirsafa, M., Aazami, H., Mohsenzadegan, M., Sedighi, G. & Bahar, M. (2017). The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Communication and Signaling, 15, 23.
Verhoeven, Y., Tilborghs, S., Jacobs, J., De Waele, J., Quatannens, D., & Deben, C., et al. (2020). The potential and controversy of targeting STAT family members in cancer. Seminars in Cancer Biology, 60, 41–56.
Lescoat, A., Lelong, M., Jeljeli, M., Piquet-Pellorce, C., Morzadec, C., & Ballerie, A., et al. (2020). Combined anti-fibrotic and anti-inflammatory properties of JAK-inhibitors on macrophages in vitro and in vivo perspectives for scleroderma-associated interstitial lung disease. Biochemical Pharmacology, 178, 114103.
Shan, X., Hu, P., Ni, L., Shen, L., Zhang, Y. & Ji, Z. et al. (2022). Serine metabolism orchestrates macrophage polarization by regulating the IGF1–p38 axis. Cellular & Molecular Immunology, 19, 1263–1278. Nature Publishing Group.
Ding, N., Wang, Y., Dou, C., Liu, F., Guan, G., & Wei, K., et al. (2019). Physalin D regulates macrophage M1/M2 polarization via the STAT1/6 pathway. Journal of Cellular Physiology, 234, 8788–8796. John Wiley & Sons, Ltd.
Panagi, I., Jennings, E., Zeng, J., Günster, R. A., Stones, C. D., & Mak, H., et al. (2020). Salmonella effector SteE converts the mammalian serine/threonine kinase GSK3 into a tyrosine kinase to direct macrophage polarization. Cell Host Microbe, 27, 41–53.e6.
Li, Q., Cheng, Y., Zhang, Z., Bi, Z., Ma, X. & Wei, Y. et al. (2022). Inhibition of ROCK ameliorates lung fibrosis by suppressing M2 macrophage polarisation through phosphorylation of STAT3. Clinical and Translational Medicine, 12, e1036.
Pedroza, M., Le, T. T., Lewis, K., Karmouty-Quintana, H., To, S., & George, A. T., et al. (2016). STAT-3 contributes to lung fibrosis through epithelial injury and fibroblast-myofibroblast differentiation. FASEB J, 30, 129–140.
Jafarzadeh, A., Nemati, M. & Jafarzadeh, S. (2021). Contribution of STAT3 to the pathogenesis of COVID-19. Microbial Pathogenesis, 154, 104836.
Paludan, S., & Bowie, A. (2013). Immune sensing of DNA. Immunity (10747613), 38, 870–880.
Di Domizio, J., et al. (2022). The cGAS-STING pathway drives type I IFN immunopathology in COVID-19. Nature, 603(7899), 145–151. https://doi.org/10.1038/s41586-022-04421-w.
Wang, Q., et al. (2022). XBP1-mediated activation of the STING signalling pathway in macrophages contributes to liver fibrosis progression. JHEP Reports: Innovation in Hepatology, 4(11), 100555 https://doi.org/10.1016/j.jhepr.2022.100555.
Sun, S.-C., Han, R., Hou, S.-S., Yi, H.-Q., Chi, S.-J., & Zhang, A.-H. (2020). Juglanin alleviates bleomycin-induced lung injury by suppressing inflammation and fibrosis via targeting sting signaling. Biomedicine & Pharmacotherapy, 127, 110119.
Wu, Y., Fang, Y., Wei, Q., Shi, H., Tan, H., Deng, Y., et al. Tumor-targeted delivery of a STING agonist improves cancer immunotherapy. Proceedings of the National Academy of Sciences of the United States of America 119:e2214278119.
Benmerzoug, S., Bounab, B., Rose, S., Gosset, D., Biet, F., & Cochard, T., et al. (2019). Sterile lung inflammation induced by silica exacerbates mycobacterium tuberculosis infection via STING-dependent type 2 immunity. Cell Reports, 27, 2649–2664.e5.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 81460001, No.82260002), the Natural Science Research Foundation of Jilin Province for Sciences and Technology (grant number 20220101353JC), and the Education Department Project of Jilin (grant number JJKH20220546KJ).
Author information
Authors and Affiliations
Contributions
Resources, Z.J.; Writing—original draft preparation, W.X.; Writing—review and editing, L.X., L.C. ; Funding acquisition, C.H., L.L. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, X., Zhou, J., Li, X. et al. The Role of Macrophages in Lung Fibrosis and the Signaling Pathway. Cell Biochem Biophys 82, 479–488 (2024). https://doi.org/10.1007/s12013-024-01253-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-024-01253-5