Abstract
Increasing evidence suggests that stem cells or stem cell-derived cells may contribute to tissue repair, not only by replacing lost tissue but also by delivering complex sets of secretory molecules, called secretomes, into host injured tissues. In recent years, extracellular vesicles (EVs) have gained much attention for their diverse and important roles in a wide range of pathophysiological processes. EVs are released from most types of cells and mediates cell–cell communication by activating receptors on target cells or by being taken up by recipient cells. EVs, including microvesicles and exosomes, encapsulate and carry proteins, nucleic acids, and lipids in the lumen and on the cell surface. Thus, EV-mediated intercellular communication has been extensively studied across various biological processes. While a number of investigations has been conducted in different tissues and body fluids, the field lacks a systematic review on stem cell-derived EVs, especially regarding their roles in stemness and differentiation. Here, we provide an overview of the pathophysiological roles of EVs and summarize recent findings focusing on EVs released from various types of stem cells. We also highlight emerging evidence for the potential implication of EVs in self-renewal, differentiation, and reprograming and discuss the benefits and limitations in translational approaches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Stem cells, including embryonic stem cells (ESCs) [1], tissue-specific stem cells (TSCs) [2], and induced pluripotent stem cells (iPSCs) [3], have the capacity to self-renew and differentiate into various different cell types. Extensive global studies over the past two decades have investigated the therapeutic potential of stem cells across a wide range of degenerative diseases, including nerve degeneration, cardiac diseases, liver failure, and retinal degeneration [4]. Despite much progress in stem cell therapies, delivery of cells into the body remains limited due to poor survival of grafted cells, immune rejection, tumor formation, genetic instability, limited cell supply, and ethical concerns [5]. Promising technological advances, including cellular reprogramming, genome editing, 3D organoid generation, and biomaterial engineering offer hope for overcoming these current drawbacks. However, alternative approaches based on the indirect therapeutic potential of stem cells may offer a safer and more effective modality that can overcome the challenges of cell-based therapy.
Recent evidence suggests that stem cells or stem cell-derived cells contribute to tissue repair, not only through lost tissue replacement but also through delivery of paracrine signals into injured host tissues [6]. Mechanistically, mesenchymal stem cells (MSCs) are though to exert a regenerative effect by promoting neovascularization and cell proliferation or by modulating immune responses via paracrine signaling [7]. Moreover, repair effect of cardiac stem cell transplantation is caused by paracrine effects rather than differentiation capacity of the stem cells [8]. Additionally, we showed that only a single injection of secreted factors from human ESC-derived hepatocytes (i.e., secretome, referred to as a complex set of secretory molecules), but not cell transplantation, was necessary to promote endogenous regeneration of injured liver tissues [9]. Additionally, we found that the secretome of umbilical cord-derived MSCs attenuates liver fibrosis by inhibiting TGF-β signaling in mice with hepatic fibrosis [10]. Additionally, previous studies on human PSC-derived cardiomyocytes [11] and endothelial cells [12] speculated about the therapeutic contributions of stem cell-derived paracrine signals. Together, these findings raise the possibility that stem cells synthesize and release therapeutic signals. Identification of these beneficial secretory factors has been hampered by the challenges of analysis of the enormous spectrum of stem cell-derived signals as well as batch-to-batch variations in secretome profiles.
Likely mediators of the beneficial paracrine effects of stem cell secretomes are extracellular vesicles (EVs) [13]. EVs can play important roles in regeneration of various tissue through modulation of apoptosis, inflammation, proliferation, and angiogenesis by delivering their internal cargo in a paracrine mode of action [14, 15]. In this review, we explore the idea that the therapeutic effects of stem cells may be mediated by EVs released from stem cells or their progeny. Here, we summarize research updates on stem cell EVs in diseases, stemness, and differentiation, and discuss their potential applications and limitations related to regenerative medicine.
2 EVs: an overview
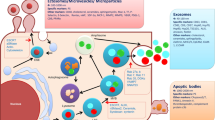
EVs are non-replicable, membrane-encapsulated particles released from most types of cells [16]. Although EVs were first recognized as “trash cans” to move unusable proteins to the outside of cells [17], they are now appreciated as crucial tools for intercellular communication. EVs are classified into exosomes, microvesicles (MVs), and apoptotic bodies based on their size or mechanism of generation (Fig. 1A). Exosomes are released by the fusion of multi-vesicular bodies with the plasma membrane [18], while MVs are directly shed from the plasma membrane, and apoptotic bodies are derived from blebbing of apoptotic cells [19]. The release of EVs into the intercellular space mediates communication either through release of EV cargo into tissue microenvironments or through activation of receptors on recipient cells by ligands displayed on the EV surface. Additionally, EVs can be taken up by specific recipient cells and release their cargo intracellularly, which allows for communication to only targeted cells [20]. EV-mediated communication is not confined to local tissues, as EVs exist in systemic circulation and deliver their contents to distant tissues [21], even across the blood–brain barrier [22]. EVs can be detected in many types of body fluids, including saliva, breast milk, semen, systemic circulation, urine, synovial fluid, and amniotic fluid [23]. EVs contain various types of proteins and nucleic acids, such as double-stranded DNA, mRNA, miRNA, and long non-coding RNA, and display conventional markers such as CD9, CD63, or CD81 in the membrane and TSG101, alix, flotillin-1/2, or heat shock protein 70 in the lumen (Fig. 1A) [16]. A schematic summary of the biogenesis, action modes, and cargos of EVs is shown in Fig. 1A. With diverse cargoes, EVs have been known to conduct many biological roles as discussed below.
Schematic of the diverse roles of EVs modulating pathophysiological and cellular processes. A Biogenesis, potential cargos, and action modes of EVs (microvesicles, exosomes, and apoptotic bodies). Exosomes are formed in early endosomes, which subsequently produce MVBs. MVBs release exosomes into the extracellular space by exocytosis. Microvesicles are formed and released by outward budding and shedding of the plasma membrane. Apoptotic bodies are enclosed vesicles produced from apoptotic cells by membrane blebbing. EVs mediate cell–cell communications by activating receptors on target cells or being taken up by recipient cells, thus releasing their cargo inside the cells. EVs are also transported through systemic circulation and deliver their contents to distant tissues. B Pathophysiological implication of EVs in inflammation, adaptive immunity, cancer, aging, and degenerative diseases. C Potential roles of stem cell-derived EVs in early development of embryos, biological processes, and stemness/differentiation of stem cells. EVs extracellular vesicles, MVB multi-vesicular bodies, SC stem cell, ESCs embryonic stem cells, iPSCs induced pluripotent stem cells, MSC multipotent stem cells, TSCs tissue-specific stem cells, HSC hematopoietic stem cells, PSC, pluripotent stem cell, NPC neural progenitor cells
2.1 Pathophysiological functions of EVs
The contents of EVs vary based on cell type of origin, and various types of cargo molecules within EVs can modulate biological process in recipient cells. For example, dendritic cell (DC)-derived EVs have MHC class I, MHC class II, and CD86 on their surface, which are involved in T cell activation [24]. Glioblastoma-derived EVs deliver mRNAs associated with cell proliferation or migration to target cells and the mRNAs are translated in recipient cells [25]. Additionally, MSC-derived EVs, like MSCs, can promote angiogenesis by transferring miRNA into endothelial cells [26].
EVs participate in various physiological and pathological processes across a wide spectrum of diseases (Fig. 1B) [27]. During inflammation, EVs play multiple roles in regulating both pro- and anti-inflammatory responses. For example, EVs derived from pro-inflammatory phagocytes, such as M1 macrophages [28] and mature dendritic cells [29] can recruit phagocytes and activate their pro-inflammatory phenotypes. In contrast, M2 macrophage- [28] or activated neutrophil-derived EVs [30] inhibit inflammation by inducing secretion of anti-inflammatory cytokines. Likewise innate immunity, adaptive immune cells are regulated by exosome for both activation and suppression [24, 29, 31]. EVs can either promote or attenuate chronic inflammation under disease conditions. In obese subjects, microRNA-34a in adipocyte-derived EVs suppresses polarization of adipose-resident macrophages into an M2 phenotype through Klf4 repression, which further aggravates obesity-induced inflammation [32]. Moreover, in colitis, T regulatory cells decrease inflammation by releasing miRNAs through EVs to suppress the activity of T helper 1 cells, which promote inflammation [33].
EVs also play a role in aging, as senescent cells release increased number of EVs, and the ability for EV uptake changes with age [34]. Chronic low-grade inflammation exists in organisms of advanced age, known as “inflammaging”. Contents within EVs from aging cells are altered toward types that promote aging progression. For example, amounts of inflammaging-related miRNAs in EVs are altered in patients with aging-related diseases. Mechanistically, EVs from senescent endothelial cells or the serum of aged subjects contain increased levels of annexin, BMP2, and calcium ions, which are all associated with vascular calcification [35, 36]. EV-enriched miRNAs from the bone marrow (BM) of aged mice induce dysfunction of MSCs by promoting cellular senescence [37]. On the contrary, EVs from young individuals can have anti-aging effects. EVs from young mice serum relieve inflammaging in old mice, in part by rejuvenating the aged thymus [38]. Additionally, EVs secreted by neonatal umbilical cord-derived MSCs reverse aging phenotypes of adult BM-derived MSCs and enhance their regenerative capacity [39].
EVs contribute significantly to the development of degenerative diseases. In inflammatory brain injury, IL-1β-stimulated astrocytes release EVs into circulation and induce transmigration of peripheral immune cells into the brain by increasing NF-kB activity through PPARα inhibition [40]. EVs in Alzheimer’s disease (AD) models show increased levels of amyloid beta42 and disrupt neuronal homeostasis [41]. Similarly, other AD-associated proteins spread through the brain via EVs, including α-synuclein, tau, and prions [42]. In the liver, integrin β-enriched EVs increase hepatic inflammation by promoting the infiltration of pro-inflammatory monocytes into the liver parenchyma in an animal model of non-alcoholic steatohepatitis (NASH) [43]. Lipotoxic hepatocyte-released EVs induce pro-fibrogenic characteristics in recipient hepatic stellate cells (HSCs) by downregulating PPARγ using miR-128-3p [44]. Additionally, palmitic acid-treated, hepatocyte-derived EVs induce pro-fibrotic phenotypes of HSCs, partly by miR-192 [45]. Although additional degenerative diseases involve EV-derived molecules, such as osteoarthritis or atherosclerosis [46, 47], EVs have also been shown to impact tissue regeneration in the brain, heart, lung, liver, bone, and skin, as will be described in more detail below [48,49,50].
Of particular importance, within tumors, EVs can act as a double-edged sword by contributing to both tumor progression and regression. Tumor-derived EVs can transfer characteristics of its origin to both tumor and non-tumor cells. In glioma, EVs transfer oncogenic epidermal growth factor receptor (EGFR) EGFRvIII from EGFRvII-positive cells to EGFRvIII-negative cells within the tumor, inducing the display of EGFRvIII on the recipient cell’s surface [51]. Furthermore, double-stranded DNA encoding the oncogenic gene H-ras is encapsulated in EVs and taken up by non-tumor cells, such as fibroblasts, which increases their proliferation [52]. Similarly, transfer of the BCR-ABL gene to neutrophils transforms them into malignant cells [53]. Interestingly, a recent finding showed that EVs obtained from indoor dust could promote lung metastasis of a melanoma cell line in mice through induction of TNF-α [54]. In contrast, uptake of tumor cell-derived EVs by DCs induces CD8+ T cell-mediated anti-tumor effects, as the DCs process and display the tumor antigen, which consequently retards tumor growth [55]. Based on their ability to deliver cargo to target tissues, even cross the blood–brain barrier, EVs have been developed as promising therapeutic vehicles for cancer [56]. These results demonstrate the critical role of EVs as molecular messengers in oncogenic transformations and anti-tumor responses. Yet, these studies also underscore the need for further molecular characterization of EVs regarding their dual beneficial and detrimental roles in tumors before meaningful progress toward translational applications can be made.
2.2 Stem cell-derived EVs
Many stem cell types use EVs for intercellular communications, including TSCs, PSCs and other progenitor cells. The cargo of stem cell-derived EVs contains pluripotency-related transcription factors, mRNA, anti-inflammatory cytokines, or miRNA, and shows beneficial effects across different diseases and tissue damage [57,58,59] (Table 1).
EVs released from stem cells may also reverse the progression of aging. A recent study revealed that miRNAs in hypothalamic stem cell-released EVs reverse aging-related symptoms in both normal aging and htNSC-ablated mice [60]. Long non-coding RNAs in EVs secreted from umbilical cord-derived MSCs ameliorate aging-induced cardiac dysfunctions via inhibition of NF-κB/TNF-α signaling [61]. Additionally, autophagy-inducing, mRNA-enriched EVs derived from young MSCs can rejuvenate aged hematopoietic stem cells [62]. Both human ESC- and iPSC-derived EVs also alleviate aging-associated phenotypes of senescent MSCs [63, 64]. These data suggest that stem cell-derived EVs could replace the therapeutic roles of stem cells in several cases of degenerative diseases or tissue injuries and may have multiple advantages over direct stem cell replacement therapies. A schematic summary of physiological implications of stem cell-derived EVs is shown in Fig. 1C, and their therapeutic potential is discussed in more detail below.
3 Therapeutic potential of stem cell-derived EVs
3.1 Multipotent stem cell-derived EVs
MSCs have emerged as a promising therapeutic regulator due to their anti-inflammatory and regenerative capacities. Currently, several ongoing studies are investigating the ability of the MSC-derived secretome to cure diverse diseases through paracrine mechanisms. For example, Xin et al. demonstrated that treatment with BM MSC-derived exosomes improved neurological recovery, neurogenesis, and angiogenesis in a rat stroke model [65]. Furthermore, treatment with the miR-17–92 cluster of enriched BM MSC-derived exosomes increases neurogenesis and oligodendrogenesis by regulating PTEN expression at the ischemic boundary zone of rats [66]. Similar to the effect of MSC transplantation in a rodent stroke model, subsequent studies have demonstrated that human MSC-derived EVs have the capacity to reduce post-ischemic neurological impairment, while attenuating post-ischemic immunosuppression for successful brain remodeling [67]. The therapeutic effect in neurological diseases has also been reported with the use of NSC-derived EVs. Recent work in murine and porcine models of ischemic stroke showed that NSC-derived EVs contribute to an efficient decrease in both lesion volume and brain swelling with a simultaneous increase in motor activity [68, 69]. In addition, exosomes secreted from adipose-derived stem cells reduce mutant huntingtin aggregates that cause Huntington’s disease (HD), activate the p-CREB-PGC1α pathway, and repress mitochondrial dysfunction in an in vitro HD model [70]. Furthermore, human astrocyte-derived EVs improve electrophysiological function and inhibit apoptosis of human iPSC-derived cortical neurons [71].
In addition to studies of neural deficits, MSC-derived EVs have also been explored as a tool for the effective treatment of acute lung injury (ALI). MSC-derived EVs exhibit therapeutic effects in restoring lung protein permeability and reducing inflammatory response, likely by inducing upregulation of keratinocyte growth factor in endotoxin-induced ALI mice [72]. MSC-derived EVs were able to attenuate the severity of bacterial pneumonia in mice by increasing bacterial phagocytosis of monocytes, possibly with the CD44 receptor mediating EV uptake in recipient immune cells [73]. Concordant with these findings, miR-145 in MSC-derived EVs could suppress multidrug resistance-associated protein 1 and also lead to leukotriene B4 production to enhance antimicrobial activity [74]. Therefore, accumulating evidence suggests that MSC EV-dependent leukotrienes play a critical role in lung injury repair. Additional studies have proposed a therapeutic effect for MSC-derived EVs in other types of lung injuries, including influenza-infected ALI [75] and alpha-1-antitrypsin deficiency-related lung diseases [76].
The therapeutic role of EVs has also been recognized in liver diseases, including liver fibrosis, non-alcoholic fatty liver disease, hepatitis, and cirrhosis. Our group previously reported that the cell-free secretome of umbilical cord-derived MSCs showed strong anti-fibrotic activity through inhibiting TGFβ1-induced activation of HSCs [10]. Similarly, several groups have reported the effect of EVs in ameliorating liver fibrosis by inhibiting EMT [77], activating autophagy through the miR-181-5p-dependent STAT3 pathway [78], and repressing hedgehog signaling through miR-486-5p enriched in tonsil-derived MSCs [79]. These findings are in line with observations that MSC-derived exosomes relieve hepatic injury in different animal models of liver injuries through multiple mechanisms, including suppression of the inflammatory response, attenuation of oxidative stress, inhibition of apoptosis, attenuation of HSC activation, and promotion of hepatic regeneration [80,81,82]. In addition, studies in a murine model of NASH suggest anti-fibrotic and anti-inflammatory effects for human hepatic stem cell-derived EVs [83]. Furthermore, a recent study reported a beneficial role of exosomes in the regeneration of liver cells, as inhibition of exosome release from human hepatic progenitors induced reactive oxygen species (ROS) production and caused cell death of immortalized hepatocytes [84].
Pathophysiological roles of MSC-derived EVs in acute kidney injury (AKI) have been suggested by a series of studies. Bruno et al. suggested that MSC-derived EVs could deliver mRNA and induce tubular cell proliferation in glycerol-induced AKI mice [85]. They also showed that MSC-derived EVs enhance renal function in cisplatin-induced AKI mice and upregulate anti-apoptotic genes in cisplatin-treated human tubular epithelial cells [86]. Tomasoni et al. further demonstrated that BM MSC-derived EVs enclosing insulin-like growth factor-1 receptor mRNA enhance proliferation of proximal tubular cells [87]. In addition, both adipose-derived MSCs (ADMSCs) and ADMSC-derived EVs can improve AKI [88].
Immunomodulatory functions of MSC-EVs have shown therapeutic effects in various disease models [89]. A recent study revealed that the anti-inflammatory effects of BM MSC-derived exosomes can be significantly enhanced by interleukin-1β (IL-1β) priming [90]. The exosome obtained from IL-1β-primed BM MSCs exhibits powerful anti-inflammatory activity in osteoarthritic SW982 cells, mediated mainly by miR-147b in exosomes. These immunomodulatory effects are also found in an ocular Sjogren’s syndrome mouse model, which recapitulates a chronic autoimmune disease, where MSC-EVs from early-passage MSCs are more therapeutic than MSC-EVs from late passage MSCs [79]. MSC-EVs containing TGF-β1, pentraxin3, let-7b-5p or miR-21-5p have high immunosuppressive effects in the autoimmune disorder [79]. Furthermore, MSC-EVs also showed therapeutic effects in skin injuries by promoting cutaneous wound healing [89, 91]. Kim et al. revealed that EVs from iPSC-derived MSCs enhance cell proliferation and collagen secretion in human keratinocytes and human dermal fibroblasts [92], suggesting a potential therapeutic application of MSC-EVs in skin regeneration.
Cardiovascular progenitor cells (CVPCs) were found to secrete EVs with a protective effect against cardiovascular diseases. An in vitro study proposed that EVs secreted from human CVPCs (hCVPC) could enhance both the survival of cardiac cells and the migration of endothelial cells in rats [93]. Similarly, injection of hCVPC-derived EVs into mice with chronic heart failure resulted in increased expression of cardioprotective genes, thereby improving cardiac function [93, 94]. It was also found that EVs secreted from CVPCs under hypoxic conditions are highly enriched with the long non-coding RNA MALAT1, which can enhance survival of cardiomyocytes and increase angiogenesis via miR-497 inhibition [94]. These compelling findings suggest a promising application of TSC-derived EVs in therapeutic treatments, without the need for cell transplantation. However, substantial efforts are required to improve methods of EV production and to identify molecular mechanisms and key contributors underlying the therapeutic action of TSC-derived EVs.
3.2 Pluripotent stem cell-derived EVs
Encompassing both human ESCs (hESCs) and human iPSCs (hiPSCs), hPSCs can self-renew and differentiate to become diverse types of terminally differentiated cells. Although transplantation of hPSCs or hPSC-derived somatic cells can ameliorate tissue injury and promote tissue regeneration, the tumorigenic potential of residual undifferentiated or unwanted cells hinder clinical applications of hPSCs. To address this issue, diverse approaches for utilizing hPSC-derived EVs as a cell-free therapeutic system to substitute for hPSC use are currently underway. Although hiPSCs are known to secrete a 16-fold higher level of EVs than MSCs [64], only a few studies have conducted on the potential therapeutic roles of PSC-derived EVs as below.
A previous study showed that mouse ESC-derived EVs could repair myocardial infarction by enhancing functions of cardiac progenitors and reducing fibrosis [95]. Similarly, this protective effect of mouse iPSC-derived EVs in ischemic myocardium was found to be due to Nanog-related miR-21 and HIF-α-related miR-210 present in iPSC-derived EVs [96]. Additionally, mouse ESC-derived exosomes induce M1 to M2 polarization of macrophages, thus alleviating inflammation-induced pyroptosis in doxorubicin-induced cardiomyopathy [97]. Furthermore, another study revealed that iPSC-derived EVs promote angiogenesis of murine endothelial cells in vitro and enhance left ventricular function in vivo. [98]. These findings suggest that hPSC-derived EVs could be utilized as a new cell-free therapy for cardiac diseases.
A recent analysis of the anti-fibrotic roles of human iPSC-derived EVs in liver fibrosis showed iPSC-derived EVs reduce chemotaxis and proliferation of HSCs and also diminish various HSC activation markers, such as α-smooth muscle actin (α-SMA), collagen type I, and tissue inhibitor of metalloproteinase-1 (TIMP1) [99]. Genomic analysis also revealed that miR-92a-3p, a well-known anti-fibrotic factor of pulmonary fibrosis [100], was the most abundant miRNA in iPSC-EVs [99]. Furthermore, the authors demonstrated the anti-fibrotic effects of iPSC-EVs in CCL4-induced liver fibrosis mice [99]. Our previous studies support these findings, as the secretomes of hPSC-derived progenies can promote endogenous regeneration of host injured tissues by delivering trophic factors [9].
Although ADMSC-derived EVs have shown therapeutic effects on AKI as described earlier [88], only recently has there been a report on PSC-derived EVs in AKI [101]. When compared to ADMSC-derived EVs, iPSC-derived EVs more efficiently inhibit cell death of human proximal tubule epithelial cells and also reduce mitochondria damage in hypoxia-reoxygeneration injury. Additionally, using an animal model of AKI, researchers showed that iPSC-EVs protect the kidney from tubular cell death, increase renal tubular cell proliferation, enhance kidney functions, and reduce macrophage infiltration.
iPSC-derived EVs are considered a tool for siRNA delivery across the blood–brain barrier to inhibit expression of pro-inflammatory genes [102]. Ju et al. delivered specific siRNAs to iPSC-derived EVs by electroporation in an attempt to silence intercellular adhesion molecule-1 (ICAM-1) in recipient cells. The modified iPSC-derived EVs reduced ICAM-1 expression and attenuated neutrophil-endothelium adhesion in lipopolysaccharide-stimulated human primary pulmonary microvascular endothelial cells [103]. These data support the idea that iPSC-EVs can serve as efficient messengers to deliver predesigned therapeutic molecules to target cells across different diseases and injuries.
4 Roles of EVs in reproduction, stemness, and differentiation
As described above, stem cell-derived EVs have been suggested as promising therapeutics for various diseases. As adult stem cells are responsible for homeostasis and regeneration in normal and injured tissues, understanding the roles of EVs and the underlying mechanisms in stem cell biology are priorities for basic research and therapeutic applications. The potential roles of stem cell-derived EVs in stemness and differentiation are summarized in Table 2.
4.1 EVs in early embryonic development
Successful implantation and early embryonic development rely on intercellular communications between the embryo and maternal tissues. Reports have recently emerged on the critical role of EVs in these communication processes [104]. Co-culture of parthenogenetic embryos with cloned embryos [105] or supplementation of embryo-derived EVs to cloned embryos [106] improved the survival and blastocyst formation of cloned embryos in vitro. Incubation of mouse embryos with outgrowth embryo-derived EVs improved both the development of pre-implantation embryos and implantation rates when transferred in utero [107]. ESC-derived EVs containing a high level of laminin and fibronectin have the capacity to activate JNK and FAK in trophoblasts and promote efficient implantation of blastocysts [108]. Additionally, endometrial exosomes containing menstrual cycle-related hormones activate the FAK signaling pathway and improve implantation efficiency through increased adhesive capacity of trophoblasts [109]. Compared to other physiological roles of EVs, contributions of EVs in mediating embryo-maternal crosstalk during implantation, pregnancy, and infertility are emerging as important issues [110, 111]. Therefore, a better understanding of the role of EVs in reproduction will facilitate development of new diagnostic tests and treatments for infertility.
4.2 EVs in the self-renewal and differentiation of stem cells
EVs derived from various cell types likely have the potential to regulate both maintenance and differentiation of stem cells by transferring proteins, mRNAs, and miRNAs or by directly activating signaling pathways through binding to cell surface molecules [112]. Acute myeloid leukemia (AML) blast-derived exosomes block normal hematopoiesis by inducing DKK1 expression [113] and transferring miR-150/155, which downregulates C-MYB in hematopoietic stem cells [114]. Erythroleukemia cell-derived exosomes deliver miR486 to target Sirt1 in hematopoietic stem cells and induce differentiation and proliferation of erythroids under hypoxia [115]. On the other hand, MSC-derived EVs can selectively support survival and clonogenic potential of hematopoietic stem cells [116]. Other reports show that MSC-EVs promote the concomitant expansion of hematopoietic stem cells into myeloid progenitors by TLR4 activation [117]. EVs also regulate G-CSF-induced migration of hematopoietic stem cells from BM into peripheral blood through transfer of miR-126 [118].
EVs derived from various cells can also regulate the proliferation and differentiation of MSCs. Endothelial cell-derived EVs promote proliferation and migration of MSCs through NF-kB signaling activation [119]. ESC-derived engineered nanovesicles increase self-renewal and pluripotency gene expression in BMSCs [120]. A recent study showed that AML-derived EVs increase the number of mesenchymal progenitor cells and inhibit osteogenic differentiation in the BM niche [113]. Lineage-specific EVs from ADMSCs [121], osteogenic MSCs [122], monocytes [123], and neurons [124] induce differentiation of MSCs into brown/white adipocytes, osteoblasts, and neuron-like cells, respectively, in vitro.
Both embryonic and adult central nervous systems harbor self-renewing pools of NSCs that reside in distinct niches. The balance between quiescence, proliferation, and differentiation of NSCs, critical for normal development and regeneration of the brain, is tightly regulated by microenvironmental factors, including EVs. Embryonic cerebrospinal fluid contains 1012 nanovesicles/ml that activate the IGF-mTORC1 pathway and promote amplification of embryonic NSCs during corticogenesis [125]. EVs also play important roles in adult neurogenesis by transferring various miRNAs (miR-let7b, -9, -34a, -124a, -128, -137, 125b) and proteins involved in signaling pathways associated with TGFβ, EGFR, and VEGF [126]. Additionally, MSC-derived EVs protect NSCs from hypoxic injury by delivering miR-210-3p and miR-133b [127].
Although still in early stages of investigation, accumulating evidence suggests that EVs have important roles in stemness maintenance and differentiation in PSCs. Like other cells, ESCs secrete and uptake EVs that contain various cargo molecules and can transfer miRNAs and proteins to different cell types [128]. Recently, ESC-derived EVs containing fibronectin were shown to help ESCs maintain their stemness and to prevent differentiation via activating the integrin-FAK pathway [129]. In contrast, HSC-derived exosomes enriched in miR-126 suppress Notch1 expression and promote hematopoietic differentiation of ESCs [130]. Therefore, elucidating the underlying mechanisms of EV-mediated PSC regulation may prove helpful for maintaining pluripotency and the precise control of directed differentiation.
4.3 EVs in cellular reprogramming
The capabilities of stem cell-derived EVs in cellular reprogramming, rejuvenation, and aging are of great interest, as stem cell-derived EVs contain stemness-related transcripts and proteins. hESC derived-MVs induce reprogramming of hematopoietic progenitors by delivering pluripotency transcripts, including Oct4, Nanog, and Wnt3 [57]. ESC-MVs can induce dedifferentiation of Müller cells by activating the retinal regeneration program through delivering both pluripotency transcripts, such as Oct4, Nanog, and Sox2, and also miR-290 [131]. Additionally, MSC-EVs convert mature murine hepatocytes into a progenitor oval cell-like phenotype that highly express OC2 and EpCAM [132].
Furthermore, ESC/iPSC-derived EVs rejuvenate senescent MSCs and skin fibroblasts via activating the IGF1/PI3K/Akt pathway [133, 134]. Hypothalamic stem cells regulate systemic aging through the secretion of exosomal miRNAs into cerebrospinal fluid [60]. In addition, endothelial progenitor cell-derived EVs activate the angiogenic program and the PI3K/Akt pathway via integrin α4 and β1 in endothelial cells [135]. In contrast, EV-mediated transfer of miR-183-5p from aged BM MSCs induced aging in young MSCs [37]. Currently, only a few studies have investigated the biological role of stem cell-derived EVs associated with pluripotency and reprogramming. However, the new insight into the role of EVs in cellular reprogramming mechanisms may provide more effective strategies for cellular reprogramming using EVs, likely without any integration of transgenes.
5 Challenges and future directions
EVs are emerging as a powerful tool for regenerative medicine. However, many issues must be overcome before their application for therapeutic purposes. A growing body of evidence on the cellular roles of EVs strongly suggests the potential for their use in treating diverse diseases and injuries across different organs. As an alternative to other approaches, cell-free EV-based therapies are currently gaining interest due to the reduced risks of tumorigenesis or other graft-mediated complications. However, how EVs contribute to therapeutic outcomes remains obscure and largely enigmatic, as less is known about the molecular mechanisms underlying their release, targeting, uptake, and intracellular dynamics. Moreover, EVs contain molecules of varying concentration and composition, both within the cell and on the cell surface. Thus, EV contents could differ depending on their cellular origin or culture conditions. These variables may cause discrepancies in therapeutic outcomes between studies and hinder the translation of EVs into the clinical setting. Moreover, in addition to beneficial molecules, EVs may contain detrimental factors that cause adverse effects and raise safety issues. Therefore, to understand EV mechanisms of action in cellular behaviors and tissue repair, more precise studies must be conducted using integrative analyses, including bioinformatic tools and other molecular approaches to identify key therapeutic factors.
Addressing the above issues is currently hampered by several technical hurdles regarding the efficient preparation and purification of EVs ranging in size from nano- to microscale. Currently, many patents address techniques aimed at improving isolation methods to secure a large amount of EVs with high reproducibility and without harmful contaminants [136]. Of particular issue is that hPSCs and TSCs are not amenable to batch or long-term culture to obtain sufficient numbers of EVs. Recent advances in 3D cultivation systems may allow mass production of stem cells without losing their properties. Although not yet demonstrated experimentally, 3D culture systems may produce more tissue-like 3D structures as compared to 2D culture conditions and may facilitate the release of EVs with higher therapeutic potential. Indeed, a recent study obtained EVs from size-controlled 3D human MSC spheroids by using both PEG hydrogel microwell arrays and orbital shaking to show that 3D MSC spheroid-derived EVs were capable of stimulating angiogenesis and neurogenesis [137].
Solving the challenges associated with the preparation and analysis of EVs may allow for broader application of EVs in regenerative medicine, as well as in vitro manipulation of stem cells. Therefore, further studies and developments for optimizing EV production, careful analysis of key EV cargos, and identifying EV mechanisms of action are warranted. Nevertheless, it has become increasingly clear that the many beneficial outcomes from stem cell-associated transplantation are mediated in a paracrine manner by EV cargo. Therefore, stem cell-derived EVs have potential value as an ideal therapeutic agent for many degenerative diseases in the near future. Deciphering the molecular complexity of the beneficial paracrine action of EVs will facilitate translation of the exciting recent findings of EVs from bench to bedside.
Data availability
The present manuscript is based on the data published and does not include original data.
References
Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19:1129–55.
Clarke D, Frisén J. Differentiation potential of adult stem cells. Curr Opin Genet Dev. 2001;11:575–80.
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72.
Volarevic V, Ljujic B, Stojkovic P, Lukic A, Arsenijevic N, Stojkovic M. Human stem cell research and regenerative medicine–present and future. Br Med Bull. 2011;99:155–68.
Choumerianou DM, Dimitriou H, Kalmanti M. Stem cells: promises versus limitations. Tissue Eng Part B Rev. 2008;14:53–60.
Baraniak PR, McDevitt TC. Stem cell paracrine actions and tissue regeneration. Regen Med. 2010;5:121–43.
Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–84.
Yang D, Wang W, Li L, Peng Y, Chen P, Huang H, et al. The relative contribution of paracine effect versus direct differentiation on adipose-derived stem cell transplantation mediated cardiac repair. PLoS One. 2013;8:e59020.
Woo DH, Kim SK, Lim HJ, Heo J, Park HS, Kang GY, et al. Direct and indirect contribution of human embryonic stem cell-derived hepatocyte-like cells to liver repair in mice. Gastroenterology. 2012;142:602–11.
An SY, Jang YJ, Lim HJ, Han J, Lee J, Lee G, et al. Milk fat globule-EGF Factor 8, secreted by mesenchymal stem cells, protects against liver fibrosis in mice. Gastroenterology. 2017;152:1174–86.
Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–24.
Cho SW, Moon SH, Lee SH, Kang SW, Kim J, Lim JM, et al. Improvement of postnatal neovascularization by human embryonic stem cell derived endothelial-like cell transplantation in a mouse model of hindlimb ischemia. Circulation. 2007;116:2409–19.
Han C, Sun X, Liu L, Jiang H, Shen Y, Xu X, et al. Exosomes and their therapeutic potentials of stem cells. Stem Cells Int. 2016;2016:7653489.
Xia J, Minamino S, Kuwabara K, Arai S. Stem cell secretome as a new booster for regenerative medicine. Biosci Trends. 2019;13:299–307.
Öztürk S, Elçin AE, Koca A, Elçin YM. Therapeutic applications of stem cells and extracellular vesicles in emergency care: futuristic perspectives. Stem Cell Rev Rep. 2021;17:390–410.
Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750.
Rashed MH, Bayraktar E, Helal GK, Abd-Ellah MF, Amero P, Chavez-Reyes A, et al. Exosomes: from garbage bins to promising therapeutic targets. Int J Mol Sci. 2017;18:538.
Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75:193–208.
Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol. 2013;113:1–11.
Vlachakis D, Mitsis T, Nicolaides N, Efthimiadou A, Giannakakis A, Bacopoulou F, et al. Functions, pathophysiology and current insights of exosomal endocrinology (Review). Mol Med Rep. 2021;23:26.
Zhang HG, Grizzle WE. Exosomes: a novel pathway of local and distant intercellular communication that facilitates the growth and metastasis of neoplastic lesions. Am J Pathol. 2014;184:28–41.
Matsumoto J, Stewart T, Sheng L, Li N, Bullock K, Song N, et al. Transmission of alpha-synuclein-containing erythrocyte-derived extracellular vesicles across the blood-brain barrier via adsorptive mediated transcytosis: another mechanism for initiation and progression of Parkinson’s disease? Acta Neuropathol Commun. 2017;5:71.
Farooqi AA, Desai NN, Qureshi MZ, Librelotto DRN, Gasparri ML, Bishayee A, et al. Exosome biogenesis, bioactivities and functions as new delivery systems of natural compounds. Biotechnol Adv. 2018;36:328–34.
Théry C, Duban L, Segura E, Véron P, Lantz O, Amigorena S. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol. 2002;3:1156–62.
Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–6.
Gong M, Yu B, Wang J, Wang Y, Liu M, Paul C, et al. Mesenchymal stem cells release exosomes that transfer miRNAs to endothelial cells and promote angiogenesis. Oncotarget. 2017;8:45200–12.
Gurunathan S, Kang MH, Jeyaraj M, Qasim M, Kim JH. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells. 2019;8:307.
Cheng L, Wang Y, Huang L. Exosomes from M1-polarized macrophages potentiate the cancer vaccine by creating a pro-inflammatory microenvironment in the lymph node. Mol Ther. 2017;25:1665–75.
Segura E, Nicco C, Lombard B, Véron P, Raposo G, Batteux F, et al. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood. 2005;106:216–23.
Gasser O, Schifferli JA. Activated polymorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis. Blood. 2004;104:2543–8.
Zhu D, Tian J, Wu X, Li M, Tang X, Rui K, et al. G-MDSC-derived exosomes attenuate collagen-induced arthritis by impairing Th1 and Th17 cell responses. Biochim Biophys Acta Mol Basis Dis. 2019;1865:165540.
Pan Y, Hui X, Hoo RLC, Ye D, Chan CYC, Feng T, et al. Adipocyte-secreted exosomal microRNA-34a inhibits M2 macrophage polarization to promote obesity-induced adipose inflammation. J Clin Invest. 2019;129:834–49.
Okoye IS, Coomes SM, Pelly VS, Czieso S, Papayannopoulos V, Tolmachova T, et al. MicroRNA-containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity. 2014;41:89–103.
D’Anca M, Fenoglio C, Serpente M, Arosio B, Cesari M, Scarpini EA, et al. Exosome determinants of physiological aging and age-related neurodegenerative diseases. Front Aging Neurosci. 2019;11:232.
Olivieri F, Albertini MC, Orciani M, Ceka A, Cricca M, Procopio AD, et al. DNA damage response (DDR) and senescence: shuttled inflamma-miRNAs on the stage of inflamm-aging. Oncotarget. 2015;6:35509–21.
Alique M, Ruíz-Torres MP, Bodega G, Noci MV, Troyano N, Bohórquez L, et al. Microvesicles from the plasma of elderly subjects and from senescent endothelial cells promote vascular calcification. Aging (Albany NY). 2017;9:778–89.
Davis C, Dukes A, Drewry M, Helwa I, Johnson MH, Isales CM, et al. MicroRNA-183-5p Increases with Age in Bone-Derived Extracellular Vesicles, Suppresses Bone Marrow Stromal (Stem) Cell Proliferation, and Induces Stem Cell Senescence. Tissue Eng Part A. 2017;23:1231–40.
Wang W, Wang L, Ruan L, Oh J, Dong X, Zhuge Q, et al. Extracellular vesicles extracted from young donor serum attenuate inflammaging via partially rejuvenating aged T-cell immunotolerance. FASEB J. 2018;32:fj201800059R.
Lei Q, Gao F, Liu T, Ren W, Chen L, Cao Y, et al. Extracellular vesicles deposit PCNA to rejuvenate aged bone marrow-derived mesenchymal stem cells and slow age-related degeneration. Sci Transl Med. 2021;13:eaaz8697.
Dickens AM, Tovar-Y-Romo LB, Yoo SW, Trout AL, Bae M, Kanmogne M, et al. Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Sci Signal. 2017;10:eaai7696.
Eitan E, Hutchison ER, Marosi K, Comotto J, Mustapic M, Nigam SM, et al. Extracellular vesicle-associated Abeta mediates trans-neuronal bioenergetic and Ca(2+)-handling deficits in Alzheimer's disease models. NPJ Aging Mech Dis. 2016;2:16019.
Saman S, Kim W, Raya M, Visnick Y, Miro S, Saman S, et al. Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J Biol Chem. 2012;287:3842–9.
Guo Q, Furuta K, Lucien F, Gutierrez Sanchez LH, Hirsova P, Krishnan A, et al. Integrin beta1-enriched extracellular vesicles mediate monocyte adhesion and promote liver inflammation in murine NASH. J Hepatol. 2019;71:1193–205.
Povero D, Panera N, Eguchi A, Johnson CD, Papouchado BG, de Araujo HL, et al. Lipid-induced hepatocyte-derived extracellular vesicles regulate hepatic stellate cell via microRNAs targeting PPAR-gamma. Cell Mol Gastroenterol Hepatol. 2015;1:646–63.e4.
Lee YS, Kim SY, Ko E, Lee JH, Yi HS, Yoo YJ, et al. Exosomes derived from palmitic acid-treated hepatocytes induce fibrotic activation of hepatic stellate cells. Sci Rep. 2017;7:3710.
Withrow J, Murphy C, Liu Y, Hunter M, Fulzele S, Hamrick MW. Extracellular vesicles in the pathogenesis of rheumatoid arthritis and osteoarthritis. Arthritis Res Ther. 2016;18:286.
Hafiane A, Daskalopoulou SS. Extracellular vesicles characteristics and emerging roles in atherosclerotic cardiovascular disease. Metabolism. 2018;85:213–22.
Kang X, Zuo Z, Hong W, Tang H, Geng W. Progress of research on exosomes in the protection against ischemic brain injury. Front Neurosci. 2019;13:1149.
Bjørge IM, Kim SY, Mano JF, Kalionis B, Chrzanowski W. Extracellular vesicles, exosomes and shedding vesicles in regenerative medicine—a new paradigm for tissue repair. Biomater Sci. 2017;6:60–78.
Szabo G, Momen-Heravi F. Extracellular vesicles in liver disease and potential as biomarkers and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2017;14:455–66.
Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10:619–24.
Lee TH, Chennakrishnaiah S, Audemard E, Montermini L, Meehan B, Rak J. Oncogenic ras-driven cancer cell vesiculation leads to emission of double-stranded DNA capable of interacting with target cells. Biochem Biophys Res Commun. 2014;451:295–301.
Cai J, Wu G, Tan X, Han Y, Chen C, Li C, et al. Transferred BCR/ABL DNA from K562 extracellular vesicles causes chronic myeloid leukemia in immunodeficient mice. PLoS One. 2014;9:e105200.
Dinh NTH, Lee J, Lee J, Kim SS, Go G, Bae S, et al. Indoor dust extracellular vesicles promote cancer lung metastasis by inducing tumour necrosis factor-alpha. J Extracell Vesicles. 2020;9:1766821.
Wolfers J, Lozier A, Raposo G, Regnault A, Théry C, Masurier C, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7:297–303.
Lim W, Kim HS. Exosomes as therapeutic vehicles for cancer. Tissue Eng Regen Med. 2019;16:213–23.
Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20:847–56.
Tavakoli Dargani Z, Singla DK. Embryonic stem cell-derived exosomes inhibit doxorubicin-induced TLR4-NLRP3-mediated cell death-pyroptosis. Am J Physiol Heart Circ Physiol. 2019;317:H460–71.
Chen B, Sun Y, Zhang J, Zhu Q, Yang Y, Niu X, et al. Human embryonic stem cell-derived exosomes promote pressure ulcer healing in aged mice by rejuvenating senescent endothelial cells. Stem Cell Res Ther. 2019;10:142.
Zhang Y, Kim MS, Jia B, Yan J, Zuniga-Hertz JP, Han C, et al. Hypothalamic stem cells control ageing speed partly through exosomal miRNAs. Nature. 2017;548:52–7.
Zhu B, Zhang L, Liang C, Liu B, Pan X, Wang Y, et al. Stem cell-derived exosomes prevent aging-induced cardiac dysfunction through a novel exosome/lncRNA MALAT1/NF-kappaB/TNF-alpha signaling pathway. Oxid Med Cell Longev. 2019;2019:9739258.
Kulkarni R, Bajaj M, Ghode S, Jalnapurkar S, Limaye L, Kale VP. Intercellular transfer of microvesicles from young mesenchymal stromal cells rejuvenates aged murine hematopoietic stem cells. Stem Cells. 2018;36:420–33.
Gong L, Chen B, Zhang J, Sun Y, Yuan J, Niu X, et al. Human ESC-sEVs alleviate age-related bone loss by rejuvenating senescent bone marrow-derived mesenchymal stem cells. J Extracell Vesicles. 2020;9:1800971.
Liu S, Mahairaki V, Bai H, Ding Z, Li J, Witwer KW, et al. Highly purified human extracellular vesicles produced by stem cells alleviate aging cellular phenotypes of senescent human cells. Stem Cells. 2019;37:779–90.
Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab. 2013;33:1711–5.
Xin H, Katakowski M, Wang F, Qian JY, Liu XS, Ali MM, et al. MicroRNA cluster miR-17-92 Cluster in exosomes enhance neuroplasticity and functional recovery after stroke in rats. Stroke. 2017;48:747–53.
Lee JY, Kim E, Choi SM, Kim DW, Kim KP, Lee I, et al. Microvesicles from brain-extract-treated mesenchymal stem cells improve neurological functions in a rat model of ischemic stroke. Sci Rep. 2016;6:33038.
Webb RL, Kaiser EE, Scoville SL, Thompson TA, Fatima S, Pandya C, et al. Human neural stem cell extracellular vesicles improve tissue and functional recovery in the murine thromboembolic stroke model. Transl Stroke Res. 2018;9:530–9.
Webb RL, Kaiser EE, Jurgielewicz BJ, Spellicy S, Scoville SL, Thompson TA, et al. Human neural stem cell extracellular vesicles improve recovery in a porcine model of ischemic stroke. Stroke. 2018;49:1248–56.
Lee M, Liu T, Im W, Kim M. Exosomes from adipose-derived stem cells ameliorate phenotype of Huntington’s disease in vitro model. Eur J Neurosci. 2016;44:2114–9.
Chun C, Smith AST, Kim H, Kamenz DS, Lee JH, Lee JB, et al. Astrocyte-derived extracellular vesicles enhance the survival and electrophysiological function of human cortical neurons in vitro. Biomaterials. 2021;271:120700.
Zhu YG, Feng XM, Abbott J, Fang XH, Hao Q, Monsel A, et al. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells. 2014;32:116–25.
Monsel A, Zhu YG, Gennai S, Hao Q, Hu S, Rouby JJ, et al. Therapeutic effects of human mesenchymal stem cell-derived microvesicles in severe pneumonia in mice. Am J Respir Crit Care Med. 2015;192:324–36.
Hao Q, Gudapati V, Monsel A, Park JH, Hu S, Kato H, et al. Mesenchymal stem cell-derived extracellular vesicles decrease lung injury in mice. J Immunol. 2019;203:1961–72.
Khatri M, Richardson LA, Meulia T. Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res Ther. 2018;9:17.
Bari E, Ferrarotti I, Di Silvestre D, Grisoli P, Barzon V, Balderacchi A, et al. Adipose mesenchymal extracellular vesicles as alpha-1-antitrypsin physiological delivery systems for lung regeneration. Cells. 2019;8:965.
Li T, Yan Y, Wang B, Qian H, Zhang X, Shen L, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013;22:845–54.
Qu Y, Zhang Q, Cai X, Li F, Ma Z, Xu M, et al. Exosomes derived from miR-181-5p-modified adipose-derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. J Cell Mol Med. 2017;21:2491–502.
Kim H, Lee MJ, Bae EH, Ryu JS, Kaur G, Kim HJ, et al. Comprehensive molecular profiles of functionally effective MSC-derived extracellular vesicles in immunomodulation. Mol Ther. 2020;28:1628–44.
Yao J, Zheng J, Cai J, Zeng K, Zhou C, Zhang J, et al. Extracellular vesicles derived from human umbilical cord mesenchymal stem cells alleviate rat hepatic ischemia-reperfusion injury by suppressing oxidative stress and neutrophil inflammatory response. FASEB J. 2019;33:1695–710.
Haga H, Yan IK, Borrelli DA, Matsuda A, Parasramka M, Shukla N, et al. Extracellular vesicles from bone marrow-derived mesenchymal stem cells protect against murine hepatic ischemia/reperfusion injury. Liver Transpl. 2017;23:791–803.
Rostom DM, Attia N, Khalifa HM, Abou Nazel MW, El Sabaawy EA. The therapeutic potential of extracellular vesicles versus mesenchymal stem cells in liver damage. Tissue Eng Regen Med. 2020;17:537–52.
Bruno S, Pasquino C, Herrera Sanchez MB, Tapparo M, Figliolini F, Grange C, et al. HLSC-Derived extracellular vesicles attenuate liver fibrosis and inflammation in a murine model of non-alcoholic steatohepatitis. Mol Ther. 2020;28:479–89.
Hyung S, Jeong J, Shin K, Kim JY, Yim JH, Yu CJ, et al. Exosomes derived from chemically induced human hepatic progenitors inhibit oxidative stress induced cell death. Biotechnol Bioeng. 2020;117:2658–67.
Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20:1053–67.
Bruno S, Grange C, Collino F, Deregibus MC, Cantaluppi V, Biancone L, et al. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One. 2012;7:e33115.
Tomasoni S, Longaretti L, Rota C, Morigi M, Conti S, Gotti E, et al. Transfer of growth factor receptor mRNA via exosomes unravels the regenerative effect of mesenchymal stem cells. Stem Cells Dev. 2013;22:772–80.
Lin KC, Yip HK, Shao PL, Wu SC, Chen KH, Chen YT, et al. Combination of adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes for protecting kidney from acute ischemia-reperfusion injury. Int J Cardiol. 2016;216:173–85.
Ha DH, Kim HK, Lee J, Kwon HH, Park GH, Yang SH, et al. Mesenchymal stem/stromal cell-derived exosomes for immunomodulatory therapeutics and skin regeneration. Cells. 2020;9:1157.
Kim M, Shin DI, Choi BH, Min BH. Exosomes from IL-1beta-primed mesenchymal stem cells inhibited IL-1beta- and TNF-alpha-mediated inflammatory responses in osteoarthritic SW982 cells. Tissue Eng Regen Med. 2021;18:525–36.
Heo JS, Kim S, Yang CE, Choi Y, Song SY, Kim HO. Human adipose mesenchymal stem cell-derived exosomes: a key player in wound healing. Tissue Eng Regen Med. 2021;18:537–48.
Kim S, Lee SK, Kim H, Kim TM. Exosomes secreted from induced pluripotent stem cell-derived mesenchymal stem cells accelerate skin cell proliferation. Int J Mol Sci. 2018;19:3119.
El Harane N, Kervadec A, Bellamy V, Pidial L, Neametalla HJ, Perier MC, et al. Acellular therapeutic approach for heart failure: in vitro production of extracellular vesicles from human cardiovascular progenitors. Eur Heart J. 2018;39:1835–47.
Wu Q, Wang J, Tan WLW, Jiang Y, Wang S, Li Q, et al. Extracellular vesicles from human embryonic stem cell-derived cardiovascular progenitor cells promote cardiac infarct healing through reducing cardiomyocyte death and promoting angiogenesis. Cell Death Dis. 2020;11:354.
Khan M, Nickoloff E, Abramova T, Johnson J, Verma SK, Krishnamurthy P, et al. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ Res. 2015;117:52–64.
Wang Y, Zhang L, Li Y, Chen L, Wang X, Guo W, et al. Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. Int J Cardiol. 2015;192:61–9.
Singla DK, Johnson TA, Tavakoli Dargani Z. Exosome treatment enhances anti-inflammatory M2 macrophages and reduces inflammation-induced pyroptosis in doxorubicin-induced cardiomyopathy. Cells. 2019;8:1224.
Adamiak M, Cheng G, Bobis-Wozowicz S, Zhao L, Kedracka-Krok S, Samanta A, et al. Induced Pluripotent Stem Cell (iPSC)-derived extracellular vesicles are safer and more effective for cardiac repair than iPSCs. Circ Res. 2018;122(2):296–309.
Povero D, Pinatel EM, Leszczynska A, Goyal NP, Nishio T, Kim J, et al. Human induced pluripotent stem cell-derived extracellular vesicles reduce hepatic stellate cell activation and liver fibrosis. JCI Insight. 2019;5:e125652.
Berschneider B, Ellwanger DC, Baarsma HA, Thiel C, Shimbori C, White ES, et al. miR-92a regulates TGF-beta1-induced WISP1 expression in pulmonary fibrosis. Int J Biochem Cell Biol. 2014;53:432–41.
Collino F, Lopes JA, Tapparo M, Tortelote GG, Kasai-Brunswick TH, Lopes GMC, et al. Extracellular vesicles derived from induced pluripotent stem cells promote renoprotection in acute kidney injury model. Cells. 2020;9:453.
El Andaloussi S, Lakhal S, Mäger I, Wood MJ. Exosomes for targeted siRNA delivery across biological barriers. Adv Drug Deliv Rev. 2013;65:391–7.
Ju Z, Ma J, Wang C, Yu J, Qiao Y, Hei F. Exosomes from iPSCs delivering siRNA attenuate intracellular adhesion molecule-1 expression and neutrophils adhesion in pulmonary microvascular endothelial cells. Inflammation. 2017;40:486–96.
Qamar AY, Mahiddine FY, Bang S, Fang X, Shin ST, Kim MJ, et al. Extracellular vesicle mediated crosstalk between the gametes, conceptus, and female reproductive tract. Front Vet Sci. 2020;7:589117.
Saadeldin IM, Kim SJ, Choi YB, Lee BC. Improvement of cloned embryos development by co-culturing with parthenotes: a possible role of exosomes/microvesicles for embryos paracrine communication. Cell Reprogram. 2014;16:223–34.
Qu P, Qing S, Liu R, Qin H, Wang W, Qiao F, et al. Effects of embryo-derived exosomes on the development of bovine cloned embryos. PLoS One. 2017;12:e0174535.
Kim J, Lee J, Lee TB, Jun JH. Embryotrophic effects of extracellular vesicles derived from outgrowth embryos in pre- and peri-implantation embryonic development in mice. Mol Reprod Dev. 2019;86:187–96.
Desrochers LM, Bordeleau F, Reinhart-King CA, Cerione RA, Antonyak MA. Microvesicles provide a mechanism for intercellular communication by embryonic stem cells during embryo implantation. Nat Commun. 2016;7:11958.
Greening DW, Nguyen HP, Elgass K, Simpson RJ, Salamonsen LA. Human endometrial exosomes contain hormone-specific cargo modulating trophoblast adhesive capacity: insights into endometrial-embryo interactions. Biol Reprod. 2016;94:38.
Das M, Kale V. Extracellular vesicles: Mediators of embryo-maternal crosstalk during pregnancy and a new weapon to fight against infertility. Eur J Cell Biol. 2020;99:151125.
Sun X, Ma X, Yang X, Zhang X. Exosomes and female infertility. Curr Drug Metab. 2019;20:773–80.
Nawaz M, Fatima F, Vallabhaneni KC, Penfornis P, Valadi H, Ekström K, et al. Extracellular vesicles: evolving factors in stem cell biology. Stem Cells Int. 2016;2016:1073140.
Kumar B, Garcia M, Weng L, Jung X, Murakami JL, Hu X, et al. Acute myeloid leukemia transforms the bone marrow niche into a leukemia-permissive microenvironment through exosome secretion. Leukemia. 2018;32:575–87.
Hornick NI, Doron B, Abdelhamed S, Huan J, Harrington CA, Shen R, et al. AML suppresses hematopoiesis by releasing exosomes that contain microRNAs targeting c-MYB. Sci Signal. 2016;9:ra88.
Shi XF, Wang H, Kong FX, Xu QQ, Xiao FJ, Yang YF, et al. Exosomal miR-486 regulates hypoxia-induced erythroid differentiation of erythroleukemia cells through targeting Sirt1. Exp Cell Res. 2017;351:74–81.
Stik G, Crequit S, Petit L, Durant J, Charbord P, Jaffredo T, et al. Extracellular vesicles of stromal origin target and support hematopoietic stem and progenitor cells. J Cell Biol. 2017;216:2217–30.
Goloviznina NA, Verghese SC, Yoon YM, Taratula O, Marks DL, Kurre P. Mesenchymal stromal cell-derived extracellular vesicles promote myeloid-biased multipotent hematopoietic progenitor expansion via toll-like receptor engagement. J Biol Chem. 2016;291:24607–17.
Salvucci O, Jiang K, Gasperini P, Maric D, Zhu J, Sakakibara S, et al. MicroRNA126 contributes to granulocyte colony-stimulating factor-induced hematopoietic progenitor cell mobilization by reducing the expression of vascular cell adhesion molecule 1. Haematologica. 2012;97:818–26.
Lozito TP, Tuan RS. Endothelial and cancer cells interact with mesenchymal stem cells via both microparticles and secreted factors. J Cell Mol Med. 2014;18:2372–84.
Jo W, Jeong D, Kim J, Park J. Self-Renewal of bone marrow stem cells by nanovesicles engineered from embryonic stem cells. Adv Healthc Mater. 2016;5:3148–56.
Jung YJ, Kim HK, Cho Y, Choi JS, Woo CH, Lee KS, et al. Cell reprogramming using extracellular vesicles from differentiating stem cells into white/beige adipocytes. Sci Adv. 2020;6:eaay6721.
Narayanan K, Kumar S, Padmanabhan P, Gulyas B, Wan ACA, Rajendran VM. Lineage-specific exosomes could override extracellular matrix mediated human mesenchymal stem cell differentiation. Biomaterials. 2018;182:312–22.
Ekström K, Omar O, Granéli C, Wang X, Vazirisani F, Thomsen P. Monocyte exosomes stimulate the osteogenic gene expression of mesenchymal stem cells. PLoS One. 2013;8:e75227.
Takeda YS, Xu Q. Neuronal Differentiation of human mesenchymal stem cells using exosomes derived from differentiating neuronal cells. PLoS One. 2015;10:e0135111.
Feliciano DM, Zhang S, Nasrallah CM, Lisgo SN, Bordey A. Embryonic cerebrospinal fluid nanovesicles carry evolutionarily conserved molecules and promote neural stem cell amplification. PLoS One. 2014;9:e88810.
Bátiz LF, Castro MA, Burgos PV, Velásquez ZD, Muñoz RI, Lafourcade CA, et al. Exosomes as novel regulators of adult neurogenic niches. Front Cell Neurosci. 2016;9:501.
Li F, Zhang J, Liao R, Duan Y, Tao L, Xu Y, et al. Mesenchymal stem cellderived extracellular vesicles prevent neural stem cell hypoxia injury via promoting miR2103p expression. Mol Med Rep. 2020;22:3813–21.
Yuan A, Farber EL, Rapoport AL, Tejada D, Deniskin R, Akhmedov NB, et al. Transfer of microRNAs by embryonic stem cell microvesicles. PLoS One. 2009;4:e4722.
Hur YH, Feng S, Wilson KF, Cerione RA, Antonyak MA. Embryonic stem cell-derived extracellular vesicles maintain ESC stemness by activating FAK. Dev Cell. 2021;56:277-91.e6.
Liao FL, Tan L, Liu H, Wang JJ, Ma XT, Zhao B, et al. Hematopoietic stem cell-derived exosomes promote hematopoietic differentiation of mouse embryonic stem cells in vitro via inhibiting the miR126/Notch1 pathway. Acta Pharmacol Sin. 2018;39:552–60.
Katsman D, Stackpole EJ, Domin DR, Farber DB. Embryonic stem cell-derived microvesicles induce gene expression changes in Muller cells of the retina. PLoS One. 2012;7:e50417.
Wu HH, Lee OK. Exosomes from mesenchymal stem cells induce the conversion of hepatocytes into progenitor oval cells. Stem Cell Res Ther. 2017;8:117.
Zhang Y, Xu J, Liu S, Lim M, Zhao S, Cui K, et al. Embryonic stem cell-derived extracellular vesicles enhance the therapeutic effect of mesenchymal stem cells. Theranostics. 2019;9:6976–90.
Oh M, Lee J, Kim YJ, Rhee WJ, Park JH. Exosomes derived from human induced pluripotent stem cells ameliorate the aging of skin fibroblasts. Int J Mol Sci. 2018;19:1715.
Deregibus MC, Cantaluppi V, Calogero R, Lo Iacono M, Tetta C, Biancone L, et al. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007;110:2440–8.
Urbanelli L, Buratta S, Sagini K, Ferrara G, Lanni M, Emiliani C. Exosome-based strategies for diagnosis and therapy. Recent Pat CNS Drug Discov. 2015;10:10–27.
Cha JM, Shin EK, Sung JH, Moon GJ, Kim EH, Cho YH, et al. Efficient scalable production of therapeutic microvesicles derived from human mesenchymal stem cells. Sci Rep. 2018;8:1171.
Acknowledgement
This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korean government (MIST) (No. 2020R1A2C2006240).
Author information
Authors and Affiliations
Contributions
HJK developed the conceptual framework of the review paper. HJK, GK, JL, YL performed bibliography search and wrote the manuscript including the figure and tables. JHK designed, supervised and wrote the manuscript. All authors commented on the manuscript and agreed with submission to Tissue Engineering and Regenerative Medicine.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Informed consent
None. No investigation involving participants or volunteers has been carried out in the present study.
Ethical statement
No animal or human studies were carried out by the authors. This paper has not been previously published elsewhere and is not currently being considered for publication elsewhere.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, H.J., Kim, G., Lee, J. et al. Secretome of Stem Cells: Roles of Extracellular Vesicles in Diseases, Stemness, Differentiation, and Reprogramming. Tissue Eng Regen Med 19, 19–33 (2022). https://doi.org/10.1007/s13770-021-00406-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-021-00406-4