Abstract
Extracellular vesicles (EVs) are minute lipid-bilayer sacs discharged by cells, encompassing a diverse array of proteins, nucleic acids, and lipids. The identification of EVs as pivotal agents in intercellular communication has sparked compelling research pathways in the realms of cell biology and neurodegenerative diseases. Utilizing EVs for medicinal reasons has garnered interest due to the adaptability of EV-mediated communication. EVs can be classified based on their physical characteristics, biochemical composition, or cell of origin following purification. This review delves into the primary sub-types of EVs, providing an overview of the biogenesis of each type. Additionally, it explores the diverse environmental conditions triggering EV release and the originating cells, including stem cells and those from the Central Nervous System. Within the brain, EVs play a pivotal role as essential mediators of intercellular communication, significantly impacting synaptic plasticity, brain development, and the etiology of neurological diseases. Their potential diagnostic and therapeutic applications in various brain-related conditions are underscored, given their ability to carry specific cargo. Specially engineered EVs hold promise for treating diverse diseases, including neurodegenerative disorders. This study primarily emphasizes the diagnostic and potential therapeutic uses of EVs in neurological disorders such as Alzheimer’s Disease, Huntington’s Disease, Parkinson’s Disease, Amyotrophic Lateral Sclerosis, and Prions disease. It also summarizes innovative techniques for detecting EVs in the brain, suggesting that EVs could serve as non-invasive biomarkers for early detection, disease monitoring, and prognosis in neurological disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extracellular vesicles (EVs) are small lipid-bilayer vesicles released by cells. They contain a variety of proteins, nucleic acids, and lipids, and are important in intercellular communication. They can be isolated and characterized by different techniques. Exosomes, microvesicles, and apoptotic bodies are some of the subtypes of EVs that can be divided based on their size and biogenesis [1]. EV uptake through endocytosis is a result of the interaction between EVs and recipient cells, which includes ligand-receptor signaling and/or fusion with the recipient cell membrane. This procedure enables the transfer of cargo molecules and EV membrane components to the target cell’s cytoplasm or nucleus, potentially affecting cellular signaling and having a variety of physiological effects. To begin with, understanding the biophysical and biochemical nature of EVs, is crucial to decipher their application in the field of medical science.
Biophysical and Biochemical Characterization of EVs
EVs can be characterized according to their physical attributes, biochemical composition, or cell of origin once they are purified. While the most frequently used technique for EV isolation is differential ultracentrifugation, various alternative techniques, such as polyethylene glycol precipitation, immunoprecipitation, size exclusion chromatography, and immuno-isolation approaches are commonly employed [2].
The biophysical features of EVs, such as vesicle size, concentration, and distribution, can be characterized by microscopy-based methods. Such methods include Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM), Atomic Force Microscopy (AFM), and Cryo-Electron microscopy. Biophysical characterization can also utilize techniques such as dynamic light scattering, nanoparticle tracking, and tunable resistive pulse sensing (TRPS) analysis. One report highlights a light microscopic single EV analysis technique for robust and multiplexed biomarker measurement in individual vesicles [3]. Recently, a group of scientists developed a multiplexed analysis technique for EVs, referred to as MASEV, to examine thousands of individual EVs through five cycles of multi-channel fluorescence staining targeting 15 different EV biomarkers [4]. In SEM, EVs exhibit a distorted cup-shaped structure and display a consistent unimodal size distribution [5, 6]. TEM is appreciated for its ability to identify and describe individual EVs. Additionally, the utilization of immunogold labeling in TEM allows for insights into the biochemical properties of EV surface proteins. Cryo-TEM is employed to overcome notable drawbacks in traditional TEM methods, such as specimen dehydration, chemical fixation, and staining of biological samples. EVs isolated from cell culture supernatant are usually stained with Cluster of Differentiation-63 (CD63), CD81, and CD9 antibodies, which in turn are coupled with gold particles [7]. In AFM, the topographic AFM reveals a circular morphology of isolated EVs, whereas phase images exhibit substructures, indicating the presence of diverse constitutive elements such as lipids and proteins within these formations. Individual EV subtypes called exosomes, exhibit reversible mechanical deformation, showing a distinctive elastic trilobed membrane measuring 70–100 nm, which contains substructures carrying specific transmembrane receptors [8]. TRPS is a resilient and adaptable technique commonly utilized for the in situ, time-dependent examination of DNA binding onto magnetic nanoparticles [9]. This method has been extensively explored for measuring the size distribution of EVs and has been engineered to deliver enzymes for combating Alzheimer’s disease (AD) (with sizes of 150–200 nm) and anticancer-miRNAs to cancerous cells [10, 11]. Nanoparticle Tracking Analysis (NTA) is a widely employed technique for the biophysical characterization of EVs. In EV analysis, NTA tracks its movement by analyzing images, and detecting the trajectory of EVs to measure particle velocity, which correlates with their sizes. NTA can analyze various vesicle types, including those with diameters as low as 30 nm. As a non-invasive method, sample preparation is straightforward, and the analysis itself requires less time. A chapter focuses on EV characterization using NS300 NTA instrument [6, 12]. Like NTA, dynamic light scattering (DLS) also uses the principle of light scattering. It is a comparatively simple method for EV size measurement without visualizing it. This method is highly effective for measuring vesicle size in red blood cells and EVs derived from ovarian cancer cells. The diameter of vesicles can be easily determined by DLS, but it does not provide any biochemical information about the biogenesis of EVs [13]. Details about the advances of this technology in EV characterization are highlighted in a recent chapter [14].
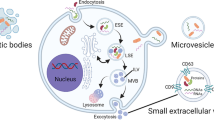
Biochemical evaluations of EVs are commonly conducted through flow cytometry, immunoblotting, lipidomic, proteomic, and nucleic acid sequencing studies, and recently, by Raman spectroscopy analysis [15]. Central to EV proteomics, Mass Spectrometry (MS) enables the identification and quantification of proteins and lipids in complex mixtures. It is often coupled with liquid chromatography (LC-MS/MS) for enhanced sensitivity. Protein and lipid identification is achieved by comparing experimental MS data to respective databases by using bioinformatic tools [16, 17]. These may help in identification of novel protein and lipid biomarkers. For instance, lipid species that are more abundant in Adipose EVs (AdEVs) in the context of obesity could potentially serve as biomarker candidates or play a role as mediators in the metabolic dysfunctions associated with obesity [18]. For nucleic acid analysis, DNA sequencing can provide information about genomic content within EVs, including potential mutations, copy number variations, and other genetic alterations. On the other hand, RNA sequencing enables the identification and quantification of RNA species within EVs, including messenger RNA (mRNA), microRNA (miRNA), long non-coding RNA (lncRNA), and other small RNAs. A current report examined small EVs from mast cells and found specific genetic molecules that are modulated when these cells become activated [19]. Flow cytometry is used both for physical and chemical composition analysis of EVs. Here, the challenge is to obtain a single particle suspension. Collaborating from the International Society for Extracellular Vesicles (ISEV), International Society for Advancement of Cytometry (ISAC), and the International Society on Thrombosis and Haemostasis (ISTH), researchers formed the EV Flow Cytometry working group. This group released an ISEV position manuscript introducing the Minimum Information about a Flow Cytometry Experiment on Extracellular Vesicles framework. However, the framework lacked sufficient background details. Consequently, another compendium was created to furnish the essential background information for designing and executing reproducible EV Flow Cytometry experiments. It encompasses information on EVs, the interplay between light and EVs, Flow Cytometry hardware, experimental design, preanalytical procedures, sample preparation, assay controls, instrument data acquisition and calibration, EV characterization, and data reporting [20, 21]. In contrast to flow cytometry, Western blotting does not enable the direct observation of intact vesicles. Instead, the vesicles undergo lysis, and the proteins are denatured and reduced as part of the sample preparation process. Although it is one of the simplest techniques, the major drawback is that multiplexing is not well-executed, and the specificity and reproducibility are constrained by the quality of the antibody employed. The Raman analysis, a vibrational spectroscopy method, has been demonstrated to guarantee precise chemical identification and rapid analytical processes within the EV domain. In a recent report, the Raman Spectroscopy analysis unveiled biochemical distinctions between serum EVs and salivary EVs, evident in variations in intensity and the presence of characteristic peaks. Significant discrepancies were identified in the amide regions, reflecting differences in both internal cargo and the protein corona, along with variations in carbohydrate components. The observed differences in carbohydrates could account for the presence of mucins within or adsorbed to salivary EVs. It is speculated that salivary EVs might carry more nucleic acids compared to serum EVs, presenting a potentially valuable cargo for RNA biomarkers [20]. In a nutshell, these studies reveal that EVs have a complex chemical composition that encompasses a plethora of lipids, proteins, nucleic acids, and various bioactive molecules. The physical and chemical composition largely depends on the type of extracellular vesicle, environmental condition under which they are released as well as the cells from which they originate. In the following section, the major sub-types of EVs have been discussed with an overview of the biogenesis of each type. Table 1 summarizes the different types of EVs with information on their parameters. The sub-types of EVs include exosomes, microvesicles, apoptotic bodies, large oncosomes, retrovirus-like particle, migrasomes, exopheres, necroptotic, autophagic, and ferroptotic vesicles [22,23,24,25,26].
Types of Extracellular Vesicles
Exosomes
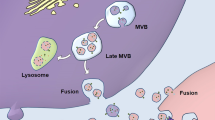
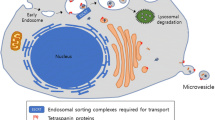
These are a subtype of membrane-contained vesicles. They help in the transport of proteins, lipids, DNA, and different types of RNA, thereby facilitating intercellular and interorgan communication. Exosomes are small vesicles that develop from the endosomal pathway and range in size from 30 to 100 nm. Early endosomes participate in inward budding to create multivesicular bodies (MVBs) that contain intraluminal vesicles (ILVs). If MVB undergoes lysosomal fusion, the cargo contained within the ILVs will undergo degradation. Conversely, if the MVB fuses with the cell membrane, the ILVs will be released into the extracellular space, transforming into exosomes. Such vesicles can travel in all body fluids and once released, they can perform a wide variety of functions. They can transmit signals and molecules to other cells as well as help in remodeling the extracellular matrix. Exosomes can be formed through both ESCRT-dependent and ESCRT-independent mechanisms. The ESCRT machinery is a multiprotein complex that orchestrates molecular binding and membrane deformation events, leading to the biogenesis of intraluminal vesicles (ILVs) and the recruitment of cargo to them. The ESCRT machinery mediates the step-by-step creation of ILVs, but sphingomyelinase can cause domain-induced inward budding of ILVs that results in the formation of exosomes [22, 27, 28]. The RNA-binding protein YBX1 (Y-box binding protein 1) was recognized by Park et al. as a crucial component of the mechanisms underlying RNA sorting into exosomes. According to the findings, YBX1 interacts with the ESCRT-II complex to make it easier to package particular RNA species into exosomes [29]. These results contributed to a deeper comprehension of the molecular mechanisms behind RNA sorting and the make-up of exosome cargo. Exosomes have a varied composition but are high in tetraspanins like CD9, CD63, and CD81, which act as specific markers for endosomes. They carry a variety of endosome-associated molecules, such as annexins and flotillins [30].
Microvesicles
These are larger than exosomes and can be 100–1000 nm in size. They are also referred to as microparticles or ectosomes. Microvesicles, as opposed to exosomes, are produced by the plasma membrane budding outward and are then discharged into the extracellular space. Hence, they are also known as shedding vesicles. The generation of microvesicles is the outcome of the dynamic interplay between the redistribution of phospholipids and the contraction of cytoskeletal proteins. Compared to exosomes, microvesicles carry a broader range of cellular components, reflecting the composition of the cell from which they originate. Increasing evidence points to the involvement of small GTPases, such as the Rho family and ARFs (ADP ribosylation factors), by regulating cytoskeletal elements [30]. Tetraspanins have been found in microvesicles and other vesicles, even though they were once thought to be specific exosome indicators. Along with the ESCRT proteins, the plasma membrane’s lipids and Ca2+-dependent enzymatic machinery, such aminophospholipid translocases and scramblases are also necessary for the formation of microvesicles [23].
Apoptotic Bodies
Another subtype of EVs, apoptotic bodies, are relatively large and range in size from 100 to 2000 nm. Apoptotic bodies are a major subset of apoptotic extracellular vesicles. While exosomes and microvesicles are secreted during normal cell processes, apoptotic bodies are released by apoptotic cells. The formation of apoptotic bodies has the potential to impact diverse biological processes, including the clearance of apoptotic cells and communication between neighboring cells. These EVs are ingested by phagocytic cells for digestion and contain fragmented subcellular organelles for degradation [31]. The biogenesis involves membrane blebbing on the cell surface. This is followed by apoptotic membrane protrusions in the form of microtubule spikes, beaded apoptopodia, and the formation of apoptotic bodies. Inhibitors of molecular factors like ROCK1, MLCK, and caspases can suppress this process, highlighting their importance in the formation of apoptotic bodies [32].
Although the above three types of EVs are the most studied, it is important to note that due to advances in research and technology, EV classification is still evolving. Numerous processes are involved in the sorting of cytosolic proteins and nucleic acids into EVs. Heat shock cognate protein (HSC70) and chaperone heat shock proteins (HSP70 and HSP90) have both been proposed to have a role in the sorting of soluble proteins to EVs. The incorporation of cytosolic proteins into EVs can also be mediated by farnesylation- or ubiquitylation-dependent processes. The sorting mechanism for RNA species is less understood, with some studies implicating the involvement of ESCRT-II and YBX1 in sorting RNA into exosomes [23].
The biogenesis and release of these EV subtypes involve distinct cellular machinery (Fig. 1).
In the next section, the focus will shift to the varied environmental conditions under which the EVs are released as well as the cells from which they originate, limiting to stem cells and cells of the Central Nervous System (CNS) only. These factors also affect the physical and chemical nature, as well as applicability of EVs. Extracellular vesicles are released by cells in both physiological and pathological conditions. Under normal physiological conditions, EV release is often triggered by specific signaling pathways, including those involving the endosomal sorting complex required for transport (ESCRT) machinery. Normal cellular processes such as cell activation or differentiation can induce EV formation as part of intercellular communication. Stem cells release EVs, including exosomes and microvesicles, containing a cargo of proteins, lipids, and nucleic acids. Stem cell-derived EVs exhibit regenerative potential, contributing to tissue repair and regeneration in various tissues and organs. They can also modulate the immune response, influencing inflammation and immune cell behavior. EVs that are secreted by Embryonic stem cells and Induced Pluripotent Stem Cells carry pluripotent and regenerative potential. EVs from Neural Stem Cells (NSCs) may promote neuroregeneration and neuroprotection. EVs released from Endothelial Progenitor Cells and Cardiac Stem Cells exhibit angiogenic properties and may contribute to vascular repair and regeneration. Other stem cells like Hematopoietic Stem Cells play a role in regulating hematopoiesis, influencing the development of blood cells. EVs from mesenchymal stem cells (MSCs) are well-known for their immunomodulatory and angiogenic properties [24]. Interestingly, EVs that are released from Cancer Stem Cells can influence the tumor microenvironment and may contribute to therapeutic resistance by transferring molecules that enhance resistance mechanisms [33].
Ongoing research continues to uncover the unique characteristics and therapeutic potential of stem cell-derived extracellular vesicles. Of all the different types of stem cells mentioned above, MSC-derived EVs have gained attention for their huge therapeutic potential in various diseases, including inflammatory disorders, neurodegenerative diseases, cardiovascular diseases, and tissue injuries. They often exhibit anti-inflammatory effects, reducing inflammation in target tissues and contribute to tissue repair and regeneration by promoting cell proliferation and reducing apoptosis. There are different types of MSCs, and EVs derived from them are known to have vast properties. Bone marrow-derived MSCs-derived EVs have shown therapeutic potential in various applications, including tissue repair, immunomodulation, anti-inflammatory effects, and osteogenesis. EVs from adipose tissue-derived MSCs have demonstrated angiogenic effects and may contribute to anti-fibrotic processes. EVs from Wharton’s Jelly-derived MSCs have shown neuroprotective and anti-inflammatory effects [25]. EVs from umbilical cord blood-derived MSCs are recognized for their immunomodulatory effects and organ disorder-treating potential, making them relevant in autoimmune diseases and transplant settings [34]. EVs derived from placenta-derived MSCs also exhibit immunomodulatory potential and anti-inflammatory properties. Dental Pulp-Derived MSCs-derived EVs have regenerative properties and may carry factors promoting odontogenesis and dental tissue regeneration. EVs from synovium-derived MSCs may play a role in chondrogenesis and cartilage regeneration and may exhibit anti-arthritic effects [34, 35].
While there are similarities in the general characteristics of EVs derived from different types of MSCs, there can also be differences. Several factors and variables contribute to the heterogeneity of EVs across various MSC sources. For instance, the tissue origin of MSCs, as described above, may influence the cargo of EVs they release. The conditions under which MSCs are cultured, including the type of media, growth factors, and supplements used, can also impact the composition of EVs. Variations in culture conditions can influence the expression of specific proteins and nucleic acids in EVs. MSCs undergo multiple passages during in vitro expansion. The passage number of MSCs can affect the characteristics of both the parent MSCs and the released EVs [36]. Apart from such variations, MSCs can be subjected to various stimuli, such as pro-inflammatory cytokines, hypoxia, or other environmental cues, which can modulate the cargo of EVs, making them responsive to specific conditions and exhibit varied properties [37]. MSCs from different donors may also exhibit variations in their characteristics and, consequently, in the EVs they release. Different isolation techniques for EVs can yield varying results. Methods such as ultracentrifugation, size exclusion chromatography, and immunoaffinity capture can influence the purity and yield of EVs [38, 39].
EVs in the CNS are generated from various cell types, including neurons. The site of generation of EVs in the CNS can vary based on the specific cell type. Neurons, particularly in the CNS, release EVs from their axonal terminals. These vesicles, known as neuronal exosomes, are involved in interneuronal communication and may carry specific cargo related to synaptic function. EVs can also be generated in the somata (cell bodies) and dendrites of neurons. The cargo of these vesicles may include molecules associated with cellular maintenance and signaling. [40]. Astrocytes, a type of glial cell in the CNS, can release EVs from their endfeet and processes. These vesicles, known as astrocyte-derived exosomes, are involved in intercellular communication with neurons and other glial cells. Microglia, the resident immune cells of the CNS, release EVs primarily from their processes. Microglial exosomes play a role in immune regulation and communication with other cells in the CNS [41]. Oligodendrocytes, responsible for myelinating axons in the CNS, can release EVs from their myelinating processes. These vesicles may contain factors related to myelin maintenance and axonal support [42]. It is important to note that the study of EVs in the CNS is a rapidly evolving field, and ongoing research may provide more detailed insights into the specific mechanisms and functions of EVs released by different cell types in the brain. Additionally, hazardous protein aggregates linked to neurodegenerative diseases have been discovered to be carried by EVs. These abnormal protein aggregates are disseminated by EVs, which aids in the spread of neurodegenerative disorders and protein misfolding.
Apart from being released under normal physiological conditions, EV release has also been linked to stress conditions like oxidative stress [43], heat shock [44], and hypoxia [45]. Such conditions stimulate EV production, with or without altered cargo composition, potentially serving as a cellular response to different types of stress situations. Thus, EVs may have the potential to deal with different types of pathological and/or stress conditions with the aid of the cargo that they carry.
EVs have shown significant promise in the realms of many diseases, mainly neuroprotection and cancer therapy, according to studies by Park et al. [29] and Chen et al. [46]. EVs are ideal candidates for therapeutic and diagnostic uses due to the variety of cargo they contain. Due to their capacity to control growth, development, and intercellular communication, EVs have been demonstrated to have therapeutic potential in many cancer models and neurological disorders. They have been connected to improvements in cognitive and motor impairments, decreased neuropathology, and anti-inflammatory and neuroprotective characteristics [47]. Out of all the different subsets of EVs, exosomes have emerged to be the most studied, as most reports highlight their significant contribution to both normal physiological processes and disease pathogenesis. Understanding their functions and harnessing their therapeutic potential has implications for advancing medical research and developing innovative treatments.
Role of EV in Cell–Cell Communication
In the past two decades, there has been a lot of research done on EVs, which play a remarkable variety of roles in cell-to-cell communication. These vesicles, which are produced by almost all cell types, are filled with a variety of bioactive substances, including proteins, lipids, and nucleic acids like mRNAs and microRNAs. EVs can carry a plethora of messages to destination cells since the cargo composition varies based on the kind of cell and the physiological or pathological environment. EVs have a particularly crucial role in the CNS, where complex interplay between glia and neurons is necessary for a variety of biological processes. Direct cell-to-cell contact and the release of soluble substances are just two of the many ways that glial cells and neurons can communicate with one another. However, the identification of EVs as intercellular communication mediators has revealed additional information regarding the complexity and effectiveness of these interactions.
The discovery of EVs as crucial mediators of intercellular communication has opened exciting avenues of research in the field of cell biology and neurodegenerative diseases. These tiny vesicles serve as potent messengers, transmitting diverse functional molecules between cells in the CNS. The ability to create novel treatments for neurological disorders and other complex diseases is made possible by understanding the mechanics and roles of EV signaling.
Engineered EVs containing specialized medicinal compounds could be used as delivery systems for targeted medicines. By delivering therapeutic cargoes like regulatory miRNAs to cell populations, specially tailored EVs have the potential to treat a variety of diseases, including neurodegenerative disorders. In the following section, we will concentrate on the role of EVs in major neurodegenerative disorders and prioritize the diagnostic and potential therapeutic application of EVs in the brain (Fig. 2).
Role of Extracellular Vesicles (EVs) in Alzheimer’s Disease (AD)
EVs have emerged as a potential therapeutic tool and a major player in the pathophysiology of AD. They provide a promising route for the delivery of targeted drugs to the brain since they can easily cross the blood–brain barrier (BBB). In neurodegenerative diseases like AD, stem cell-derived EVs have demonstrated neuroprotective and immunomodulatory characteristics [48,49,50]. Studies have shown that EVs originate from MSCs that overexpress neprilysin, a protease that aids in the breakdown or degradation of Aβ, preventing plaque development in AD mouse models [51]. Additionally, MSC-derived EVs have demonstrated neuroprotective properties by defending and preventing neurons from oxidative stress and synaptic damage brought on by Aβ [52, 53]. The release of specific cargo, such as miR-29c-3p, which suppresses beta-site amyloid precursor protein cleaving enzyme 1 expression and stimulates the Wnt/β-catenin pathway in neurons, is thought to be responsible for this neuroprotection [54]. Furthermore, EVs produced from neural stem cells have demonstrated neuroprotective properties, including the restoration of fear extinction memory consolidation and a decrease in anxiety-related behaviors [55]. Increasing EVs in the brain through exercise is another potential therapeutic approach that has been proposed to combat cerebral illnesses like AD [56]. Interesting engineering techniques are being developed to improve the precise targeting of EVs for efficient medicinal interventions [57]. The necessary modifications can be made to the parent cells and then included in the produced EVs, enabling targeted administration [58]. Amyloid β (Aβ) plaques and hyperphosphorylated tau protein buildup in the brain to produce neurofibrillary tangles, which are the key pathological features of AD [59]. These pathogenic proteins have been linked to the spread of EVs. Studies have shown that β-cleavage of the amyloid precursor protein occurs in early endosomes, followed by the delivery of Aβ to multivesicular bodies within EVs. Exosomes generated from neurons and astrocytes have been recovered from the plasma of AD patients, and their contents have been shown to be altered. For example, neuron-derived exosomes (NDEs) from AD patients exhibited elevated levels of Aβ1-42, the toxic form of Aβ, suggesting a potential biomarker for AD. Additionally, it was discovered that AD patients have lower levels of cellular survival factors in NDEs, which may account for the reduced neuronal resistance to neurotoxic proteins. The quantities of complement proteins and pro-inflammatory substances found in astrocyte-derived exosomes (ADEs) from AD patients were also indicative of the disease progression [60]. These findings demonstrate the complex role of EVs in AD, exhibiting both neurotoxic and neuroprotective effects.
EVs have the potential to be used as diagnostic indicators for AD in the creation of biomarkers. Changes in Aβ1-42, cellular survival factors, and complement protein levels in NDEs and ADEs have been linked to the onset and staging of AD [60]. Dysregulated miRNAs in EVs have also been identified as potential biomarkers for AD. For instance, differential expression of miR-9-5p and miR-598 in exosome-enriched cerebrospinal fluid (CSF) samples from AD patients compared to controls has been observed [59]. Furthermore, a panel of plasma exosome-derived miRNAs has shown high accuracy in predicting the state of AD. A recent report highlighted a protein module containing an abundance of astrocyte-specific extracellular vesicle (EV) markers and exhibited a strong association with AD pathology and cognitive decline, indicating a crucial role in the progression of AD. The central protein within this module, integrin-β1, demonstrated a noteworthy increase in astrocyte-specific EVs isolated from total brain-derived AD EVs. Additionally, it was linked to the accumulation of brain β-amyloid and tau in separate cohorts [42]. Additionally, the study by Badhwar et al. demonstrated that brain-secreted EVs also contained tau protein, another critical component in AD pathology. Increased levels of phosphorylated tau (p-tau) were detected in EVs derived from AD patients, suggesting their potential role in carrying and transferring pathological forms of tau between cells [61]. The presence of abnormal tau is associated with the formation of neurofibrillary tangles, a characteristic feature of AD. Hence, EVs have emerged as important players in AD pathophysiology, offering therapeutic potential, diagnostic biomarkers, and insights into disease mechanisms. They hold promise for targeted drug delivery, neuroprotection, and immunomodulation. Additionally, EVs can serve as diagnostic indicators through the analysis of their cargo and miRNA profiles. Understanding the complex role of EVs in AD is crucial for the development of effective therapeutic strategies and early detection.
Role of Extracellular Vesicles (EVs) in Huntington’s Disease (HD)
Huntington’s disease (HD) is a progressive neurodegenerative disorder that results in the loss of neural cells in the caudate, putamen, and cerebral cortex, leading to motor, cognitive, and emotional impairments. According to Lee et al., the IT-15 (interesting transcript 15) gene’s dominant mutation increases the number of CAG repeats in the huntingtin protein’s polyglutamine (polyQ) sequence [3]. Over 100 additional proteins interact with the mutant huntingtin protein containing polyQ repeats, demonstrating its pleiotropic activities. Less than 35 repeats are present in healthy people, while more than 37 repeats are present in HD patients. The pathogenic gene IT15 produces the mutant huntingtin protein (mHTT), which has 3144 amino acids and a relative molecular weight of 350,000 Da. The presence of inclusion bodies and aggregates created by mHTT in the CNS’s neurons is the primary pathogenic alteration seen in HD. Zhang et al.’s experiment, using human embryonic kidney 293T cells as donor cells for EVs revealed that the released EVs contained polyQ and RNA with CAG repeats, indicating that EVs may have the ability to transmit the repeated RNA of a toxically amplified trinucleotide from one cell to another. Although further research is required to be sure, these EVs may act as biomarkers for disease states and therapy responses. The hallmark of HD pathology is the formation of intraneuronal fibrillar aggregates of mutant huntingtin protein, forming intracellular inclusion bodies. Like other amyloid-related neurodegenerative diseases, the abundance of polar glutamine renders huntingtin proteins prone to aggregate by adopting a β-sheet-rich conformation. Wild-type proteins are recruited to these aggregates by mutant huntingtin protein. The presence of inclusion bodies with altered huntingtin protein has not been directly linked to neurotoxicity; rather, they may act as a protective mechanism. Nevertheless, the buildup of protein aggregates can impair neuronal function and the reduction of normal huntingtin protein may possibly be a factor in the pathophysiology of the disease [62]. In addition, NfL in plasma is a well-studied prognostic blood biomarker of disease onset and progression in HD [63] and its level differs significantly across cohorts [64].
Emerging evidence indicates that EVs play a significant role in the pathogenesis of HD, providing valuable insights into disease progression and potential therapeutic strategies. EVs have been attributed to the transmission of HD pathogenesis and the intercellular growth of mHTT. According to studies, EVs produced from mHTT-expressing cells can transmit the protein to recipient cells, causing intracellular aggregation and aiding in the spread of the illness. The probability of a cell-to-cell spread of pathogenic proteins, which would affect the development and course of HD, is increased by this EV-mediated transmission mechanism. In HD, EVs are also employed to carry additional cargo, such as miRNAs associated with the condition. According to research by Abels et al. [65], EVs from HD patient-derived cells contained disease-associated miRNAs that might be transferred to recipient cells to affect gene expression and contribute to HD pathogenesis. These miRNAs have the power to affect several cellular functions, including synapse function, neuronal survival, and neuroinflammation, all of which are dysregulated in HD. Thus, the cargo carried by EVs can influence disease progression and serve as potential targets for diagnosis and therapy. Recent advancements have explored the therapeutic potential of engineered EVs for HD. The modification of EVs produced from MSCs to overexpress neprilysin, a protease implicated in the destruction of amyloid-beta protein, is one strategy. According to Katsuda et al. [51], these modified EVs had increased amyloid-beta-degrading activity, which reduced plaque formation in HD animal models. The potential for effective HD treatment approaches lies in the tailored administration of medicinal compounds via EVs. Exercise-induced EVs have also become recognized as possible HD treatment options. Recent research indicates that EVs generated during exercise may contribute to the positive benefits of exercise on HD pathogenesis. According to Zhang et al. [56], exercise raised the concentrations of EVs in the brain and peripheral circulation, which facilitated the transfer of advantageous molecules and miRNAs that support neuroprotection and lessen neuroinflammation. Harnessing the therapeutic potential of exercise-induced EVs may provide new avenues for intervention in HD.
Role of Extracellular Vesicles (EVs) in Parkinson’s disease (PD)
Parkinson’s disease (PD) is a progressive neurodegenerative disorder characterized by the loss of dopaminergic neurons in the substantia nigra and the presence of intracellular protein aggregates called Lewy bodies. Even though the actual cause of PD is still unknown, mounting evidence points to a critical role for EVs in the pathogenesis and development of the illness. A variety of cell types, including neurons, astrocytes, microglia, and oligodendrocytes, release tiny membrane-bound vesicles known as EVs. The transfer of proteins, lipids, and nucleic acids between cells is made possible by these vesicles, which also allow for the exchange of molecular data and the functional modulation of recipient cells. Numerous in vitro and in vivo studies have demonstrated the release of α-synuclein (α-syn)-containing EVs from pathogenic processes occurring in the cells, such as poor proteasomal and mitochondrial dysfunction, lysosomal malfunction, and suppression of glucocerebrosidase 1 activity [66]. It has been established that these EVs transport α-synuclein in both monomeric and oligomeric forms, enabling the development of α-synuclein disease throughout the nervous system. The release of EVs containing α-syn is increased when intracellular protein trafficking through lysosomes is blocked, confirming the role of lysosomal dysfunction in PD pathology. Studies have shown that α-syn can be released into the extracellular space via EVs, facilitating its intercellular transfer and propagation. For instance, Loria et al. [67] demonstrated that EVs derived from neuronal cells carrying pathological α-syn could induce α-syn aggregation and neurotoxicity in recipient cells. It is presumed that EVs secreted by cells in pathological states carry and spread misfolded, toxic, pathological molecules, including α-syn, which plays a central role in PD. Additionally, EVs released from microglia and astrocytes have been shown to contribute to α-syn propagation and neuroinflammation in PD. These findings highlight the potential of EVs as mediators of protein aggregation and disease progression in PD. Furthermore, EV-bound α-syn has been shown to induce oligomerization, which was evident from the ability of α-syn in EVs isolated from the CSF to promote oligomerization in a dose-dependent manner. The presence of high levels of gangliosides (GM1 and GM3) within EVs has been suggested to make α-syn more prone to aggregation. Studies carried out in vitro on SH-SY5Y neuroblastoma cells in culture have shown that EVs produce both monomeric and oligomeric forms of α -syn. It’s significant because EVs containing α-syn are absorbed by recipient cells more effectively than soluble α-syn in conditioned medium, resulting in neurodegeneration. The immune cells in the brain known as microglia have also been linked to the spread of α-synucleinopathy via EVs. When microglia are subjected to human α-syn preformed fibrils, they release EVs that include α-syn, which cause α-syn aggregation in recipient neurons. These EVs further enhance α-syn aggregation in neurons when proinflammatory cytokines, such as Tumor Necrosis Factor-α (TNF-α), Interleukin-1 (IL-1), or Interleukin-6 (IL-6), are present. Direct injection of EVs from microglia treated with phosphorylated phospho α-syn into the mouse striatum causes dopaminergic cell degeneration, motor impairments, and phosphorylated phosphor α-syn aggregation in multiple brain areas linked to the striatum. Additionally, it has been revealed that microglia-derived EVs observed in the CSF of PD patients contain α-syn oligomers that can trigger α-syn aggregation in neurons [68].
Additionally, EVs have been linked to the transmission of genes carrying genetic mutations or variations that increase the likelihood of developing PD. For example, EVs can release mutant leucine-rich repeat kinase 2 (LRRK2), associated to late-onset PD. Increased genetic risks for PD have been linked to the tau gene microtubule-associated protein tau, which is involved in AD’s etiology. Increased EV release and extracellular α-syn levels may also be caused by the mutant vacuolar protein sorting-associated protein 35, which has been linked to late-onset autosomal dominant PD. The identification of reliable biomarkers for early PD diagnosis is crucial for timely intervention and disease management. EVs carrying disease-related proteins and miRNAs have emerged as potential biomarkers for PD. Characterization of EVs isolated from the urine, blood, saliva, or CSF of PD patients has shown promising results in predicting disease progression and facilitating the application of disease-modifying treatments. For example, studies have explored the potential of α-syn as a biomarker for PD using EVs isolated from the CSF of patients. It has been found that proteins associated with PD, such as DJ-1 and LRRK2, have been detected in EVs from PD patients’ CSF. Additionally, EVs isolated from urine samples of PD patients have been shown to contain α-syn and other PD-related proteins, suggesting their potential as non-invasive biomarkers for PD [69]. EVs can transport miRNAs, proteins, and lipids that are particular to a certain disease, making them a prospective source of diagnostic biomarkers. Numerous studies have shown that EVs from PD patients have different miRNA profiles than EVs from healthy controls [70]. For instance, Gui et al. found elevated levels of miR-19b and miR-29c in EVs from PD patients, and these levels were correlated with the severity of the disease [70]. The unique characteristics of EVs, including their innate potential to cross the BBB and their cargo-loading capacity, make them desirable candidates for therapeutic delivery in PD. The therapeutic potential of EVs in PD models has been investigated in a number of trials. For instance, Cooper et al. showed that in a preclinical PD model, EVs generated from MSCs could reduce neuroinflammation and safeguard dopaminergic neurons [71]. Moreover, EVs designed to carry particular cargo, including neurotrophic factors or anti-inflammatory agents, have shown promise in reducing PD-related pathology [72]. Similar to this, EVs generated by activated glial cells that carry inflammatory mediators can spread and prolong the inflammatory response, exacerbating neuroinflammation and facilitating the development of disease. These findings indicate that the analysis of EVs in body fluids holds promise for the development of reliable prognostic markers for PD, and EV-based therapeutic strategies enabling early detection and intervention. Further research in this area is needed to validate and optimize the use of EVs as biomarkers in clinical settings.
Role of Extracellular Vesicles (EVs) in Amyotrophic Lateral Sclerosis (ALS)
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disorder characterized by the degeneration of motor neurons in the brain and spinal cord. Accumulation of aberrant proteins, mitochondrial dysfunction, glutamate excitotoxicity, oxidative stress, and neuroinflammation are all factors in the pathogenesis of ALS. Super Oxide Dismutase1 (SOD1), Transactive Response DNA Binding Protein, Heterogeneous nuclear ribonucleoproteins A2/B1/A1, senataxin, profilin1, TATA-binding protein associated factor 15, ataxin2, C9ORF72, ubiquilin-2, optineurin, valosin-containing protein, and Fused in Sarcoma (FUS) genes are among those with ALS-related gene abnormalities [48]. EVs have become key players in the pathophysiology of ALS. They are microscopic, membrane-bound vesicles that are expelled by cells and contain a variety of biomolecules, such as proteins and nucleic acids. Studies have demonstrated that EVs contribute to the development of the disease by spreading toxic proteins linked to ALS, including as SOD1, TAR DNA-binding protein 43 (TDP-43), and FUS [73]. It has been determined that the primary sources of EVs harboring pathogenic ALS proteins are neurons and astrocytes. It has been discovered that EVs made from astrocytes from ALS patients exhibit a neurotoxic profile, which causes motor neuron degeneration. Axonal development, neuronal survival, and inflammation are all impacted by dysregulated miRNAs, including miR-494-3p and miR-124, which have been found in EVs produced in patients with ALS [48]. In addition to their role in protein and miRNA transfer, EVs have shown potential as biomarkers for ALS. EVs produced from ALS patients have been found to have altered miRNA profiles, showing that they may have diagnostic relevance. Based on their differential expression in EVs produced from ALS patients, miR-218, miR-155, and miR-124 have been identified as possible ALS biomarkers.
Furthermore, EVs have also demonstrated neuroprotective qualities in ALS when produced from other cell types, including MSCs and NSCs. It has been discovered that MSC-derived EVs can influence immunological responses, lessen oxidative stress, and increase motor neuron viability. By encouraging neuronal survival and lowering neuroinflammation, EVs generated from NSCs overexpressing the insulin-like growth factor 1 receptor have been found to alleviate the neurologic impairments in ALS mice models [48]. However, there are still a number of significant issues in EV-mediated ALS studies that require attention. For the creation of efficient therapeutic treatments, it is necessary to comprehend the delicate balance between the removal of intracellular harmful proteins and the possible detrimental effects of their uptake by surrounding cells. To find effective targets for preventing the propagation process, it is important to explore the interactions between surface proteins on EVs and the plasma membranes of motor neurons. Further research is required to characterize non-neuronal EV cargos and analyze EVs originating from motor neurons in order to better comprehend the phenotypic impacts and the entire communication network in ALS [73]. Researchers have been investigating the therapeutic potential of EV manipulation for the treatment of ALS in recent years. Techniques include creating EVs to target particular cell types or locations of the CNS, altering the cargo of EVs to increase their neuroprotective qualities, and using EVs as delivery systems for therapeutic compounds. In preclinical research, loading EVs with particular miRNAs or anti-inflammatory drugs, for instance, has demonstrated promise for attenuating ALS-related pathology and enhancing motor function. Additionally, EVs derived from stem cells have received considerable attention in ALS research. By encouraging neuroprotection, lowering inflammation, and improving motor neuron lifespan, EVs generated from various stem cell sources, such as MSCs and NSCs, have shown therapeutic effects in ALS models [48]. These stem cell-derived EVs can be modified to enhance their therapeutic potential by incorporating specific molecules or engineering them to target affected regions in the CNS. Despite the progress made in understanding the role of EVs in ALS and their potential as therapeutic tools, there are still challenges and limitations that need to be addressed. It is crucial to standardize EV isolation and characterization techniques in order to compare research results and apply them in clinical settings. Additionally, optimizing EV cargo loading and delivery techniques as well as addressing issues about the security and potential negative effects of EV-based therapies are crucial factors to consider.
Role of Extracellular Vesicles (EVs) in Prions Disease
Prion diseases, also known as transmissible spongiform encephalopathies, are rare and fatal neurodegenerative disorders characterized by the accumulation of abnormal prion proteins in the CNS. EVs have been implicated in the propagation and spread of abnormal prion proteins, serving as vehicles for their intercellular transfer and dissemination. EVs can release abnormal prion proteins, or PrPSc (scrapie Prion protein), into the extracellular environment, promoting their transmission to nearby cells. It has been demonstrated that EVs generated from prion-infected cells include PrPSc, which can cause the transformation of PrPC (cellular prion protein) into the pathogenic PrPSc form. In vitro and animal studies have shown that EVs can transfer PrP-Sc, indicating their potential role in the spread of prion proteins. Studies have also shown that EVs play a significant role in the CNS accumulation and amplification of aberrant prion proteins. It has been shown that EVs released from prion-infected neuronal cells spread PrPSc to recipient cells, facilitating the transformation of PrPC into the pathogenic variant. EVs produced from prion-infected microglia have also been connected to the spread of prion pathology, promoting neuroinflammation and the progression of disease. The structure of PrPSc, the isoform associated with disease, is different from that of PrPC. Generally found on the cell surface, PrPC is a glycosylphosphatidylinositol-anchored protein, whereas PrPSc has an aberrant β-sheet-rich conformation and aggregation propensity.
EVs not only contribute to prion protein propagation but may also participate in prion protein clearance. EVs released by astrocytes and microglia have been shown to facilitate the removal of PrPSc from the CNS by promoting its degradation within lysosomes. This clearance mechanism serves as a protective response against prion accumulation and neurotoxicity. In physiological and pathological conditions, the association of PrPC with EVs is crucial. A recent study found that brain-derived EVs have elevated levels of enriched prion protein, which increased following transient ischemia [74]. Additionally, in the same study, brain EVs from wild-type and PrPC knockout mice were extracted, and the EV absorption by primary neurons, astrocytes, and microglia from the PrPC knockout mouse was substantially quicker and more effective. This suggests that the association of PrPC with EVs is crucial for the pathophysiological and physiological conditions that may be the target of therapeutic intervention. A hydrophilic interaction between the EV prion protein and the neuronal prion protein is necessary for the majority of EVs to migrate along the neuronal surface, according to a recent study focusing on astroglial communication and the movement of EVs [75]. Additionally, it has been discovered that PrPC-associated EVs isolated from human plasma have anti-inflammatory effects on macrophages, indicating their potential therapeutic significance [76]. Experiments involving exosomes from prion-infected neuronal cells have shown that these exosomes have the capacity to transmit infectivity to healthy cells, both neuronal and non-neuronal, in culture. Studies conducted in vivo have demonstrated that injecting mice with exosomes expressing PrPSc causes the onset of clinical prion disease. Prion infectivity has been found in EVs isolated from a variety of biofluids, such as blood, milk, urine, and CSF, indicating that EVs may be an important way of disease transmission. Recently in sCJD (Sporadic Creutzfeldt–Jakob disease) cases, PrPTSE (transmissible spongiform encephalopathy-associated prion protein) was discovered to accumulate in the eye, raising the possibility that tear EVs from prion-infected patients could also contain prion infectivity [77]. Using in vitro models, it was discovered that the ESCRT-dependent and independent routes of exosome release were related to cellular prion infectivity and exosomal release of prion infectivity. Additionally, multiple prion strains that exhibit distinct differential release of PrPTSE linked with exosomes were identified using the transgenic RK13 cell line expressing ovine, mouse, and vole PrPC. This work showed that the majority of prion infectivity was linked to exosomes from conditioned medium containing prion particles, and that exosomes from RK13 infected with ovine 127 S released 20–40 times more PrPTSE than those from murine 22 L and vole prions. Together, these studies imply that EVs may be a significant source of prion infectivity and emphasize the potential for new insights into dysregulated biochemical processes and disease processes by a deeper understanding the mechanisms of prion EV cargo loading and cellular uptake.
EVs have also been studied as a potential source of prion biological markers. They can be separated from readily available biofluids like blood plasma and serum and carry a variety of biomolecules, including proteins, lipids, and microRNAs (miRNAs). It has been discovered that EVs derived from the CNS carry brain-related miRNAs, pointing to their potential as diagnostic biomarkers. The potential of EVs as carriers of prion-related compounds and biomarkers for prion pathologies has been suggested by several studies. For instance, it has been demonstrated that PrPSc, the aberrant version of the prion protein, is present in EVs generated from prion-infected neuronal cells. Fevrier et al. showed that PrPSc-associated exosomes are released by prion-infected cells, indicating their role in the dissemination of prion pathology [78]. Investigations have also been done towards PrPSc detection in EVs that have been extracted from biological fluids. PrPSc was found in EVs made from the plasma of prion-infected mice. Similar to this, PrPSc-containing EVs have been found in the CSF of individuals with Creutzfeldt-Jakob disease (CJD). Thus, EVs have a variety of functions in prion disorders. They enhance intercellular communication, aid in the spread and dispersal of aberrant prion proteins, may help clear out prion proteins, and show promise as a source of biomarkers for early disease detection and monitoring. Further investigation into the mechanisms governing the roles and payload of prion EVs may shed light on the pathophysiology of prion disease and suggest new therapeutic targets.
Diagnostic and Potential Therapeutic Applications of EV in Brain
EVs have a significant role in the treatment of some cancers and neurological diseases. Proteins and nucleic acids, which are indicative of the pathological alterations in their parent cells, are among the disease-specific cargo carried by these tiny vesicles. They make good candidates for non-invasive biomarkers due to their capacity to pass through numerous biological barriers and circulate in bodily fluids. In this context, we will focus on the potential diagnostic and therapeutic applications of EVs in AD, HD, PD, ALS and Prion disease. EVs provide useful insights for early detection, disease monitoring, and prognosis, bringing hope for more effective and personalized diagnostic and therapeutic approaches.
Diagnostic Applications
Alzheimer’s Disease
For effective treatment and disease control, AD must be diagnosed as early as possible. Owing to its ability to encapsulate and transport a variety of disease-specific chemicals, EVs have emerged as promising candidates for AD biomarkers. These insights into the pathophysiology of AD are extremely helpful. In addition to the well-known biomarkers Aβ42 and p-tau181, scientists have looked at the diagnostic value of other cargo elements found in EVs. The protein known as neurofilament light chain (NfL), which is mostly present in nerve cells, is one such element. As a result of axonal injury and neurodegeneration, NfL is released into the extracellular space, making it a possible biomarker for several neurological conditions, including AD. A study conducted by Winston et al. investigated the levels of NfL (Neurofilament light chain) in EVs isolated from both plasma and CSF of AD patients [79]. Comparing AD patients to healthy controls, the scientists found that AD patients’ EVs had higher levels of NfL. NfL levels in EVs also showed a correlation with disease severity, indicating its usefulness as a biomarker for tracking the development of AD. Synaptic proteins have also drawn investigation as potential EV-based biomarkers for AD in addition to NfL. AD is characterized by synaptic dysfunction, and changes in synaptic proteins may be a sign of early neuronal injury. Synaptic proteins are carried by EVs generated from brain cells, and they can offer important insights into the function and health of synapses in AD. Further studies are ongoing to validate the utility of these EV cargo components, including NfL and synaptic proteins, as reliable biomarkers for AD diagnosis and progression. The accuracy and sensitivity of AD detection may be increased by combining numerous biomarkers within EVs, allowing for early intervention and better patient outcomes.
Huntington’s Disease (HD)
A mutation in the huntingtin gene (HTT), which results in the synthesis of mutant huntingtin protein, causes HD, a fatal neurological illness (mHTT). Progressive motor dysfunction, decreasing cognitive function, and mental symptoms are the hallmarks of HD. For the treatment of the disease and prospective therapeutic approaches, an early and precise diagnosis of HD is essential. EVs, which can transport illness-specific cargo like mHTT and reflect the pathophysiology of the disease, have become potential diagnostic biomarkers for HD. Investigation of mHTT levels in various biofluids, including plasma, CSF, and urine, is one of the key subjects of HD research. These biofluids’ EVs have been investigated for their potential as mHTT and other disease-related molecule carriers. In a 2016 study, Busch et al. investigated the amounts of mHTT in plasma-derived EVs from HD patients [80]. In contrast to healthy controls, the researchers discovered higher quantities of mHTT in the plasma exosomes of HD patients. This study suggests that plasma exosome mHTT has the potential to serve as a biomarker for the diagnosis of HD. Additionally, studies have examined the levels of mHTT in EVs taken from CSF. The results have been mixed, and further research is needed to substantiate CSF exosome mHTT as a meaningful HD biomarker. Apart from mHTT, NfL is also a diagnostic biomarker for detecting the onset and progression of HD [63]. Researchers are investigating additional cargo elements within EVs, such as microRNAs and other disease-specific proteins, that could function as possible biomarkers for HD diagnosis and disease progression in their ongoing search for accurate EV-based biomarkers in HD.
Parkinson’s Disease (PD)
PD, a progressive neurodegenerative disorder, is characterized by tremors, bradykinesia, rigidity, and postural instability. For early treatment and disease management, a timely and correct diagnosis of Parkinson’s disease is essential. EVs, which have the capacity to contain illness-specific cargo reflecting the pathophysiology of the disease, have become recognized as potential diagnostic biomarkers for PD in recent years. α-syn levels in EVs from different biofluids, such as plasma, CSF, saliva, and urine, have been one of the main areas of interest in PD research. α-syn is a crucial protein in the pathophysiology of PD, and the disease is characterized by the aggregation of this protein [81] investigated the concentrations of α-syn in EVs generated from plasma of PD patients. When compared to healthy controls, PD patients had considerably greater plasma exosome α-syn levels, which suggests that this molecule may one day serve as a diagnostic biomarker for the disease. The study also found an inverse relationship between plasma exosome α-syn levels and illness severity, suggesting that this compound may serve as a marker for the development of the disease. The diagnostic potential of salivary EVs in PD was examined in a different study by Cao et al. [82]. In saliva-derived EVs from PD patients, the researchers discovered α-syn oligomers, which may be used as a diagnostic marker for the disease. Saliva collection is a convenient and non-invasive method for discovering possible PD biomarkers. To diagnose PD, α-syn levels in CSF-derived EVs have also been studied. In contrast to healthy controls, PD patients have higher quantities of p-tau and α-syn in their CSF exosomes, according to a study by Goetzl et al. [60]. These results point to the possibility of p-tau and CSF exosome α-syn as PD diagnostic indicators. The diagnostic value of urine-derived EVs in PD has also been studied. Ser(P)-1292 LRRK2, a phosphorylated version of LRRK2, was found in urine exosomes and may be used as a diagnostic marker for male PD patients, according to a study by Wang et al. [83]. Researchers have examined into microRNAs (miRNAs) in EVs as potential diagnostic biomarkers for PD in addition to α-syn. It has been discovered that a number of miRNAs, including miR-1, miR-19b-3p, miR-153, miR-409-3p, and miR-10a-5p, are differentially expressed in the CSF and plasma exosomes of PD patients, suggesting that they may one day serve as diagnostic markers for the disease. EVs provide PD biomarker research with a potentially positive beginning. The development of non-invasive and sensitive techniques for early PD detection and disease monitoring holds considerable promise for the study of α-syn, miRNAs, and other disease-specific molecules inside EVs from various biofluids. The clinical relevance of EV-based biomarkers in PD diagnosis and therapy, however, requires additional validation and larger-scale investigations.
Amyotrophic Lateral Sclerosis (ALS)
The devastating neurodegenerative disorder ALS, often known as Lou Gehrig’s disease, is characterized by the gradual degradation of motor neurons, which causes muscle weakness, paralysis, and, finally respiratory failure. For the appropriate medical interventions and therapies to be started, an early and precise diagnosis of ALS is essential. Due to their capacity to encapsulate illness-specific cargo, like as proteins and nucleic acids, which can represent the pathophysiological changes associated with the disease, EVs have recently come to be recognized as potential diagnostic biomarkers for ALS. Research of disease-related proteins, in particular SOD1, in EVs from CSF and other biofluids has been one of the main areas of attention in ALS research. SOD1 levels in CSF-derived exosomes from ALS patients were examined in a study by Zea Roca et al. [84]. SOD1 has the potential to serve as a diagnostic biomarker for ALS, according to the researchers, who discovered higher levels of SOD1 in CSF exosomes of ALS patients compared to controls. SOD1 is a crucial protein linked to the pathology of ALS, and its presence in EVs may be an indicator of the disease’s progression in the brain. Other research studies have investigated the ability of miRNAs in EVs to diagnose ALS in addition to SOD1. Small non-coding RNAs called microRNAs are crucial in post-transcriptional gene regulation and have been implicated in various disease processes, including ALS. The possibility for miRNAs to serve as ALS diagnostic markers was highlighted by a study by Freischmidt et al. [85] that found differently expressed miRNAs in CSF-derived exosomes of ALS patients. Additionally, analysis of plasma-derived EVs revealed particular miRNAs, including miR-338-3p and miR-1246, as potential diagnostic indicators for ALS [86]. In addition to CSF and plasma, urine-derived EVs have also been investigated for use as ALS biomarkers. In a 2019 study, Katsu et al. [87] examined urine exosomes and found that phosphorylated TDP-43 (p-TDP-43), a crucial pathogenic protein in ALS, was present at higher levels in ALS patients compared to controls. These results raise the possibility of using urinary exosome p-TDP-43 as a non-invasive ALS diagnostic test. Together, EVs offer a viable method for locating ALS biomarkers. EVs from diverse biofluids can be used to study disease-related proteins, miRNAs, and other potential disease-specific molecules, which may lead to the development of non-invasive, sensitive ALS diagnostic tools. To establish the clinical relevance of EV-based biomarkers in the diagnosis and treatment of ALS, more validation and larger-scale research are required. Additionally, there is ongoing research into the utilization of EVs as delivery systems for possible therapeutic drugs in ALS, opening new possibilities for the development of focused therapies for this debilitating neurodegenerative condition.
Prions Disease (PrD)
In a study by Liu et al., the idea of designing EVs for focused treatment is further demonstrated [88]. It was shown by the researchers that EVs produced from astrocytes transfected with a miR-29 mimic may successfully lessen brain ischemia-reperfusion injury in rats. Nuclear factor kappa B/nucleotide-binding domain and leucine-rich repeat-containing family, pyrin domain-containing 3 were downregulated in order to achieve this. This proof of concept implies that EVs can be customized for treatment by adding particular microRNAs, a method that offers scalability and minimally intrusive delivery and holds tremendous promise for treating PrD. EVs offer an effective line of research in the specific scenario of PrD when efficient therapeutic strategies are desperately needed. Minikel et al. have demonstrated the use of prion protein lowering antisense oligonucleotides (ASOs) as a recently developed technique [89]. According to the study, ASOs significantly reversed the illness phenotype and enhanced longevity across different prion strains in a dose-dependent manner by downregulating the amounts of prion protein. Despite drawbacks with nucleic acid-based treatments, including poor biodistribution and instability, these problems are solved by taking advantage of EVs’ intrinsic qualities. EVs are a very promising option for delivering ASOs in PrD treatment because they have a better bioavailability and can be tailored for targeted administration. In addition, because ASOs are only ten nucleotides long, they are perfect candidates to be engineered into EVs using methods like electroporation. This guarantees a less invasive method of delivering medicinal substances to the brain while also improving their efficiency. Extracellular vesicles—exosomes in particular—show tremendous promise as therapeutic vehicles for treating the intricacies of neurodegenerative illnesses like Parkinson’s disease. EVs’ capacity to overcome biological barriers, control immune responses, and transport customized payloads makes them adaptable instruments for focused therapies. In addition to offering hope for improving treatment approaches, the current investigation of EVs in PrD opens the door to more individualized and efficient medicinal interventions in the upcoming years.
Potential Therapeutic Application
Alzheimer’s Disease (AD)
Due to their capacity to carry disease-specific cargo that reflects the underlying pathophysiology of AD, EVs in the setting of AD have demonstrated significant potential as diagnostic biomarkers. For instance, studies have looked at the components of EVs’ cargo, such as tau and Aβ, to find changes that correspond with the development of the disease. Notably, higher amounts of Aβ42 and tau phosphorylated at threonine 181 (p-tau181), which are important clinical markers of AD, have been detected in CSF-derived exosomes from AD patients [60]. Additionally, the diagnostic potential of other EV cargo parts such synaptic proteins and NfL chain has been studied. NfL-carrying EVs have been linked to neurodegeneration in AD, offering prospective biomarkers to gauge the severity and progression of the condition [78].
Huntington’s Disease (HD)
EVs show promise as RNA interference therapy delivery systems in HD. A CAG repeat expansion in the HTT, which results in the production of mHTT protein, is the cause of HD. Studies have used EVs to deliver siRNAs that target mHTT in order to reduce its expression in neurons. In HD mice models, Wang et al. [90] showed that EVs loaded with mHTT-targeting siRNA efficiently decreased mHTT protein levels in neuronal cells and attenuated disease-related neurodegeneration. To sustain neuronal survival and enhance motor function in HD animals, EVs can also carry neuroprotective substances, including brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF) [91].
Parkinson’s Disease (PD)
In PD, EV-based treatments have shown promise for neuroprotection and disease modulation. Studies have investigated the utilization EVs produced from MSCs to deliver neuroprotective factors (MSCs). For instance, miR-133b-3p-enriched MSC-derived EVs protected dopaminergic neurons from -synuclein-induced toxicity, indicating their potential as a PD treatment [26]. Additionally, EVs may be used therapeutically to lessen the burden of toxic α-synuclein aggregates in PD by facilitating their clearance through the autophagy-lysosome pathway [92]. Additionally, anti-inflammatory substances might be added to EVs from immune cells like microglia to lessen neuroinflammation and safeguard dopaminergic neurons [56].
Amyotrophic Lateral Sclerosis (ALS)
Researchers have looked at the neuroprotective and immunomodulatory effects of EVs in ALS. In an ALS animal model, EVs made from MSCs that were loaded with vascular endothelial growth factor increased motor neuron survival and slowed disease progression [93]. Additionally, in ALS mice models, regulatory T cells (Tregs) decreased neuroinflammation and promoted neurogenesis, suggesting the therapeutic potential of these cells in slowing the progression of the disease [94]. Furthermore, it is possible to modify gene expression and cellular responses related to ALS development by engineering EVs to transport particular microRNAs (miRNAs) [95].
Prions Disease (PrD)
EVs, in particular exosomes, have become a potential area of study in the field of biomedicine because of their ability to operate as both functional entities that transport signals to destination cells and carriers of biological substances for protection. Their therapeutic potential has garnered considerable interest due to their versatility, particularly in the context of neurodegenerative disorders such as prion diseases (PrD). A notable area of research explores the potential of EVs produced from MSCs to treat neurodegenerative diseases. In mice models of multiple sclerosis, a recent study [89] demonstrated the therapeutic effectiveness of intravenously delivered EVs produced from human placenta-derived MSCs. Increased myelination, less DNA damage, and better impulse control were the outcomes of this intervention. These results highlight EVs’ capacity to negotiate the complexities of neurodegenerative pathogenesis. Comparably, another study examined the immunomodulatory properties of human bone marrow-derived MSC-derived EVs. The study showed that intra-arterial injection of MSC-derived EVs reduced neuroinflammation in rats with focal brain damage that had been generated. This demonstrates how EVs can cross biological barriers like the blood-brain barrier and regulate the immune response within the brain (BBB). These qualities make EVs attractive delivery systems for medicines intended to target particular proteins linked to PD (PrD), like the prion protein (PrPC). Moreover, EVs’ therapeutic potential includes treating issues unique to PrD. Researchers investigated how mice’s neuroinflammation and cognitive performance were affected by EVs produced from human neural stem cells [88]. The findings showed that injecting these EVs intravenously to immunocompetent mice improved their cognitive performance and reduced neuroinflammation. Notably, the protective effects of miR-124 were found to be mostly dependent on it, demonstrating the possibility of creating engineered EVs through the transfection of cells with particular microRNAs. This method might provide therapeutic miRNAs to enrich EV cargo, offering a customized approach to treating PrD.
Emerging Technology on Detection of EV in Brain
Emerging technologies are further propelling our understanding of EV biology and its role in brain function and neurological disorders. The imaging of EVs at almost atomic resolution using cryo-electron microscopy provides information about their structural variety and cargo arrangement [96]. Technologies for RNA modification profiling offer a thorough perspective of RNA alterations in EV-derived RNAs, highlighting their selective packing and regulatory functions [97]. With the development of CRISPR/Cas9 genome editing in EVs, there are now more options for modifying their contents and functions. With the aid of this technology, researchers can modify EVs to contain therapeutic compounds, making them effective tools for directing therapies in neurological illnesses. Additionally, single EV sorting and modification methods allow for the isolation and examination of single EVs, revealing details about their heterogeneity and functional dynamics [98]. With the aid of in vivo imaging techniques like intravital microscopy and bioluminescence imaging, EVs can be seen and tracked in real-time in living animal models, revealing important details on their biodistribution and interactions with neuronal cells in the whole brain environment. High-resolution imaging of EVs in the brain is made possible by fluorescence nanoscopy, which uses methods like stimulated emission depletion microscopy and stochastic optical reconstruction microscopy [99]. Through techniques that go above the diffraction limit, it is now possible to study EV-mediated signaling and connections between neurons and glial cells as well as EV distribution and cargo in cellular and subcellular compartments.
The investigation of EV cargo has been revolutionized by mass spectrometry-based proteomics, which has produced invaluable data concerning the protein makeup of EVs obtained from the brain [100]. The finding of possible biomarkers for neurological illnesses is made easier by the identification and quantification of EV-associated proteins using advanced MS methods. Quantitative proteomics also sheds light on EV-mediated cellular responses in the brain by revealing changes in the EV proteome under various physiological situations. A non-invasive method to examine EVs in biofluids including blood and CSF is using liquid biopsies. Microfluidic platforms, immune-affinity capture, and size exclusion chromatography are some of the methods that make it possible to separate and enrich brain-specific EVs from complicated biofluids [101]. As EV-derived biomarkers in biofluids may represent brain diseases, liquid biopsies hold potential for the diagnosis and monitoring of neurodegenerative disorders. Another significant study by Chen et al. in 2023 [46] focused on exploring the diagnostic potential of brain-specific EVs in the CSF for early detection of multiple sclerosis (MS). Utilizing liquid biopsy techniques, the researchers identified specific EV biomarkers that could serve as early indicators of MS onset and disease progression. This discovery holds great promise for the development of non-invasive and sensitive diagnostic tools to detect MS at an early stage, enabling timely intervention and management. By thoroughly assessing the RNA content of brain-derived EVs, EV RNA-Seq may identify the presence of mRNAs, microRNAs, and other small RNAs [102]. This method sheds light on the possibility of functional RNA cargo being transferred from EVs to destination cells, thereby altering gene expression and biological functions. Finding EV-specific non-coding RNAs with regulatory functions in illness and neural development is made easier with the help of EV RNA-Seq.
In a study conducted by Kim et al. in 2023 [103], bioluminescence imaging was employed to track the biodistribution of EVs in the brain in real time. By introducing bioluminescent EVs into live animal models, the researchers gained valuable insights into the spatial and temporal distribution of these vesicles in different regions of the brain. The findings shed light on the intricate trafficking and interactions of EVs with neural cells, providing a deeper understanding of their role in intercellular communication within the CNS.
In addition to the above studies, a recent investigation by Li et al. in 2023 [104] delved into the therapeutic applications of engineered EVs for the treatment of AD. By packing EVs with therapeutic cargo including anti-inflammatory chemicals and neuroprotective medicines, the researchers showed that these customized EVs can penetrate the BBB and target certain brain regions affected by AD. With the help of this study, new directions in the development of precision medicine strategies for the treatment of neurodegenerative illnesses are now possible. Furthermore, Rodriguez et al. proposed a study in 2023 that looked at the function of EVs in promoting neuronal healing following traumatic brain damage (TBI) [105]. The essential signaling molecules and microRNAs involved in supporting neuro-regeneration and tissue repair were found by the researchers by analyzing the cargo of EVs generated by activated glial cells in response to TBI. These results offer insightful information on the potential therapeutic use of TBI. The combination of these emerging technologies with the increasing knowledge of EV biology is advancing our understanding of the roles of EVs in brain development, synaptic plasticity, neural repair, and disease pathogenesis. As these techniques continue to evolve, they hold great promise for uncovering new biomarkers and therapeutic targets, ultimately paving the way for innovative approaches to diagnose and treat neurological disorders.
Conclusion
EVs have become key mediators of intercellular communication inside the brain, contributing significantly to synaptic plasticity, brain development, and the etiology of neurological diseases. They present interesting possibilities for diagnostic and therapeutic applications in numerous brain-related illnesses due to their capacity to carry specific cargo, such as proteins, nucleic acids, and microRNAs. In this context, we investigated the potential of EVs as therapeutic agents and diagnostic biomarkers in ALS, PD, HD, AD, and PrD. In the realm of diagnostics, EVs hold promise as non-invasive biomarkers for early detection, disease monitoring, and prognosis in neurological disorders. Studies have shown that specific cargo components, such as NfL chain and synaptic proteins in AD, mHTT, and NfL in HD, α-syn in PD, and SOD1 in ALS, are potential biomarkers that can provide valuable insights into disease progression and severity. Therapeutically, EVs provide a flexible platform for the delivery of specific drugs for a range of neurological illnesses. EVs have the potential to slow down disease-related neurodegeneration and boost neuronal survival when they are loaded with therapeutic cargo like siRNAs that target disease-causing genes or neuroprotective substances like BDNF and GDNF. Additionally, immune cell-derived EVs have the ability to control neuroinflammation and promote neurogenesis, offering prospective therapeutic approaches for the management of disease. The understanding of EV biology in the brain has been advanced by emerging technologies. Investigating EV structure, cargo, and functional dynamics is made possible by cutting-edge imaging methods, proteomics, RNA-Seq, and liquid biopsies. Customized therapeutic applications are now possible with the use of CRISPR/Cas9 genome editing in EVs. Future avenues for individualized medical strategies in neurological illnesses are excitingly provided by these technologies. To successfully implement EV-based diagnostics and treatments in clinical practice, a few issues must be resolved. To ensure the reliability and accuracy of EV biomarkers in disease diagnosis and monitoring, standardization and validation are essential. For clinical applications, it is also critical to develop effective and scalable techniques for EV isolation, purification, and therapeutic cargo loading. The potential immunogenicity of EVs and their interactions with the immune system requires careful consideration. As a result, the investigation of EVs in brain function and neurological ailments has uncovered intriguing prospects for early disease identification, focused treatments, and precision medicine approaches. EV-based diagnostics and therapies have significant promise for transforming the field of neurology and enhancing patient outcomes in a variety of brain-related disorders with continued research and technical improvements. In the upcoming years, cooperation between researchers, doctors, and industry stakeholders will be essential to overcoming obstacles and realizing the full potential of EVs.
Data availability
No datasets were generated or analyzed during the current study.
References
Théry, C., Witwer, K. W., Aikawa, E., Alcaraz, M. J., Anderson, J. D., Andriantsitohaina, R., & Zuba-Surma, E. K. (2018). Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. Journal of Extracellular Vesicles, 7(1), 1535750.
Tiwari, S., Kumar, V., Randhawa, S., & Verma, S. K. (2021). Preparation and characterization of extracellular vesicles. American Journal of Reproductive Immunology, 85(2), e13367. https://doi.org/10.1111/aji.13367.
Lee, K., Fraser, K., Ghaddar, B., Yang, K., Kim, E., Balaj, L., & Weissleder, R. (2018). Multiplexed profiling of single extracellular vesicles. ACS Nano, 12(1), 494–503. https://doi.org/10.1021/acsnano.7b07060.
Spitzberg, J. D., Ferguson, S., & Yang, K. S., et al. (2023). Multiplexed analysis of EV reveals specific biomarker composition with diagnostic impact. Nature Communications, 14, 1239 https://doi.org/10.1038/s41467-023-36932-z.
Théry, C., Amigorena, S., Raposo, G., & Clayton, A. (2006). Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Current Protocols in Cell Biology, Chapter 3, Unit 3.22. https://doi.org/10.1002/0471143030.cb0322s30.
Shao, H., Chung, J., Balaj, L., Charest, A., Bigner, D. D., Carter, B. S., & Lee, H. (2012). Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy. Nature Medicine, 18(12), 1835–1840. https://doi.org/10.1038/nm.2994.
Cizmar, P., & Yuana, Y. (2017). Detection and characterization of extracellular vesicles by transmission and cryo-transmission electron microscopy. Methods in Molecular Biology, 1660, 221–232. https://doi.org/10.1007/978-1-4939-7253-1_18.
Sharma, S., Rasool, H. I., Palanisamy, V., Mathisen, C., Schmidt, M., Wong, D. T., & Gimzewski, J. K. (2010). Structural-mechanical characterization of nanoparticle exosomes in human saliva, using correlative AFM, FESEM, and force spectroscopy. ACS Nano, 4(4), 1921–1926. https://doi.org/10.1021/nn901824n.
Vogel, R., Pal, A. K., & Jambhrunkar, S., et al. (2017). High-resolution single particle zeta potential characterisation of biological nanoparticles using tunable resistive pulse sensing. Scientific Reports, 7(1), 17479.
Shimbo, K., Miyaki, S., & Ishitobi, H., et al. (2014). Exosome-formed synthetic microRNA-143 is transferred to osteosarcoma cells and inhibits their migration. Biochemical and Biophysical Research Communications, 445(2), 381–387.
Katsuda, T., Kosaka, N., Takeshita, F., & Ochiya, T. (2013). The therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Proteomics, 13(10-11), 1637–1653.
Longjohn, M. N., & Christian, S. L. (2022). Characterizing extracellular vesicles using nanoparticle-tracking analysis. Methods in Molecular Biology, 2508, 353–373. https://doi.org/10.1007/978-1-0716-2376-3_23.
Lawrie, A. S., Albanyan, A., Cardigan, R. A., Mackie, I. J., & Harrison, P. (2009). Microparticle sizing by dynamic light scattering in fresh-frozen plasma. Vox Sanguinis, 96(3), 206–212.
Khan, M. A., Anand, S., Deshmukh, S. K., Singh, S., & Singh, A. P. (2022). Determining the size distribution and integrity of extracellular vesicles by dynamic light scattering. Methods in Molecular Biology, 2413, 165–175. https://doi.org/10.1007/978-1-0716-1896-7_17.
Doyle, L. M., & Wang, M. Z. (2019). Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells, 8(7), 727.
Lischnig, A., Bergqvist, M., Ochiya, T., & Lässer, C. (2022). Quantitative proteomics identifies proteins enriched in large and small extracellular vesicles. Molecular & Cellular Proteomics, 21(9), 100273. https://doi.org/10.1016/j.mcpro.2022.100273.
Kreimer, S., Belov, A. M., Ghiran, I., Murthy, S. K., Frank, D. A., & Ivanov, A. R. (2015). Mass-spectrometry-based molecular characterization of extracellular vesicles: lipidomics and proteomics. Journal of Proteome Research, 14(6), 2367–2384. https://doi.org/10.1021/pr501279t.
Blandin, A., Dugail, I., & Hilairet, G., et al. (2023). Lipidomic analysis of adipose-derived extracellular vesicles reveals specific EV lipid sorting informative of the obesity metabolic state. Cell Reports, 42(3), 112169. https://doi.org/10.1016/j.celrep.2023.112169.
Lin, L., Liang, Y., Cao, T., Huang, Y., Li, W., Li, J., & Li, L. (2023). Transcriptome profiling and ceRNA network of small extracellular vesicles from resting and degranulated mast cells. Epigenomics, 15(17), 845–862. https://doi.org/10.2217/epi-2023-0175.
Welsh, J. A., Arkesteijn, G. J. A., Bremer, M., Cimorelli, M., Dignat-George, F., Giebel, B., & van der Pol, E. (2023). A compendium of single extracellular vesicle flow cytometry. Journal of Extracellular Vesicles, 12(2), e12299. https://doi.org/10.1002/jev2.12299.
Mangolini, V., Gualerzi, A., Picciolini, S., Rodà, F., Del Prete, A., Forleo, L., & Bedoni, M. (2023). Biochemical characterization of human salivary extracellular vesicles as a valuable source of biomarkers. Biology, 12(2), 227. https://doi.org/10.3390/biology12020227.
Krylova, S. V., & Feng, D. (2023). The machinery of exosomes: biogenesis, release, and uptake. International Journal of Molecular Sciences, 24(2), 1337. https://doi.org/10.3390/ijms24021337.
Raghav, A., Tripathi, P., Mishra, B. K., Jeong, G. B., Banday, S., Gautam, K. A., & Ahmad, J. (2021). Mesenchymal stromal cell-derived tailored exosomes treat bacteria-associated diabetes foot ulcers: A customized approach from bench to bed. Frontiers in Microbiology, 12, 712588. https://doi.org/10.3389/fmicb.2021.712588.
Kim, H. J., Kim, G., Lee, J., Lee, Y., & Kim, J. H. (2022). Secretome of stem cells: Roles of extracellular vesicles in diseases, stemness, differentiation, and reprogramming. Tissue Engineering and Regenerative Medicine, 19(1), 19–33. https://doi.org/10.1007/s13770-021-00406-4.
Gupta, S., Krishnakumar, V., Soni, N., Rao, E. P., Banerjee, A., & Mohanty, S. (2022). Comparative proteomic profiling of Small Extracellular vesicles derived from iPSCs and tissue-specific mesenchymal stem cells. Experimental Cell Research, 420(2), 113354. https://doi.org/10.1016/j.yexcr.2022.113354.
Jahanbani, et al. (2021). miR-133b-3p in extracellular vesicles from bone marrow mesenchymal stem cells alleviates Parkinson’s disease via regulating MERTK-mediated nuclear autophagy. Theranostics, 11(23), 11550–11567. https://doi.org/10.7150/thno.61016.
Trajkovic, K., et al. (2008). Endocytosis regulates exosome secretion and selective accumulation of miRNAs in colorectal cancer cells. Nature Communications, 4, 1229. https://doi.org/10.1038/ncomms2328
Théry, C., et al. (2018). Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. Journal of Extracellular Vesicles, 7(1), 1535750. https://doi.org/10.1080/20013078.2018.1535750.
Park, S. Y., Kim, D. S., Kim, H. M., Lee, J. K., Hwang, D. Y., Kim, T. H., You, S., & Han, D. K. (2022). Human mesenchymal stem cell-derived extracellular vesicles promote neural differentiation of neural progenitor cells. International Journal of Molecular Sciences, 23(13), 7047. https://doi.org/10.3390/ijms23137047.
Rädler, J., Gupta, D., Zickler, A., & Andaloussi, S. E. (2023). Exploiting the biogenesis of extracellular vesicles for bioengineering and therapeutic cargo loading. Molecular Therapy, 31(5), 1231–1250. https://doi.org/10.1016/j.ymthe.2023.02.013.
Santavanond, J. P., Rutter, S. F., Atkin-Smith, G. K., & Poon, I. K. H. (2021). Apoptotic bodies: mechanism of formation, isolation and functional relevance. Sub-Cellular Biochemistry, 97, 61–88. https://doi.org/10.1007/978-3-030-67171-6_4.
Li, M., Liao, L., & Tian, W. (2020). Extracellular vesicles derived from apoptotic cells: An essential link between death and regeneration. Frontiers in Cell and Developmental Biology, 8, 573511. https://doi.org/10.3389/fcell.2020.573511.
Naghibi, A. F., Daneshdoust, D., Taha, S. R., Abedi, S., Dehdezi, P. A., Zadeh, M. S., & Soleymani-Goloujeh, M. (2023). Role of cancer stem cell-derived extracellular vesicles in cancer progression and metastasis. Pathology Research and Practice, 247, 154558. https://doi.org/10.1016/j.prp.2023.154558.
Li, Q., Yu, H., Sun, M., Yang, P., Hu, X., Ao, Y., & Cheng, J. (2021). The tissue origin effect of extracellular vesicles on cartilage and bone regeneration. Acta Biomaterialia, 125, 253–266. https://doi.org/10.1016/j.actbio.2021.02.039.
Matsuzaka, Y., & Yashiro, R. (2022). Therapeutic Strategy of mesenchymal-stem-cell-derived extracellular vesicles as regenerative medicine. International Journal of Molecular Sciences, 23(12), 6480. https://doi.org/10.3390/ijms23126480.
Patel, D. B., Gray, K. M., Santharam, Y., Lamichhane, T. N., Stroka, K. M., & Jay, S. M. (2017). Impact of cell culture parameters on production and vascularization bioactivity of mesenchymal stem cell-derived extracellular vesicles. Bioengineering and Translational Medicine, 2(2), 170–179. https://doi.org/10.1002/btm2.10065.
Kang, M., Huang, C. C., Gajendrareddy, P., Lu, Y., Shirazi, S., Ravindran, S., & Cooper, L. F. (2022). Extracellular vesicles From TNFα preconditioned MSCs: Effects on immunomodulation and bone regeneration. Frontiers in Immunology, 13, 878194. https://doi.org/10.3389/fimmu.2022.878194.
Mushahary, D., Spittler, A., Kasper, C., Weber, V., & Charwat, V. (2018). Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry Part A, 93(1), 19–31. https://doi.org/10.1002/cyto.a.23242.
Tsiapalis, D., & O’Driscoll, L. (2020). Mesenchymal stem cell derived extracellular vesicles for tissue engineering and regenerative medicine applications. Cells, 9(4), 991. https://doi.org/10.3390/cells9040991.
Blanchette, C. R., Scalera, A. L., Harris, K. P., Zhao, Z., Dresselhaus, E. C., Koles, K., & Rodal, A. A. (2022). Local regulation of extracellular vesicle traffic by the synaptic endocytic machinery. Journal of Cell Biology, 221(5), e202112094. https://doi.org/10.1083/jcb.202112094.
Hering, C., & Shetty, A. K. (2023). Extracellular vesicles derived from neural stem cells, astrocytes, and microglia as therapeutics for easing TBI-induced brain dysfunction. Stem Cells Translational Medicine, 12(3), 140–153. https://doi.org/10.1093/stcltm/szad004.
You, Y., Muraoka, S., Jedrychowski, M. P., Hu, J., McQuade, A. K., Young-Pearse, T., & Ikezu, T. (2022). Human neural cell type-specific extracellular vesicle proteome defines disease-related molecules associated with activated astrocytes in Alzheimer’s disease brain. Journal of Extracellular Vesicles, 11(1), e12183. https://doi.org/10.1002/jev2.12183.
Chiaradia, E., Tancini, B., Emiliani, C., Delo, F., Pellegrino, R. M., Tognoloni, A., & Buratta, S. (2021). Cells. Cells, 10(7), 1763. https://doi.org/10.3390/cells10071763.
Van den Broek, B., et al. (2021). Advances in Drug Delivery Reviews.
Theranostics, 12(13), 5776-5802. (2022, July 18). https://doi.org/10.7150/thno.73931.
Chen, Y., Zhou, C., Zhao, X., Che, R., Wu, Y., Wan, S., & Hua, X. (2023). Extracellular vesicles derived from human umbilical cord mesenchymal stem cells promote trophoblast cell proliferation and migration by targeting TFPI2 in preeclampsia. Stem Cells International, 2023, 7927747. https://doi.org/10.1155/2023/7927747.
Pan, J., Sheng, S., Ye, L., Xu, X., Ma, Y., Feng, X., & Zheng, J. C. (2022). Extracellular vesicles derived from glioblastoma promote proliferation and migration of neural progenitor cells via PI3K-Akt pathway. Cell Communication and Signaling, 20(1), 7 https://doi.org/10.1186/s12964-021-00760-9.
Niu, J., & Li, Z. (2020). The potential therapeutic roles of extracellular vesicles in Alzheimer’s disease. Journal of Alzheimer’s Disease, 78(4), 1383–1393. https://doi.org/10.3233/JAD-201040.
Garcia-Contreras, M., & Thakor, A. S. (2021). Stem cell-derived extracellular vesicles in neurodegenerative disorders: A focus on Alzheimer’s disease. Stem Cell Research & Therapy, 12(1), 142. https://doi.org/10.1186/s13287-021-02179-2.
Kim, D. K., Nishida, H., & An, S. Y., et al. (2021). Extracellular vesicles, especially derived from mesenchymal stem cells, promote therapeutic effects in Alzheimer’s disease. Advanced Drug Delivery Reviews, 173, 532–545. https://doi.org/10.1016/j.addr.2021.03.010.
Katsuda, T., Tsuchiya, R., & Kosaka, N., et al. (2013). Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Scientific Reports, 3, 1197 https://doi.org/10.1038/srep01197.
de Godoy, M. A., Saraiva, L. M., & de Carvalho, L. R. P., et al. (2018). Mesenchymal stem cells and cell-derived extracellular vesicles protect hippocampal neurons from oxidative stress and synapse damage induced by amyloid-beta oligomers. Journal of Biological Chemistry, 293(6), 1957–1975. https://doi.org/10.1074/jbc.RA117.000315.
Ma, T., Chen, Y., & Vingtdeux, V., et al. (2020). Regulation of memory formation by the transcription factor XBP1. Cell Reports, 30(9), 2865–2874.e3. https://doi.org/10.1016/j.celrep.2020.02.010.
Sha, Y., & Han, Q. G. Y., et al. (2021). MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials Science, 9(8), 2621–2633. https://doi.org/10.1039/D0BM02050E.
Apodaca, L. A., Baddour, A. A., & Aghajan, M., et al. (2021). Extracellular vesicles from neural stem cells transfer IFN-γ via Ifngr1 to activate Stat1 signaling in target cells. Molecular Cell, 81(12), 2615–2630.e7. https://doi.org/10.1016/j.molcel.2021.05.035.
Zhang, Y., Kim, M. S., & Jia, B., et al. (2021). Hypothalamic stem cells control ageing speed partly through exosomal miRNAs. Nature, 548(7665), 52–57. https://doi.org/10.1038/nature23282.
Jang, S. C., Kim, O. Y., & Yoon, C. M., et al. (2013). Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano, 7(9), 7698–7710. https://doi.org/10.1021/nn402232g.
Dooley, K., McConnell, R. E., & Xu, K., et al. (2021). Engineering enhanced therapeutic exosomes. Advances in Drug Delivery Reviews, 175, 113761. https://doi.org/10.1016/j.addr.2021.113761.
Alzheimer’s Association. (2021). Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 17(2), 195-225. https://doi.org/10.1002/alz.12328.
Goetzl, E. J., Schwartz, J. B., & Abner, E. L., et al. (2018). High complement levels in astrocyte-derived exosomes of Alzheimer’s disease. Annals of Neurology, 83(3), 544–552. https://doi.org/10.1002/ana.25176.
Badhwar, A., Haqqani, A. S., & Biomberg, M., et al. (2019). Increased tau phosphorylation and impaired presynaptic function in hypertriglyceridemic ApoB-100 transgenic mice. Alzheimer’s & Dementia: Translational Research & Clinical Interventions, 5, 814–823. https://doi.org/10.1016/j.trci.2019.09.007.
Dragatsis, I., Levine, M. S., & Zeitlin, S. (2000). Inactivation of Hdh in the brain and testis results in progressive neurodegeneration and sterility in mice. Nature Genetics, 26(3), 300–306. https://doi.org/10.1038/81593.
Byrne, L. M., Rodrigues, F. B., Blennow, K., Durr, A., Leavitt, B. R., Roos, R. A. C., & Wild, E. J. (2017). Neurofilament light protein in blood as a potential biomarker of neurodegeneration in Huntington’s disease: a retrospective cohort analysis. The Lancet Neurology, 16(8), 601–609. https://doi.org/10.1016/S1474-4422(17)30124-2.
Parkin, G. M., Thomas, E. A., & Corey-Bloom, J. (2023). Plasma NfL as a prognostic biomarker for enriching HD-ISS stage 1 categorization: A cross-sectional study. EBioMedicine, 93, 104646. https://doi.org/10.1016/j.ebiom.2023.104646.
Abels, E. R., & Breakefield, X. O. (2016). Introduction to extracellular vesicles: Biogenesis, RNA cargo selection, content, release, and uptake. Cellular and Molecular Neurobiology, 36(3), 301–312. https://doi.org/10.1007/s10571-016-0366-z.
Gao, L., Chen, C., & Wang, H., et al. (2020). Extracellular vesicles: Emerging diagnostic and therapeutic tools in pharmacology. Journal of Clinical Pharmacology, 60(6), 651–658. https://doi.org/10.1002/jcph.1586.
Loria, F., Vargas, J. Y., & Bousset, L., et al. (2017). α-Synuclein transfer between neurons and astrocytes indicates that astrocytes play a role in degradation rather than in spreading. Acta Neuropathologica, 134(5), 789–808. https://doi.org/10.1007/s00401-017-1739-9.
Bliederhaeuser, C., Zondler, L., & Grozdanov, V., et al. (2016). Age-dependent defects of alpha-synuclein oligomer uptake in microglia and monocytes. Acta Neuropathologica, 131(3), 379–391. https://doi.org/10.1007/s00401-015-1508-5.
Zhao, Z. H., Chen, Z. T., & Zhou, R. L., et al. (2020). Increased DJ-1 and α-synuclein in plasma neural-derived exosomes as potential markers for Parkinson’s disease. Frontier in Aging Neuroscience, 12, 570549. https://doi.org/10.3389/fnagi.2020.570549.
Gui, Y., Liu, H., & Zhang, L., et al. (2015). Altered microRNA profiles in cerebrospinal fluid exosome in Parkinson disease and Alzheimer disease. Oncotarget, 6(35), 37043–37053. https://doi.org/10.18632/oncotarget.6159.
Cooper, J. M., Wiklander, P. B., & Nordin, J. Z., et al. (2014). Systemic exosomal siRNA delivery reduced alpha-synuclein aggregates in brains of transgenic mice. Movement Disorders, 29(13), 1476–1485. https://doi.org/10.1002/mds.25978.
Sun, C., Yu, Y., & Wang, L., et al. (2018). Grafted bone marrow stromal cells: a contributor to glial repair after spinal cord injury. Neuroscientist, 24(2), 152–162. https://doi.org/10.1177/1073858417691000.
Silverman, J. M., Christy, D., & Shyu, C. C., et al. (2019). CNS-derived extracellular vesicles from superoxide dismutase 1 (SOD1)(G93A) ALS mice originate from astrocytes and neurons and carry misfolded SOD1. Journal of Biological Chemistry, 294(10), 3744–3759. https://doi.org/10.1074/jbc.RA118.007229.
Brenna, G., Sagona, A. P., & Gullo, C., et al. (2020). Prion protein modulates endosomal-nuclear translocation of transferrin receptor by regulating Rab11A-WDR44-mediated coalescence. Advanced Science, 7(20), 2001114. https://doi.org/10.1002/advs.202001114.
D’Arrigo, D., Tabernacki, T., & Mottahedeh, J., et al. (2021). EV tracking and heterogeneous mobility of EVs reveals that EVs are integral components of the extracellular microenvironment. Journal of Extracellular Vesicles, 10(1), e12083. https://doi.org/10.1002/jev2.12083.
Mantuano, E., Szychowski, J., & Murchie, R., et al. (2022). Circulating exosomal prions: A new target for early blood-based diagnosis of prion infection. PLOS Pathogens, 18(1), e1010002. https://doi.org/10.1371/journal.ppat.1010002.
Orrù, C. D., Soldau, K., & Cordano, C., et al. (2018). Prion seeds distribute throughout the eyes of sporadic Creutzfeldt-Jakob disease patients. mBio, 9(6), e02095-18. https://doi.org/10.1128/mBio.02095-18.
Février, B., Vilette, D., & Archer, F., et al. (2004). Cells release prions in association with exosomes. Proceedings of the National Academy of Sciences of the United States of America, 101(26), 9683–9688. https://doi.org/10.1073/pnas.0308413101.
Winston, C. N., Goetzl, E. J., & Akers, J. C., et al. (2019). Prediction of conversion from mild cognitive impairment to dementia with neuronally derived blood exosome protein profile. Alzheimer’s & Dementia, 15(5), 617–627. https://doi.org/10.1016/j.jalz.2018.09.003.
Busch, J. I., Unger, T. L., & Jain, N., et al. (2016). Increased expression of the frontotemporal dementia risk factor TMEM106B causes C9orf72-dependent alterations in lysosomes. Human Molecular Genetics, 25(13), 2681–2697. https://doi.org/10.1093/hmg/ddw116.
Shi, M., Liu, C., & Cook, T. J., et al. (2014). Plasma exosomal α-synuclein is likely CNS-derived and increased in Parkinson’s disease. Acta Neuropathologica, 128(5), 639–650. https://doi.org/10.1007/s00401-014-1314-y.
Cao, Z., Wu, Y., & Liu, G., et al. (2018). α-Synuclein in salivary extracellular vesicles as a potential biomarker of Parkinson’s disease. Neuroscience Letters, 699, 104–110. https://doi.org/10.1016/j.neulet.2018.03.009.
Wang, S., Liu, Z., & Ye, T., et al. (2017). LRRK2 regulates dynamic profile of mitochondria in rat kidney from prenatal to aging with urinary space dilation. Scientific Reports, 7(1), 717 https://doi.org/10.1038/s41598-017-00837-w.
Zea Roca, J., Koszmarek-Weiss, U., Michalak, M., et al. (2019). Elevated SOD1 in extracellular vesicles drives motor neuron stresses to initiate paralytic ALS. bioRxiv, 870473. https://doi.org/10.1101/870473.
Freischmidt, A., Müller, K., & Zondler, L., et al. (2014). Serum microRNAs in patients with genetic amyotrophic lateral sclerosis and pre-manifest mutation carriers. Brain, 137(11), 2938–2950. https://doi.org/10.1093/brain/awu244.
Feneberg, E., Steinacker, P., & Lehnert, S., et al. (2014). Limited role of free TDP-43 as a diagnostic tool in neurodegenerative diseases. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, 15(5-6), 351–356. https://doi.org/10.3109/21678421.2014.905421.
Katsu, M., Hama, Y., & Utsumi, J., et al. (2019). MicroRNA expression profiles of neuron-derived extracellular vesicles in plasma from patients with amyotrophic lateral sclerosis. Neuroscience Research, 160, 43–49. https://doi.org/10.1016/j.neures.2019.08.003.
Liu, et al. (2021). Engineered extracellular vesicles for precision therapy in non-small cell lung cancer. Journal of Nanobiotechnology, 19(1), 108 https://doi.org/10.1186/s12951-021-00896-z.
Leavitt, M. G., Appel, S. H., & Nassif, M. (2020). Human neural stem cells-derived extracellular vesicles mitigate hallmarks of Alzheimer’s disease. Journal of Stem Cells and Regenerative Medicine, 16(1), 14–18.
Wang, et al. (2021). Extracellular vesicles microRNA-10b-5p delivered by mesenchymal stem cells-derived exosomes promotes the neurogenesis of damaged neurons through Smad4-mediated reduction of ischemia/reperfusion injury in mice. Stem Cell Research & Therapy, 12(1), 232. https://doi.org/10.1186/s13287-021-02314-3.
Yang, et al. (2020). Intranasal delivery of brain-derived neurotrophic factor gene-modified MSCs in the treatment of traumatic brain injury. Journal of Neurotrauma, 37(15), 1947–1957. https://doi.org/10.1089/neu.2019.6782.
Han, et al. (2020). Extracellular vesicles from genetically modified mesenchymal stem cells attenuate microglia activation and neuroinflammation as well as ameliorate neuropathic pain. Journal of Neuroinflammation, 17, 149 https://doi.org/10.1186/s12974-020-01846-5.
Boido, et al. (2019). Extracellular vesicles from neural stem cells transfer IFN-γ via Ifngr1 to activate Stat1 signaling in target cells. Molecular and Cellular Neurosciences, 97, 43–51. https://doi.org/10.1016/j.mcn.2019.06.008.
Yin, et al. (2021). Regulatory T cell-derived extracellular vesicles modify microglial phenotype and attenuate neuroinflammation for Parkinson’s disease and epilepsy therapy. Journal of Nanobiotechnology, 19(1), 88 https://doi.org/10.1186/s12951-021-00876-3.
Khadka, A., Spiers, J. G., Cheng, L., & Hill, A. F. (2023). Extracellular vesicles with diagnostic and therapeutic potential for prion diseases. Cell and Tissue Research, 392(1), 247–267.
Chabanon, et al. (2020). Cryo-EM structure of vesicular stomatitis virus in complex with a neutralizing nanobody. Nature, 579(7797), 581–585.
Nombela, et al. (2021). Profiling of RNA modifications and their response to the microbial patterns recognition in peripheral blood mononuclear cells from rheumatoid arthritis patients. Scientific Reports, 11(1), 17757.
Lee, et al. (2019). The isolation of exosome from blood plasma, urine and cell culture media through ultracentrifugation. Current Protocols in Stem Cell Biology, 50(1), e82.
Gilleron, et al. (2019). Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking, and endosomal escape. Nature Biotechnology, 38(1), 43–53.
Wang, et al. (2020). Profiling and quantification of the circular RNA in the porcine developing heart. Frontiers in Genetics, 11, 529.
Kovachich, et al. (2020). Enrichment technologies for extracellular vesicles: Advances and challenges. Trends in Analytical Chemistry, 124, 115781.
Safaei, et al. (2021). EVmiRNA: A database of miRNA profiling in extracellular vesicles. Scientific Reports, 11(1), 5640.
Kim, et al. (2023). Real-time imaging of biodistribution and trafficking of bioluminescent exosomes in mouse brain. Journal of Controlled Release, 334, 1–9.
Li, et al. (2023). Engineered extracellular vesicles for blood–brain barrier crossing and therapeutic cargo delivery to the Alzheimer’s brain. Molecular Therapy, 31(10), 5253–5268.
Rodriguez, et al. (2023). Glial-cell-derived extracellular vesicles as regulators of neuronal damage and repair: New insights and therapeutic opportunities after traumatic brain injury. International Journal of Molecular Sciences, 24(3), 1607.
Author information
Authors and Affiliations
Contributions
S.S. wrote the main text and prepared the figures and tables. S.P. conceptualized the idea and reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sarkar, S., Patranabis, S. Emerging Role of Extracellular Vesicles in Intercellular Communication in the Brain: Implications for Neurodegenerative Diseases and Therapeutics. Cell Biochem Biophys 82, 379–398 (2024). https://doi.org/10.1007/s12013-024-01221-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-024-01221-z