Abstract
This study was conducted to compare the efficacy of the mouse hepatic and renal antioxidant systems against inflammation-induced oxidative stress. Increased Il-1 and Il-6 expressions, markers of inflammation, were represented by inflammation models in mouse liver and kidney tissues injected intraperitoneally with LPS. After establishing the model, the GSH level and the GSH/GSSG ratio, which are oxidative stress markers, were investigated in both tissues treated with LPS and the control group. The expression of Trx1, TrxR, and Txnip genes increased in the liver tissues of LPS-treated mice. In the kidney tissue, while Trx1 expression decreased, no change was observed in TrxR1 expression, and Txnip expression increased. In the kidneys, TRXR1 and GR activities decreased, whereas GPx activity increased. In both tissues, the TRXR1 protein expression decreased significantly, while TXNIP expression increased. In conclusion, different behaviors of antioxidant system members were observed during acute inflammation in both tissues. Additionally, it can be said that the kidney tissue is more sensitive and takes earlier measures than the liver tissue against cellular damage caused by inflammation and inflammation-induced oxidative stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammation, divided into acute and chronic, is a protective response of the organism’s immune system to pathogens, damaged cells, and chemical or physical agents [1, 2]. Acute inflammation is the first response to restore homeostasis by supporting the repair of injured tissues and the destruction of pathogens and is self-limiting [3]. However, when this process exceeds the duration and severity threshold, reactive oxygen species (ROS) appear, inducing oxidative stress that promotes inflammation [4, 5]. When the antioxidant system does not eliminate the excessive ROS, many diseases associated with chronic inflammation occur, e.g., diabetes, cancer, obesity, cardiovascular and neurodegenerative disorders, and aging [6,7,8,9].
ROS, such as hydroxyl (·OH), hydrogen peroxide (H2O2), peroxide (·O2−2), and superoxide (O2−), produced in organisms at low or medium levels are signal molecules activating cellular differentiation, proliferation pathways, immunological responses, xenobiotic inflammation, and neurotransmitter catabolism [10,11,12]. Endogenous and exogenous agents induced the level of ROS, and the balance between the formation and neutralization of ROS is protected by the basic enzymatic systems, including glutathione and thioredoxin systems that protect cells against oxidative stress. In the glutathione system, glutathione peroxidase (GPx) converts hydrogen peroxide (H2O2) into water using glutathione (GSH). Glutathione disulfide (GSSG), which is formed by GPx reactions, is transformed back into two GSHs by glutathione reductase (GR) utilizing nicotinamide adenine dinucleotide phosphate (NADPH) [13,14,15]. The thioredoxin system, containing thioredoxin 1 (TRX1), thioredoxin reductase 1 (TRXR1), and thioredoxin interacting protein (TXNIP), maintains the cellular redox homeostasis and cellular physiology. TRXR1 uses NADPH as an electron source and converts oxidized TRX1 into reduced TRX1 [16].
The thioredoxin system is related to fundamental biological pathways, such as cell proliferation, DNA synthesis, growth triggering, control of transcription factors, protection against apoptosis, and regulation of cellular signaling pathways. Irregularities in this system cause many diseases that may be closely associated with inflammation [17,18,19,20]. Many studies have investigated the thioredoxin system’s preventive effect, especially TRX1, against acute inflammation. It was reported that Trx1 overexpression in mouse spleen tissue suppressed methamphetamine-induced inflammation [21]. In another study, the overexpression of Trx1 in mouse kidney tissue resisted inflammatory-related diseases, such as hyperglycemia, osteopenia, and nephropathy, through the effective removal of oxidative stress [22]. Additionally, the decreased expression of TrxR1 was observed in the mouse model with Parkinson’s disease, and it was commented that this condition might support oxidative stress and the pathology of diseases [23]. Data from many studies suggest that TRXR1 may be a promising anti-inflammatory target that plays an important role during inflammation [24].
The liver performs many central functions, particularly detoxifying toxic, substances, distributing nutrients, synthesizing fatty acids and cholesterol, metabolites, and regulating blood glucose levels [25]. The liver is also an important part of the immunological activity and the most dominant organ in the struggle against infections, especially inflammation, as a central immunological organ [26]. Hence, the liver is a significant organ for examing the relationship between inflammation, enzymatic antioxidants, and oxidative stress [27]. Many liver diseases may coexist with kidney diseases since many systemic conditions affect both the liver and the kidneys. This study clarifies how the antioxidant systems in liver and kidney tissues, especially the thioredoxin system, respond in case of inflammation and the difference between liver and kidney responses in this process.
Materials and methods
Experimental design and animal care
Young adult (3–4-month-old) male BALB/c mice (Mus musculus; n = 10) were obtained from the Medical Experimental Application and Research Center of Atatürk University. Before starting the experimental procedure, all mice were acclimated to our facilities for a week at 22 ± 2 °C temperature, 40–60% humidity, and 12/12 h light-dark cycle conditions. The mice were randomly divided into two groups as control and treatment. Treatment group mice (n = 5) were injected intraperitoneally with lipopolysaccharide (LPS) (5 mg/kg) dissolved in distilled water to perform inflammation [28]. In parallel, an equal amount of sterile saline solution (0.15 mol / L NaCl) was injected into the control group mice. Six hours after injection, the mice were sacrificed by breaking the neck. Liver and kidney tissues were removed and immediately stored at −80 °C. Animal experiments were performed following the National Research Council’s Guide for the Care and Use of Laboratory Animals and approval for them was obtained from the local Animal Care Committee of Atatürk University (Protocol no: 55885869-381).
Measurement of reduced glutathione (GSH) and oxidized glutathione (GSSG)
Mouse liver and kidney tissues were homogenized using Benchmark Scientific BeadBlaster, as described by Budak et al. [14]. The quantity (mM) of GSH and GSSG was measured using Ellman’s reagent [5,5-dithiobis-2-nitrobenzoic acid (DTNB) solution] [29].
RNA isolation, cDNA synthesis, and gene expression analysis
Total RNA was isolated following the method described by Budak et al. [14]. RNA concentrations and quality were checked by RNA gel electrophoresis. The purity and concentration of RNA samples were confirmed by measuring their absorbance at 260 and 280 nm with a spectrophotometer (Thermo Scientific, Multiskan GO, USA) [30]. The ProtoScript® First Strand cDNA Synthesis Kit (Cat No: E6300S, BioLabs) was used for the synthesis of complementary DNA (cDNA) with a PCR instrument (BIO-RAD C1000TM Thermal Cycler).
Gene-specific primer sets and TaqMan probes (Table 1) were designed using the Primer3 software program (v. 0.4.0) (http://frodo.wi.mit.edu/) and commercially purchased from Metabion. Their gene symbols and GenBank accession numbers were as follows: Il-1 (NM_010554.4), Il-6 (NM_031168.1), Trx1 (NM_011660.3), TrxR1 (NM_001042513.1), Txnip (NM_001009935.2), and β-actin (NM_007393.4). β-actin was used as an internal control gene since it was stable in all treatments. Amplification reactions were carried out, as described by Ceylan et al. [30]. The qPCR data were analyzed, as described by Livak et al. [31].
Measurement of Enzyme Activities
Specific enzyme activities of TRXR1
The preparation of tissue homogenates and the TRXR1 enzyme activity assay were performed according to Aydın et al. [28]. The protein quantification was determined in accordance with the Bradford protein assay using BSA (1 mg/mL) as a standard [32].
Specific enzyme activities of GR and GPx
The preparation of tissue homogenates and the GR and GPx enzyme activities were performed as in the study by Budak et al. [14]. The protein quantification was determined in accordance with the Bradford protein assay using BSA (1 mg/mL) as a standard [33].
Western blot analysis
The preparation of protein homogenates from liver and kidney tissues and then Western blot analysis was performed following the method described by Aydın et al. [28]. TRXR1 (Sc-28321, 1:1000, Santa Cruz, Dallas, Texas, USA) and TXNIP (Sc-271237, 1:1000, Santa Cruz, Dallas, Texas, USA) were used as primary antibodies. β‐actin (Sc-47778) was used as an internal control (1:5000 dilution). Horseradish-coupled secondary antibodies (Santa Cruz Biotechnology, sc-2005) were used as secondary antibodies [34, 35].
Statistical analysis
The LPS and control groups contained five animals, and all measurements were made three times for each animal. The statistical analysis of the results was performed by the unpaired t-test and by using GraphPad Prism Software version 7.0 (GraphPad Software, San Diego, CA, USA) for Windows. Statistical significance was assessed as follows: *P < 0.05 (significant); **P < 0.01 (very significant); ***P < 0.001 and ****P < 0.0001 (extremely significant).
Results
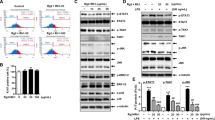
Lipopolysaccharide (LPS) induces the activation of proinflammatory cytokines in liver and kidney tissues
LPS, a pathogenic component in the outer membrane of all Gram-negative bacteria, potentially stimulates the inflammatory response by producing inflammatory mediators, cytokines, and growth factors [36, 37]. At the initial stage of acute inflammation, especially due to environmental exposure, some cancers, and bacterial infection, chemokines produced by infected or damaged cells expel cytokines, such as Il-1 and Il-6, by attracting macrophages to the damaged areas [38, 39]. Thus, the gene expression of Il-1 and Il-6, considered markers of acute inflammation, was examined by qPCR in liver and kidney tissues to demonstrate our LPS-induced inflammation model. The results showed that Il-6 and Il-1 expressions significantly increased in both tissues of LPS-treated mice (Fig. 1). The formation of an acute inflammation model in mouse tissues was determined by the increase in gene expression levels of these cytokines.
Activation of proinflammatory cytokines in the liver and kidney triggers the formation of oxidative stress
Any changes in the GSH level and the GSH/GSSG ratio indicate whether inflammation in the liver and kidney induces oxidative stress [40, 41]. Therefore, the GSH level and the GSH/GSSG ratio were investigated in the liver and kidney tissues of LPS-treated mice and the control group. In the liver and kidney tissues, there was a remarkable decrease in the GSH level and GSH/GSSG ratio the in LPS-treated mice, supporting the formation of oxidative stress (Fig. 2).
The formation of inflammation affects the hepatic and renal gene expression of the thioredoxin system
The quantitative gene expression of Trx1, TrxR1, and Txnip was measured by qPCR in the liver and kidney tissues of LPS-treated mice and the control group and compared with each other. It was seen that the expressions of Trx1, TrxR1, and Txnip genes increased in the liver tissues of the LPS group. In the kidney tissue, Trx1 expression decreased, but no change was observed in TrxR1 expression, and Txnip expression increased (Fig. 3).
Inflammation stimulates the enzymatic activity of the hepatic and renal antioxidant system members
The enzymatic activities of the antioxidant system, containing TRXR1, GPx, and GR were investigated in the liver and kidney tissues of LPS-treated mice and the control group and compared with each other. The results showed no change in TRXR activity in the liver tissue of LPS-treated mice, while GPx and GR enzyme activities decreased significantly. In the kidney tissue, a critical decrease was observed in TRXR1 and GR activities, whereas GPx activity increased (Fig. 4).
Impact of inflammation on the protein expression of hepatic and renal TRXR and TXNIP
The protein expression of TRXR1 and TXNIP was compared quantitatively by Western blot analysis in the liver and kidney tissues of LPS-treated mice. The expression level of TRXR1 protein significantly decreased in both tissues, while TXNIP expression increased significantly compared to the control group (Fig. 5).
Discussion
An inflammation model was created according to the protocol described by Singh et al. [42] in mice to examine how the thioredoxin and glutathione systems act during inflammation in liver and kidney tissues and compare them with each other. The expression of Il-1 and Il-6, considered inflammation markers, was investigated in mouse liver and kidney tissues. Clinton et al. demonstrated that LPS treatment induced inflammation and increased Il-1 gene expression in rabbit vascular tissue [43]. Starr et al. showed that LPS treatment caused the elevated Il-6 gene expression in mouse heart, liver, kidney, and spleen tissues [44]. Likewise, Li et al. demonstrated the increased Il-6 expression in rat testis tissues after LPS injection [45]. In the present study, the elevated Il-1 and Il-6 gene expressions in liver and kidney tissues obtained by qPCR indicated the formation of inflammation in the LPS-injected group.
The cellular redox potential is critical for the physiological balance of the cell, and the GSH/GSSG ratio is an important indicator of this redox potential. Any change in the ratio of the GSH metabolite, a substrate for the glutathione system’s critical enzymes, to its oxidized form (GSSG) demonstrates ROS accumulation [46]. Budak et al. showed that prolonged exposure to toxic iron levels in rat liver promoted oxidative stress by causing a decrease in the GSH level and GSH/GSSG ratio [47]. Additionally, Aydın demonstrated that oxidative stress in mouse liver tissue was associated with a decrease in the GSH and GSSG levels and the GSH/GSSG ratio [28]. However, another study indicated the increased GSH level as a sign of oxidative stress in rat gastric tissue [48]. After showing the inflammation pattern, this study spectroscopically investigated the GSH level and GSH/GSSG ratio in mouse liver and kidney tissues. It was observed that the resulting inflammation disrupted the cellular redox balance and caused oxidative stress by decreasing the GSH level and GSH/GSSG ratio in both tissues.
The thioredoxin system is engaged in different cellular events, such as proliferation, migration, angiogenesis, immune function, inflammatory modulation, protection against apoptosis, and cell regulation, apart from antioxidant functions [49,50,51]. The TRX system acts as a double-edged sword in many biological processes [28, 52]. The thioredoxin system members have been identified to be critical for cell viability [53]. The absence of TRX expression has appeared to be embryonically lethal at the early stages of mouse development, which indicates the necessity of TRX expression for early differentiation and morphogenesis [54]. Moreover, Nonn et al. stated that deleting Trx1 and Trx2 was also embryonically lethal to mice [55]. Deletion of TrxR also causes severe growth retardation and early embryonic death [56]. Furthermore, increased Trx expression protects against inflammation-induced obesity, aging, diabetes, and diabetic retinal damage in mice [57, 58]. Many studies have reported that the overexpression of Trx reduces hepatic fibrosis caused by thioacetamide, acute hepatitis, and LPS-induced liver injury [59, 60]. On the other hand, increasing Trx and TrxR expressions may prevent many diseases related to oxidative stress, but the overexpression of the TRX system and decreased Txnip expression may increase uncontrolled survival and cancer risk [61,62,63]. However, high TRX levels reduced by TRXR act as an autocrine growth factor in tumor diseases and synergize with Il-1 [64]. TrxR and Trx overexpression are known to be associated with worse prognosis in many tumor types, including stomach, lung, colon, breast, pancreas, and liver tumors [65,66,67,68,69]. Reduced or suppressed Txnip expression has also been reported in numerous human cancer cell lines [70, 71]. The overexpression of Txnip has been shown to induce apoptosis of pancreatic β-cells in animal models [72]. In addition to being a marker of apoptosis, TXNIP plays many important roles in tissues, such as regulating glucose metabolism in peripheral tissues and affecting in inflammation as part of inflammatory complexes [73].
The expression of the thioredoxin system members was examined in kidney tissue by qPCR. The results showed a decrease in Trx1 expression and an increase in Txnip expression but no change in TrxR1 expression. In the literature, it has been stated that increased TrxR1 expression protects rat kidney tissues against lead toxicity by a cellular mechanism [74]. Kasuna et al. demonstrated that the increased Trx expression in transgenic mice might protect against kidney damage [75]. TXNIP constitutes a major connection between oxidative stress and inflammation, and increased Txnip expression is linked to the pathology of kidney diseases [76, 77]. A study stated the attenuation of renal function impairment and fibrosis in TXNIP-inactivated mice [78]. Another study showed that the increased mRNA expression of Txnip was correlated with streptozotocin-induced diabetic nephropathy [79]. Additionally, TXNIP may induce apoptosis and inhibit cell proliferation by inhibiting the thioredoxin system [80]. qPCR outcomes suggest that the increased expression of Txnip, a marker in kidney diseases [81], contributes to the initiation of the apoptotic process by suppressing the antioxidant capacity from the gene level to prevent pathological changes. According to the literature, Trx and TrxR gene expression levels are expected to increase for ensuring antioxidant defense in case of oxidative stress. Although the results obtained for Trx1 and TrxR1 in the present study contradicted the literature, Txnip expression was compatible with the literature. To explain this situation better, TRXR and TXNIP protein expressions were also examined by Western blot analysis in kidney tissues.
TRXR provides the continuity of antioxidant activity by reducing oxidized TRX. On the contrary, TXNIP binds to oxidized TRX, inhibits the mentioned activity of TRXR, reduces antioxidant activity, and causes apoptosis [82]. Therefore, when the expression of these two proteins is examined, information can be obtained at the protein level about the behavior of the kidney tissue against inflammation-induced oxidative stress. In this study, while the protein expression of TRXR decreased considerably, the TXNIP level increased significantly and was correlated with gene expression. Enzymatic changes may also provide information about the coordination between the thioredoxin and glutathione systems in inflammation. Thus, TRXR1, GPx, and GR activities were measured spectrometrically. Here, we observed that the TRXR and GR enzyme activities, providing reduction, decreased critically in the kidney tissue, whereas GPx activity, providing oxidation, increased. Considering our results and the literature together, the decreased TRXR protein expression and enzyme activity under inflammation-induced oxidative stress suggest that oxidized thioredoxin increases, and changes in GR and GPx activity also support this idea, because GPx and GR enzymes act as a compensatory system when TRXR activity is reduced due to TRXR inhibition, knockout, or knockdown [65, 83]. It is known that the accumulation of oxidized TRX in the cytoplasm causes apoptosis [28, 84]. The suppression of TRXR at the level of genes, proteins, and enzyme activity, the lack of GR activity, and the increase in the amount of TXNIP indicate that the kidney tissue has given up its antioxidant defense. Since kidney tissue disorders may affect many tissues, this is considered an early precaution to prevent pathological changes in other non-target organs.
The expression of the thioredoxin system members was also examined in the liver tissue by qPCR. The results showed an increase in Trx1, TrxR1, and Txnip expressions. A study showed that the mRNA expression of Trx1 was induced in the case of oxidative stress induced by arsenic in rat liver tissue. It was interpreted that increased Trx1 might protect the liver from oxidative damage by regulating transcription factors and cell signaling pathways [85].
In the present study, we observed the inconsistency between the increase in TrxR1 gene expression and the decrease in TRXR1 protein expression in the liver tissue. It is known that the thioredoxin system members are regulated by post-translational modifications which may explain the discrepancy. A study demonstrated that acetylation increased TRXR activity, whereas deacetylation decreased it [86,87,88]. Carbonimidothioate compounds exert their cytotoxic effects against colon cancer cells by suppressing cell invasion and activity of TRXR by Lys48- and Lys63-linked polyubiquitination [89]. It was observed that low concentrations of CysNO effectively inactivated TrxR1 [90]. Qin et al. showed that high glucose/palmitate-mediated palmitoylation induced oxidative stress by causing Trx1/TrxR1 inhibition in HepG2 cells [91]. Yuan et al. found that TRX1 was rapidly glycated and irreversibly inhibited by LPS. The overexpression of exogenous supplementation of TRX1 might suppress LPS-induced TRX1 glycation, thereby protecting organs from LPS damage [92]. It was observed that the increased TXNIP protein level correlated with gene expression. TXNIP plays a critical role in the liver. Studies have demonstrated that TXNIP knockout mice fail to protect blood glucose levels through glucose production [93,94,95]. TXNIP may function as part of a complex called inflammasome, which, when activated, promotes inflammation and pyroptosis by releasing inflammatory factors. Lian et al. defined that, in the case of oxidative stress, TXNIP could separate from TRX and activate the inflammasome directly in liver disease [96]. Thus, the induction of TRX system members at the gene expression level may be the response against the increased gene and protein levels of TXNIP, causing liver damage in the event of inflammation-induced oxidative stress.
The liver is involved in detoxifying LPS. Thus it is important to examine its response to enzymatic activity [97]. In this study, no change was observed TRXR activity, but the GPx and GR enzyme activities decreased critically in the liver. The present findings suggest that inflammation causes promote oxidative stress and cellular damage by suppressing TRXR1 activity and decreasing GR and GPx activities. Park et al. showed that the inactivation of TRXR by peroxynitrite caused a signal-inducing TrxR gene expression in HUVEC [98]. Likewise, in this study, it was thought that no change in TRXR activity and a critical decrease in protein level might cause the induction of gene expression to maintain antioxidant defense in the liver tissue. The critical decrease in the GR and GPx enzymes, which are the backups of the TRX system, also supports this idea.
When the results were evaluated together, it was observed that both tissues directed the response to inflammation-induced oxidative stress differently. The differential response of the activity of the key enzyme TRXR1 and the inverse correlation in the activity of GPx in both tissues indicated that the kidney tissue was more sensitive to inflammation-induced oxidative stress than the liver tissue. Especially the lack of change in TRXR1 activity with the contrast in the gene and protein expressions of TRXR1 and TXNIP members suggested that the main response occurred at the protein level in liver tissue. Furthermore, it can be stated that the kidney tissue is more sensitive and takes earlier measures than the liver tissue against cellular damage caused by inflammation and inflammation-induced oxidative stress.
Data availability
The data created and analyzed during this study were added to the additional information files.
References
Wynn, T. A., & Vanella, K. M. (2016). Macrophages in tissue repair, regeneration, and fibrosis. Immunity, 44(3), 450–462.
Jeljeli, M., Riccio, L. G. C., Doridot, L., Chêne, C., Nicco, C., Chouzenoux, S., Deletang, Q., Allanore, Y., Kavian, N., & Batteux, F. (2019). Trained immunity modulates inflammation-induced fibrosis. Nat Commun, 10(1), 5670.
Wilhelmsen, K., Farrar, K., Tran, A., Khakpour, S., Sundar, S., Prakash, A., Wang, J., Gray, N. S., & Hellman, J. (2015). Extracellular signal-regulated kinase 5 promotes acute cellular and systemic inflammation. Sci Signal, 8(391), ra86.
Ying, Y., Jiang, C., Zhang, M., Ge, S., & Wang, X. (2019). Phloretin protects against cardiac damage and remodeling via restoring SIRT1 and anti-inflammatory effects in the streptozotocin-induced diabetic mouse model. Aging, 11(9), 2822–2835.
Kolaczkowska, E. (2016). The older the faster: aged neutrophils in inflammation. Blood, 128(19), 2280–2282.
Luo, B., Wang, J., Liu, Z., Shen, Z., Shi, R., Liu, Y., Liu, Y., Jiang, M., Wu, Y., & Zhang, Z. (2016). Phagocyte respiratory burst activates macrophage erythropoietin signalling to promote acute inflammation resolution. Nat Commun, 7, 12177.
Amor, S., Puentes, F., Baker, D., & Van der Valk, P. (2010). Inflammation in neurodegenerative diseases. Immunology, 129, 154–169.
Furman, D., Campisi, J., Verdin, E., Carrera-Bastos, P., Targ, S., Franceschi, C., Ferrucci, L., Gilroy, D. W., Fasano, A., Miller, G. W., Miller, A. H., Mantovani, A., Weyand, C. M., Barzilai, N., Goronzy, J. J., Rando, T. A., Effros, R. B., Lucia, A., Kleinstreuer, N., & Slavich, G. M. (2019). Chronic inflammation in the etiology of disease across the life span. Nat Med, 12, 1822–1832.
Greten, F. R., & Grivennikov, S. I. (2019). Inflammation and cancer: triggers, mechanisms, and consequences. Immunity, 51(1), 27–41.
Tremellen, K. (2008). Oxidative stress and male infertility–a clinical perspective. Hum Reprod Update, 14(3), 243–58.
Sena, L. A., & Chandel, N. S. (2012). Physiological roles of mitochondrial reactive oxygen species. Mol Cell, 48(2), 158–67.
Andrisic, L., Dudzik, D., Barbas, C., Milkovic, L., Grune, T., & Zarkovic, N. (2018). Short overview on metabolomics approach to study pathophysiology of oxidative stress in cancer. Redox Biol, 14, 47–58.
Förstermann, U. (2008). Oxidative stress in vascular disease: causes, defense mechanisms and potential therapies. Nat Clin Pract Cardiovasc Med, 5(6), 338–49.
Budak, H., Gonul, N., Ceylan, H., & Kocpinar, E. F. (2014). Impact of long-term Fe3+ toxicity on expression of glutathione system in rat liver. Environ Toxicol Pharmacol, 37(1), 365–70.
Robaczewska, J., Kedziora-Kornatowska, K., Kozakiewicz, M., Zary-Sikorska, E., Pawluk, H., Pawliszak, W., & Kedziora, J. (2016). Role of glutathione metabolism and glutathione-related antioxidant defense systems in hypertension. J Physiol Pharmacol, 67(3), 331–7.
Prigge, J. R., Coppo, L., Martin, S. S., Ogata, F., Miller, C. G., Bruschwein, M. D., Orlicky, D. J., Shearn, C. T., Kundert, J. A., Lytchier, J., Herr, A. E., Mattsson, Å., Taylor, M. P., Gustafsson, T. N., Arnér, E. S. J., Holmgren, A., & Schmidt, E. E. (2017). Hepatocyte hyperproliferation upon liver-specific co-disruption of Thioredoxin-1, Thioredoxin Reductase-1, and Glutathione Reductase. Cell Rep, 19(13), 2771–2781.
Chong, C. R., Chan, W. P. A., Nguyen, T. H., Liu, S., Procter, N. E. K., Ngo, D. T., Sverdlov, A. L., Chirkov, Y. Y., & Horowitz, J. D. (2014). Thioredoxin-interacting protein: pathophysiology and emerging pharmacotherapeutics in cardiovascular disease and diabetes. Cardiovasc Drugs Ther, 28(4), 347–60.
Lundberg, M., Mattsson, Å., Reiser, K., Holmgren, A., & Curbo, S. (2019). Inhibition of the thioredoxin system by PX-12 (1-methylpropyl 2-imidazolyl disulfide) impedes HIV-1 infection in TZM-bl cells. Sci Rep, 9(1), 5656.
Silva-Adaya, D., Gonsebatt, M. E., & Guevara, J. (2014). Thioredoxin system regulation in the central nervous system: experimental models and clinical evidence. Oxid Med Cell Longev, 2014, 590808.
Zhang, J., Li, X., Han, X., Liu, R., & Fang, J. (2017). Targeting the thioredoxin system for cancer therapy. Trends Pharmacol Sci, 38(9), 794–808.
Wu, X. L., Li, X., Li, Y., Kong, L. P., Fang, J. L., Zhou, X. S., Li, M., Jia, J. J., & Bai, J. (2016). The overexpression of Thioredoxin-1 suppressing inflammation induced by methamphetamine in spleen. Drug Alcohol Depend, 159, 66–71.
Hamada, Y., Fujii, H., Kitazawa, R., Yodoi, J., Kitazawa, S., & Fukagawa, M. (2009). Thioredoxin-1 overexpression in transgenic mice attenuates streptozotocin-induced diabetic osteopenia: a novel role of oxidative stress and therapeutic implications. Bone, 44(5), 936–41.
Liu, Z., Jing, Y., Yin, J., Mu, J., Yao, T., & Gao, L. (2013). Downregulation of thioredoxin reductase 1 expression in the substantia nigra pars compacta of Parkinson’s disease mice. Neural Regen Res, 8(35), 3275–83.
Rusetskaya, N. Y., Fedotov, I. V., Koftina, V. A., & Borodulin, V. B. (2019). [Selenium compounds in redox regulation of inflammation and apoptosis]. Biomed Khim, 65(3), 165–179.
Chiang, J. (2014). Pathobiology of Human Disease: A Dynamic Encyclopedia of Disease Mechanisms. Rootstown, OH, USA: Northeast Ohio Medical University.
Kubes, P., & Jenne, C. (2018). Immune responses in the liver. Annu Rev Immunol, 36, 247–277.
Robinson, M. W., Harmon, C., & O’Farrelly, C. (2016). Liver immunology and its role in inflammation and homeostasis. Cell Mol Immunol, 3, 267–76.
Sönmez Aydın, F., Hukkamlı, B., & Budak, H. (2021). Coaction of hepatic thioredoxin and glutathione systems in iron overload-induced oxidative stress. J Biochem Mol Toxicol, 35(4), e22704.
Ellman, G. L. (1959). Tissue sulfhydryl groups. Arch Biochem Biophys, 82(1), 70–7.
Ceylan, H., Budak, H., Kocpinar, E. F., Baltaci, N. G., & Erdogan, O. (2019). Examining the link between dose-dependent dietary iron intake and Alzheimer’s disease through oxidative stress in the rat cortex. J Trace Elem Med Biol, 56, 198–206.
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods, 25(4), 402–8.
Ozgencli, I., Kilic, D., Guller, U., Ciftci, M., Kufrevioglu, O. I., & Budak, H. (2018). A comparison of the inhibitory effects of anti-cancer drugs on thioredoxin reductase and glutathione s-transferase in rat liver. Anticancer Agents Med Chem, 18(14), 2053–2061.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochem, 72, 248–54.
Mahmood, T., & Yang, P. C. (2012). Western blot: technique, theory, and trouble shooting. N Am J Med Scİ, 4(9), 429–34.
Güller, P., Budak, H., Şişecioğlu, M., & Çiftci, M. (2020). An in vivo and in vitro comparison of the effects of amoxicillin, gentamicin, and cefazolin sodium antibiotics on the mouse hepatic and renal glutathione reductase enzyme. J Biochem Mol Toxicol, 34(7), e22496.
Ni, J., Zhao, Y., Su, J., Liu, Z., Fang, S., Li, L., Deng, J., & Fan, G. (2020). Toddalolactone protects lipopolysaccharide-induced sepsis and attenuates lipopolysaccharide-induced inflammatory response by modulating HMGB1-NF-κB translocation. Front Pharmacol, 11, 109.
Ahmed, M. B., Islam, S. U. I., & Lee, Y. S. (2020). Decursin negatively regulates LPS-induced upregulation of the TLR4 and JNK signaling stimulated by the expression of PRP4 in vitro. Anim Cells Syst, 24(1), 44–52.
Bieghs, V., & Trautwein, C. (2013). The innate immune response during liver inflammation and metabolic disease. Trends Immunol, 34(9), 446–52.
Ciliberto, G., Arcone, R., Wagner, E. F., & Rüther, U. (1987). Inducible and tissue-specific expression of human C-reactive protein in transgenic mice. EMBO J, 6(13), 4017–22.
Rose, S., Melnyk, S., Pavliv, O., Bai, S., Nick, T. G., Frye, R. E., & James, S. J. (2012). Evidence of oxidative damage and inflammation associated with low glutathione redox status in the autism brain. Transl Psychiatry, 2(7), e134.
Nguyen, S., Castellanos, K. A., Abraham, A., & Ferrini, M. G. (2021). Reduction of oxidative stress markers in the corpora cavernosa and media of penile dorsal artery in middle-aged rats treated with COMP-4. Int J Impot Res, 33(1), 67–74.
Singh, M., Hamid, A. A., Maurya, A. K., Prakash, O., Khan, F., Kumar, A., Aiyelaagbe, O. O., Negi, A. S., & Bawankule, D. U. (2014). Synthesis of diosgenin analogues as potential anti-inflammatory agents. J Steroid Biochem Mol Biol, 143, 323–33.
Clinton, S. K., Fleet, J. C., Loppnow, H., Salomon, R. N., Clark, B. D., Cannon, J. G., Shaw, A. R., Dinarello, C. A., & Libby, P. (1991). Interleukin-1 gene expression in rabbit vascular tissue in vivo. Am J Pathol, 138(4), 1005–14.
Starr, M. E., Evers, B. M., & Saito, H. (2009). Age-associated increase in cytokine production during systemic inflammation: adipose tissue as a major source of IL-6. J Gerontol A Biol Sci Med Sci, 64(7), 723–30.
Li, L., Ma, P., Liu, Y., Huang, C., O, W., Tang, F., & Zhang, J. V. (2013). Intermedin attenuates LPS-induced inflammation in the rat testis. PLoS One, 8(6), e65278.
Zitka, O., Skalickova, S., Gumulec, J., Masarik, M., Adam, V., Hubalek, J., Trnkova, L., Kruseova, J., Eckschlager, T., & Kizek, R. (2012). Redox status expressed as GSH:GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol Lett, 4(6), 1247–1253.
Budak, H., Kocpinar, E. F., Gonul, N., Ceylan, H., Erol, H. S., & Erdogan, O. (2014). Stimulation of gene expression and activity of antioxidant-related enzyme in Sprague Dawley rat kidney induced by long-term iron toxicity. Comp Biochem Physiol C Toxicol Pharmacol, 166, 44–50.
Suleyman, H., Dursun, H., Bilici, M., Cadirci, E., Halici, Z., Gulaboglu, M., & Albayrak, F. (2009). Relation of adrenergic receptors, which have roles in gastroprotective and anti-inflammatory effect of adrenal gland hormones, with cyclooxygenase enzyme levels in rats. J Physiol Pharmacol, 60(4), 129–34.
Mahmood, D. F. D., Abderrazak, A., El Hadri, K., Simmet, T., & Rouis, M. (2013). The thioredoxin system as a therapeutic target in human health and disease. Antioxid Redox Signal, 19(11), 1266–303.
Arnér, E. S. J., & Holmgren, A. (2006). The thioredoxin system in cancer. Semin Cancer Biol, 16(6), 420–6.
Bian, M., Fan, R., Zhao, S., & Liu, W. (2019). Targeting the thioredoxin system as a strategy for cancer therapy. J Med Chem, 62(16), 7309–7321.
Lu, J., & Holmgren, A. (2014). The thioredoxin antioxidant system. Free Radic Biol Med, 66, 75–87.
Watson, W. H., Yang, X., Choi, Y. E., Jones, D. P., & Kehrer, J. P. (2004). Thioredoxin and its role in toxicology. Toxicol Sci, 78(1), 3–14.
Matsui, M., Oshima, M., Oshima, H., Takaku, K., Maruyama, T., Yodoi, J., & Taketo, M. M. (1996). Early embryonic lethality caused by targeted disruption of the mouse thioredoxin gene. Dev Biol, 178(1), 179–85.
Nonn, L., Williams, R. R., Erickson, R. P., & Powis, G. (2003). The absence of mitochondrial thioredoxin 2 causes massive apoptosis, exencephaly, and early embryonic lethality in homozygous mice. Mol Cell Biol, 23(3), 916–22.
Lee, S., Kim, S. M., & Lee, R. T. (2013). Thioredoxin and thioredoxin target proteins: from molecular mechanisms to functional significance. Antioxid Redox Signal, 18(10), 1165–207.
Kamimoto, Y., Sugiyama, T., Kihira, T., Zhang, L., Murabayashi, N., Umekawa, T., Nagao, K., Ma, N., Toyoda, N., Yodoi, J., & Sagawa, N. (2010). Transgenic mice overproducing human thioredoxin-1, an antioxidative and anti-apoptotic protein, prevents diabetic embryopathy. Diabetologia, 53(9), 2046–55.
Salmon AB, Flores LC, Li Y, Remmen HV, Richardson A, Ikeno Y. (2012). Reduction of glucose intolerance with high-fat feeding is associated with anti-inflammatory effects of thioredoxin 1 overexpression in mice. Pathobiol Aging Age Relat Dis, 2.
Okuyama, H., Nakamura, H., Shimahara, Y., Araya, S., Kawada, N., Yamaoka, Y., & Yodoi, J. (2003). Overexpression of thioredoxin prevents acute hepatitis caused by thioacetamide or lipopolysaccharide in mice. Hepatology, 37(5), 1015–25.
Okuyama, H., Nakamura, H., Shimahara, Y., Uyama, N., Kwon, Y. W., Kawada, N., Yamaoka, Y., & Yodoi, J. (2005). Overexpression of thioredoxin prevents thioacetamide-induced hepatic fibrosis in mice. J Hepatol, 42(1), 117–23.
Shalev, A. (2014). Minireview: Thioredoxin-interacting protein: regulation and function in the pancreatic β-cell. Mol Endocrinol, 28(8), 1211–20.
Alhawiti, N. M., Mahri, S. A., Aziz, M. A., Malik, S. S., & Mohammad, S. (2017). TXNIP in metabolic regulation: physiological role and therapeutic outlook. Curr Drug Targets, 18(9), 1095–1103.
Jia, J., Geng, W., Wang, Z., Chen, L., & Zeng, X. (2019). The role of thioredoxin system in cancer: strategy for cancer therapy. Cancer Chemother Pharmacol, 84(3), 453–470.
Becker, K., Gromer, S., Schirmer, R. H., & Müller, S. (2000). Thioredoxin reductase as a pathophysiological factor and drug target. Eur J Biochem, 267(20), 6118–25.
Grogan, T. M., Fenoglio-Prieser, C., Zeheb, R., Bellamy, W., Frutiger, Y., Vela, E., Stemmerman, G., Macdonald, J., Richter, L., Gallegos, A., & Powis, G. (2000). Thioredoxin, a putative oncogene product, is overexpressed in gastric carcinoma and associated with increased proliferation and increased cell survival. Hum Pathol, 31(4), 475–81.
Kakolyris, S., Giatromanolaki, A., Koukourakis, M., Powis, G., Souglakos, J., Sivridis, E., Georgoulias, V., Gatter, K. C., & Harris, A. L. (2001). Thioredoxin expression is associated with lymph node status and prognosis in early operable non-small cell lung cancer. Clin Cancer Res, 7(10), 3087–91.
Cadenas, C., Franckenstein, D., Schmidt, M., Gehrmann, M., Hermes, M., Geppert, B., Schormann, W., Maccoux, L. J., Schug, M., Schumann, A., Wilhelm, C., Freis, E., Ickstadt, K., Rahnenführer, J., Baumbach, J. I., Sickmann, A., & Hengstler, J. G. (2010). Role of thioredoxin reductase 1 and thioredoxin interacting protein in prognosis of breast cancer. Breast Cancer Res, 12(3), R44.
Bhatia, M., Lovitt, C. J., Raninga, P. V., Avery, V. M., Di Trapani, G., & Tonissen, K. F. (2016). Expression of the thioredoxin system in an in vivo-like cancer cell environment upon auranofin treatment. Eur J Cell Biol, 95(10), 378–388.
Lei, H., Wang, G., Zhang, J., & Han, Q. (2018). Inhibiting TrxR suppresses liver cancer by inducing apoptosis and eliciting potent antitumor immunity. Oncol Rep, 40(6), 3447–3457.
Zhou, J., & Chng, W. J. (2012). Roles of thioredoxin binding protein (TXNIP) in oxidative stress, apoptosis and cancer. Mitochondrion, 13(3), 163–9.
Sheth, S. S., Bodnar, J. S., Ghazalpour, A., Thipphavong, C. K., Tsutsumi, S., Tward, A. D., Demant, P., Kodama, T., Aburatani, H., & Lusis, A. J. (2006). Hepatocellular carcinoma in Txnip-deficient mice. Oncogene, 25(25), 3528–36.
Minn, A. H., Hafele, C., & Shalev, A. (2005). Thioredoxin-interacting protein is stimulated by glucose through a carbohydrate response element and induces beta-cell apoptosis. Endocrinology, 146(5), 2397–405.
Alhawiti, N. M., Al Mahri, S., Aziz, M. A., Malik, S. S., & Mohammad, S. (2017). TXNIP in metabolic regulation: physiological role and therapeutic outlook. Curr Drug Targets, 18(9), 1095–1103.
Conterato, G. M. M., Quatrin, A., Somacal, S., Ruviaro, A. R., Vicentini, J., Augusti, P. R., Sobieski, R., Figueiredo, C., Dos Santos, C. M. M., Pereira, T. C., Bogo, M. R., Flores, E. M. M., & Emanuelli, T. (2014). Acute exposure to low lead levels and its implications on the activity and expression of cytosolic thioredoxin reductase in the kidney. Basic Clin Pharmacol Toxicol, 114(6), 476–84.
Kasuno, K., Nakamura, H., Ono, T., Muso, E., & Yodoi, J. (2003). Protective roles of thioredoxin, a redox-regulating protein, in renal ischemia/reperfusion injury. Kidney InT, 64(4), 1273–82.
Hou, X., Yang, S., & Yin, J. (2019). Blocking the REDD1/TXNIP axis ameliorates LPS-induced vascular endothelial cell injury through repressing oxidative stress and apoptosis. Am J Physiol Cell Physiol, 316(1), C104–C110.
De Marinis, Y., Cai, M., Bompada, P., Atac, D., Kotova, O., Johansson, M. E., Garcia-Vaz, E., Gomez, M. F., Laakso, M., & Groop, L. (2016). Epigenetic regulation of the thioredoxin-interacting protein (TXNIP) gene by hyperglycemia in kidney. Kidney Int, 89(2), 342–53.
He, Q., Li, Y., Zhang, W., Chen, J., Deng, W., Liu, Q., Liu, Y., & Liu, D. (2021). Role and mechanism of TXNIP in ageing-related renal fibrosis. Mech Ageing Dev, 196, 111475.
Hamada, Y., & Fukagawa (2007). A possible role of thioredoxin interacting protein in the pathogenesis of streptozotocin-induced diabetic nephropathy. M Kobe J Med Sci, 53(1-2), 53–61.
Chen, Y., Ning, J., Cao, W., Wang, S., Du, T., Jiang, J., Feng, X., & Zhang, B. (2020). Research progress of TXNIP as a tumor suppressor gene participating in the metabolic reprogramming and oxidative stress of cancer cells in various cancers. Front Oncol, 10, 568574.
Huang, C., Zhang, Y., Kelly, D. J., Tan, C. Y., Gill, A., Cheng, D., Braet, F., Park, J. S., Sue, C. M., Pollock, C. A., & Chen, X. M. (2016). Thioredoxin interacting protein (TXNIP) regulates tubular autophagy and mitophagy in diabetic nephropathy through the mTOR signaling pathway. Sci Rep, 6, 29196.
Qayyum, N., Haseeb, M., Kim, M. S., & Choi, S. (2021). Role of Thioredoxin-interacting protein in diseases and its therapeutic outlook. Int J Mol Sci, 22(5), 2754.
Cebula, M., Schmidt, E. E., & Arnér, E. S. J. (2015). TrxR1 as a potent regulator of the Nrf2-Keap1 response system. Antioxid Redox Signal, 23(10), 823–853.
Branco, V., Coppo, L., Solá, S., Lu, J., Rodrigues, C. M. P., Holmgren, A., & Carvalho, C. (2017). Impaired cross-talk between the thioredoxin and glutathione systems is related to ASK-1-mediated apoptosis in neuronal cells exposed to mercury. Redox Biol, 13, 278–287.
Li, Y., Zhang, Y., Gao, Y., Zhang, W., Cui, X., Liu, J., & Wei, Y. (2018). Arsenic induces Thioredoxin 1 and Apoptosis in human liver HHL-5 Cells. Biol Trace Elem Res, 181(2), 234–241.
Ratnayake, S., Dias, I. H. K., Lattman, E., & Griffiths, H. R. (2013). Stabilising cysteinyl thiol oxidation and nitrosation for proteomic analysis. J Proteomics, 92, 160–70.
Ungerstedt, J., Du, Y., Zhang, H., Nair, D., & Holmgren, A. (2012). In vivo redox state of human thioredoxin and redox shift by the histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA). Free Radic Biol Med, 53(11), 2002–7.
Wright, D. E., Altaany, Z., Bi, Y., Alperstein, Z., & O’Donoghue, P. (2018). Acetylation regulates thioredoxin reductase oligomerization and activity. Antioxid Redox Signal, 29(4), 377–388.
Ooi, K. K., Yeo, C. I., Ang, K. P., Akim, A. M., Cheah, Y. K., Halim, S. N., Seng, H. L., & Tiekink, E. R. (2015). Phosphanegold(I) thiolates, Ph3PAu[SC(OR)=NC6H4Me-4] for R = Me, Et and iPr, induce apoptosis, cell cycle arrest and inhibit cell invasion of HT-29 colon cancer cells through modulation of the nuclear factor-κB activation pathway and ubiquitination. J Biol Inorg Chem, 20(5), 855–73..
Engelman, R., Weisman-Shomer, P., Ziv, T., Xu, J., Arnér, E. S. J., & Benhar (2013). Multilevel regulation of 2-Cys peroxiredoxin reaction cycle by S-nitrosylation. M J Biol Chem, 288(16), 11312–24.
Qin, H., Liang, W., Xu, Z., Ye, F., Li, X., & Zhong, L. (2015). Mechanistic insights into the inhibitory effects of palmitoylation on cytosolic thioredoxin reductase and thioredoxin. Biochimie, 110, 25–35.
Yuan, Y., Jiao, X., Lau, W. B., Wang, Y., Christopher, T. A., Lopez, B. L., Ramachandrarao, S. P., Tao, L., & Ma, X. L. (2010). Thioredoxin glycation: A novel posttranslational modification that inhibits its antioxidant and organ protective actions. Free Radic Biol Med, 49(3), 332–8.
Sheth, S. S., Castellani, L. W., Chari, S., Wagg, C., Thipphavong, C. K., Bodnar, J. S., Tontonoz, P., Attie, A. D., Lopaschuk, G. D., & Lusis, A. J. (2005). Thioredoxin-interacting protein deficiency disrupts the fasting-feeding metabolic transition. J Lipid Res, 46(1), 123–34.
Yoshioka, J., Imahashi, K., Gabel, S. A., Chutkow, W. A., Burds, A. A., Gannon, J., Schulze, P. C., MacGillivray, C., London, R. E., Murphy, E., & Lee, R. T. (2007). Targeted deletion of thioredoxin-interacting protein regulates cardiac dysfunction in response to pressure overload. Circ Res, 101(12), 1328–38.
Hui, T. Y., Sheth, S. S., Diffley, J. M., Potter, D. W., Lusis, A. J., Attie, A. D., & Davis, R. A. (2004). Mice lacking thioredoxin-interacting protein provide evidence linking cellular redox state to appropriate response to nutritional signals. J Biol Chem, 279(23), 24387–93.
Lian, D., Dai, L., Xie, Z., Zhou, X., Liu, X., Zhang, Y., Huang, Y., & Chen, Y. (2018). Periodontal ligament fibroblasts migration injury via ROS/TXNIP/Nlrp3 inflammasome pathway with Porphyromonas gingivalis lipopolysaccharide. Mol Immunol, 103, 209–219.
Jirillo, E., Caccavo, D., Magrone, T., Piccigallo, E., Amati, L., Lembo, A., Kalis, C., & Gumenscheimer, M. (2002). The role of the liver in the response to LPS: experimental and clinical findings. J Endotoxin Res, 8(5), 319–27.
Park, Y. S., Fujiwara, N., Koh, Y. H., Miyamoto, Y., Suzuki, K., Honke, K., & Taniguchi, N. (2002). Induction of thioredoxin reductase gene expression by peroxynitrite in human umbilical vein endothelial cells. Biol Chem, 383(3-4), 683–91.
Author contributions
Each of the authors contributed to the paper. The preparation of the materials, the experiments, and the collection and analysis of the data were done by BH, BD, FSA, and HB. The research text was written by BH and HB. Furthermore, all authors confirmed the recent version of the article.
Funding
This work was financed by the Scientific and Technological Research Council of Türkiye (TÜBİTAK) (Project No. 114Z277) and the Scientific Research Projects Coordination Commission of Atatürk University (Project no. PRJ2016/151). Furthermore, one author (Feyza Sönmez Aydın, Ph.D.) was supported by the Council of Higher Education (CoHE) Scientific and Technological Research of Türkiye (TÜBİTAK) 2211- A Ph.D. Scholarship Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Humans were not used in the experiments in this study. Experiments using animals were carried out following the Laboratory Animal Care and Use Guidelines after approval was obtained from the local animal care committee of Atatürk University (Protocol no: 55885869-381).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hukkamlı, B., Dağdelen, B., Sönmez Aydın, F. et al. Comparison of the efficacy of the mouse hepatic and renal antioxidant systems against inflammation-induced oxidative stress. Cell Biochem Biophys 81, 299–311 (2023). https://doi.org/10.1007/s12013-023-01126-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-023-01126-3