Abstract
The field of tissue engineering is an emerging discipline which applies the basic principles of life sciences and engineering to repair and restore living tissues and organs. The purpose of this study was to investigate the effect of cold and non-thermal plasma surface modification of poly (ϵ-caprolactone) (PCL) scaffolds on fibroblast cell behavior. Nano-fiber PCL was fabricated through electrospinning technique, and some fibers were then treated by cold and non-thermal plasma. The cell–biomaterial interactions were studied by culturing the fibroblast cells on nano-fiber PCL. Scaffold biocompatibility test was assessed using an inverted microscope. The growth and proliferation of fibroblast cells on nano-fiber PCL were analyzed by MTT viability assay. Cellular attachment on the nano-fiber and their morphology were evaluated using scanning electron microscope. The result of cell culture showed that nano-fiber could support the cellular growth and proliferation by developing three-dimensional topography. The present study demonstrated that the nano-fiber surface modification with cold plasma sharply enhanced the fibroblast cell attachment. Thus, cold plasma surface modification greatly raised the bioactivity of scaffolds.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tissue engineering or regenerative medicine arose from the interactive fields of biology, engineering, materials science and medicine, and now it has a special place in the interdisciplinary field [1]. Astonishing advances have been made in recent decades in this field, which have led to formation of tissues with the ability to grow in the human body. Tissues such as skin and bone are now designed to be used in the human body or have entered clinical trials as bladder and blood vessels. The aim of tissue engineering is to restore normal function in damaged tissue, through the transfer of vital factors that can be incorporated in the animal body.
This goal which is envisaged by the relentless efforts of biologists, material and polymer scientists, immunologists and physicians may achieve the production of new tissues with physiological functions. So basically, the aim of skin tissue engineering is to repair, restore, maintain or improve the function of damaged tissues or organs [2]. Among the important components in tissue engineering, the scaffold technology is of prime importance. To prepare a three-dimensional microenvironment similar to normal tissue, a scaffold was designed and fabricated by nano-fibers similar to natural extracellular matrix [3]. The success of a tissue engineering scaffold is associated with the following features: biocompatibility, biodegradability, hydrophilicity, suitable surface topography for efficient transmission of respiratory gases and waste, mechanical integrity, storage and release of active molecules, the ability to absorb and integrate in human body [4]. Furthermore, the selection of a suitable method for production of nanoscale scaffolds is a key factor in the success in tissue engineering. A nano-fiber, fabricated through electrospinning technique, is used as a scaffold in tissue engineering. Topology of three-dimensional nano-structure is similar to fibers in ECM (extracellular matrix) proteins in the body. In this context, nanotechnology, as a powerful tool in tissue engineering, has provided the possibility to design and produce nanoscale microenvironments as in original ECM. In addition, the cells are sensitive to local nanoscale topographic pattern. Subsequent control of cellular function by nanoscale topographic guidance and engineered layers with different characteristics has been readily accepted [5]. Moreover, the use of suitable construction methods to produce scaffolds, choice of material for the preparation of scaffolds is an important part in ensuring the success of skin tissue engineering. Different natural and artificial materials are used to produce tissue engineering scaffolds. The natural materials include: collagen, chitosan, xylene, gelatin and hyaluronic acid, and the artificial materials are polylactic acid (PLA) and poly (ϵ-caprolactone) (PCL). The fabrication of scaffolds based on synthetic polymers has been widely exploited, because of many variations in material properties [6–8]. Synthetic polymers have superior mechanical properties compared to natural polymers and have the ability to modify unacceptable properties of polymers [9]. Therefore, modification of biomaterial can be carried out by wet (acid, alkali), dry (plasma) and radiation treatments (ultraviolet radiation, laser) without affecting the material properties. Surface modification with plasma has various advantages compared to wet-chemical techniques [10–12]. This method is a superior technique in producing safe products, and it is biocompatible with applicable quality control [13, 14]. The term “plasma” assigns to a partially ionized medium, generally gas in physics. In summary, plasma creates not only electrons and atoms from various ions, but rather uncharged atoms and molecules, as free radicals, excited atoms having high chemical reactivity and the ability to emit UV (ultraviolet). Gas temperature, in thermal plasma, can attain several thousand degrees Kelvin. In contrast, in cold plasma, gas can be maintained at more or less room temperature [15, 16]. Cold plasma surface modifications utilize various gases like Ar, H2, O2, N2 which are used to modify and influence initial cell attachment, growth and proliferation. Thus, a cold plasma surface treatment may be useful to assess the influence of surface wettability cell behavior [13]. The objective of this study was to investigate the behavior of mouse fibroblast cells on electrospun PCL nano-fibers, as unmodified and modified surfaces under cold and non-thermal plasma.
Experimental
Nano-fiber Preparation
For preparation of scaffolds used in this study, solution of PCL in a solvent containing formic acid and acetic acid was prepared at a concentration of 13 %. Then, the obtained solution was collected as nano-fibers on aluminum foil by electrospinning technique using a CO881007NYI machine (Asia Nanostructure/Iran) available at Iran Polymer and Petrochemical Institute.
Morphology of PCL Nano-fiber
For analysis of the morphology and structure of PCL electrospun nano-fibers, scanning electron microscopy was used, before and after surface treatment by cold atmospheric plasma, to determine the most appropriate nano-fibers in tissue engineering application.
Cold atmospheric plasma is a novel technique for surface modification, so we had to check the nano-fiber morphology after surface treatment. The sample was sputter-coated with gold layer and then examined by a VEGA//TESCAN scanning electron spectroscope (SEM) at an accelerating voltage of 20 kV.
Arrangement of Instrument in Surface Modification Procedure
In this study, plasma surface modification was used to improve the hydrophilic properties of the scaffolds. Non-thermal O2 plasma was applied onto the surface of the scaffold by Diener Electronic (Nano/Germany) available at Institute for Stem Cell Research. Nano-fibers were placed in the chamber of the plasma cleaner, and plasma discharge was applied for 5 min with low-frequency power set as 30 W under vacuum mode. Cold plasma gas using helium atmosphere (He 99.99 and 5 % oxygen) was employed for surface modification. Hydrogen gas was directly charged through a Pyrex nozzle, at ambient temperature under sterilized condition, with no vacuum and 1 cm distance from the substrate for 30 s. The gas was injected through the nozzle at the rate of 2 L/s. A copper electrode of 6 mm width around the tube was installed to convert the gas into plasma. The applied voltage on the electrodes was 10 kV with a frequency of 6 kHz and 30-microsecond pulse width. Figure 1 shows a schematic cold atmospheric plasma instrument.
Wettability Measurements
The wettability of a surface is often considered as biocompatibility factor. The change of surface hydrophilicity of nano-fiber PCL before and after plasma treatment was characterized by measuring the contact angle. All contact angle measurements were carried out under ambient conditions. Measurement of contact angle was performed using deionized water.
Determination of Surface Chemistry
The surface chemistry of cold and non-thermal plasma treatment was characterized through an Equinox 55 ATR-FTIR (Fourier transform infrared) (Bruker, Germany). The spectra were recorded from 400 to 4000 cm−1 with a 4 cm−1 resolution.
Cell Culture
Anchorage-dependent L929 mouse fibroblast cells were maintained in 50-cm3 tissue culture flasks at 37 °C in a humidified 5 % CO2 atmosphere in incubator, using DMEM (Dulbecco’s modified Eagle’s medium) culture media (Gibco, Germany) with 10 % FBS (fetal bovine serum) (Gibco, Germany). The cells were passaged every 2–3 days depending on cell proliferation rates. After washing the cells with phosphate-buffered saline solution (PBS), they were treated with trypsin (0.25 %) and ethylenediamine tetra-acetic acid (EDTA) (0.1 %) for 2 min at 37 °C. The detached cells were centrifuged and suspended in DMEM10 % FBS.
Biocompatibility and Cytotoxicity of PCL Nano-fibers
For biocompatibility test of the scaffolds toward living tissues, the following procedures were conducted: After sterilization by UV, the cells were seeded into a 24-well plate at a seeding density of 40,000 cells/well on 0.5 × 0.5 cm2 dimension of scaffold and incubated for 2 h to ensure seeding cells will be located on nano-fibers. Then, the cell culture medium was added to each well. After 24 h, the cells’ tendency toward the scaffolds, for their biocompatibility, was examined and analyzed by an inverted microscope.
Cell Proliferation by MTT Assay
The mitochondrial activity of the cultured cells was determined using a colorimetric evaluation, an indication of cell viability. The salt MTT (3-[4,5-dimethylthiazol-2yl]-2,5-diphenyl tetrazolium bromide) was reduced by mitochondrial dehydrogenase in living cells and became insoluble formazan purple crystal. Formazan crystals were soluble in DMSO (dimethyl sulfoxide). Because dead cells were unable to convert MTT to formazan, the amount of formazan produced was proportional to the number of existing living cells. Color intensity was measured by spectrophotometer at 570 nm.
For sterilization of the samples, each side of untreated and plasma-treated nano-fibers was placed under UV light for 20 min. Then, fibroblast cells were seeded into a 24-well plate containing sterilized scaffolds at seeding density of 40,000 cells/well. An MTT assay was used to distinguish the proliferation of fibroblast cells in the course of a 3-day cell culture period. Cell viability was calculated as percentage of the control group. The results were analyzed with analysis of variance (ANOVA) by SPSS software (sign test, p < .0005).
DAPI Staining
The fibroblast cells were trypsinized and cultured on PCL nano-fibers scaffolds (103 cells/each scaffold) and incubated for 72 h. The scaffolds were washed three times with PBS at pH 7.4, transferred into a new 24-well plate and fixed with 3 % paraformaldehyde solution in PBS (pH 7.4) for 10 min and then washed with PBS for 2 min. They were then permeabilized with 0.1 % triton X−100 in PBS for 2 min, aspirated with triton X−100 and washed with PBS for 2 min. DAPI (4′,6-diamidino-2-phenylindole) stain was added and then incubated in dark for 5 min. The scaffolds were washed three times with PBS (2 min each) and maintained in the dark before examining by a fluorescent microscope.
Analysis of Cell Adhesion Pattern by Scanning Electron Microscopy (SEM)
After 72 h of culturing the cells on the scaffolds, they were rinsed twice with PBS (pH 7.4) and immersed in PBS containing 4.5 % glutaraldehyde for 3 h to fix the cells. The scaffolds were rinsed twice with PBS for being dehydrated through a graded series of ethanol (from 60 to 100 %) and dried overnight. Finally, the samples were sputtered with a gold layer. The samples were mounted on sample holder, and a scanning electron microscope (SEM) (VEGA//TESCAN) was used to characterize the morphology of the attached cells and evaluated the cell proliferation.
Statistical Analysis
Data were evaluated by a Dunnet one-way analysis of variance (ANOVA) using software SPSS version 16.0. The a priori alpha value was set at 0.05 with the level of significance for all statistical analyses p < 0.05.
Results
Preparation and Observation of Morphology of PCL Nano-fibers
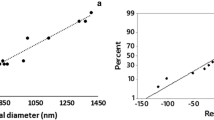
Nano-fibers used in this study were prepared by electrospinning technique. One of the characteristics of PCL-made scaffolds was its inherent hydrophobicity of the polymer. Because of this property, there was a limitation in the use of this polymer as scaffold in tissue engineering. In this study, to modify this property, cold and non-thermal plasma treatments were performed. Application of cold and thermal plasma on the surface of PCL nano-fibers resulted in the formation of hydrophilic nano-fibers. To check the morphology of the electrospinning nano-fibers, images were prepared by scanning electron microscope (SEM) (Fig. 2). According to the SEM images, the electrospun nano-fibers, before and after modification by cold atmospheric plasma, produced fibers fitted for tissue engineering application.
Wettability Measurements
The water contact angle measurement was used to estimate the degree of hydrophobicity or hydrophilicity of the material surface. Table 1 shows the change in wettability of the surface of treated and untreated PCL nano-fibers. According to the contact angle data, decrease in contact angel verified that cold and non-thermal plasma treatment led to significant hydrophilicity. It is noteworthy that by applying helium gas as cold atmospheric plasma for surface modification there would be increases in CH functional group on nano-fiber surface. While the presence of this functional group can result in incremental value of water contact angle than oxygen non-thermal plasma, the surface treatment by oxygen non-thermal plasma results in zero-degree water contact angle.
Determination of Surface Chemistry
The pristine, cold atmospheric and non-thermal plasma treatments of PCL nano-fibers were assayed by FTIR to assess the conversion in the functional groups, as shown in Fig. 3. The combination curves of FTIR results show that plasma surface modification, particularly cold atmospheric plasma, can resonate and ameliorate the functional groups on the surface of nano-fibers. The intensities of 1720 and 1420 cm−1 peaks corresponded to C=O and –COO functional groups, respectively. Therefore, intensification in functional groups due to surface modification by He and oxygen plasma seems quite impressive. As shown in Fig. 3, the peaks related to helium plasma are more explicit than the others.
Biocompatibility of PCL Nano-fibers
To assess the biocompatibility of PCL nano-fibers, before and after surface modification, the cells were cultured at seeding density of 40,000 cells on modified nano-fibers by O2 non-thermal plasma, He cold plasma and unmodified nano-fibers in CO2 incubator at 37 °C with 95 % humidity for 24 h. According to the images taken by inverted microscope, after 24 h of cells passaging on PCL nano-fibers, the cell orientation was evident toward scaffolds and cells on nano-fibers showed significantly better growth compared to positive control. Consequently, it can be said that modified and unmodified surfaces of PCL electrospun nano-fibers have good biocompatibility (Fig. 4).
Inverted microscopy images of cell proliferation besides the scaffolds after 24, 48 and 72 h of culture. The dark areas of the images in (b), (c) and (d) as shown by arrows are the scaffolds. a Monolayer culture without scaffold as control, b the cells grown on the treated by non-thermal plasma nano-fiber scaffold, c the cells grown on the untreated nano-fiber scaffold, d the cells grown on the cold atmospheric plasma-treated nano-fiber scaffold (Scale bar = 100 µm)
Cell Proliferation by MTT Assay
The results of MTT assay at 24, 48 and 72 h showed that the surface modification by He cold plasma atmospheric was improved and the progressive growth and proliferation rates were compared to positive control and surface modification by O2 non-thermal plasma and unmodified nano-fibers (Fig. 5).
DAPI Staining
DAPI (4′,6-diamidino-2-phenylindole) is a fluorescent stain that binds strongly to A-T-rich regions in DNA. This stain is used for staining viable cells and specifically for the qualitative determination of the cells [17]. The results of DAPI staining showed that He cold plasma surface modification of nano-fibers was better for attachment and proliferation of cells compared to the non-thermal plasma surface modification (Fig. 6).
Fluorescent microscopic images from DAPI staining on the scaffolds at day 3. For study of the cell proliferation on the scaffolds, we perform staining of cell nucleus. The cell growth on electrospun nano-fiber PLC scaffold treated by cold atmospheric plasma (a), non-thermal plasma (b). SEM photomicrographs of fibroblast cells on nano-fiber PCL, cultured for 72 h. Untreated PCL nano-fiber (c), treated PCL nano-fiber by non-thermal plasma (d), treated PCL nano-fiber by cold atmospheric plasma (e)
Analysis of Cell Adhesion Pattern by Scanning Electron Microscope (SEM)
The results of electron microscopy showed that after 72 h, the cultured cells were mainly spread out and adhered specially to He cold plasma-modified surface (Fig. 6). In summary, attachment, viability and proliferation of fibroblast cells were significantly promoted and enhanced by He cold plasma-modified surface in comparison with non-thermal plasma, untreated and positive control.
Discussion
In recent years, there has been ongoing study on surface modification of biomaterials by treatment to improve and enhance cellular adhesion and proliferation [18]. Plasma techniques for surface modification of biomaterials are becoming increasingly common in tissue engineering [19, 20]. The most important advantages of plasma surface modifications are: ability to change the surface properties selectively, improving biocompatibility and mimicking the local tissue environment without altering the bulk attributes. Plasma thus provides a versatile and effective means to modify surfaces. It also enhances physicochemical properties and optimizes the biofunctionality [21]. The skin, as the largest organ of human body, accounts for 16 % of total body weight with a surface area of 1.8 m2 [22]. Treatment of skin lesions such as extreme tissue damage (burns), skin defects (old wounds) or congenital defects and diseases is comprised of multiple topics in different areas of skin recovery [23]. The aim of skin tissue engineering is to repair skin through biological tools such as cells with the use of effective artificial tools. The ultimate goal is to restore both structural and functional properties of tissue [24]. In a healthy tissue, the cells exist in a three-dimensional environment that is surrounded by other cells and ECM. Cell behavior results from a combined response of several signaling interactions that occur between adjacent cells, ECM and surrounding dissolved molecules [5]. Scaffolds, as platforms on which the cells are cultured, are the most important components of tissue engineering.
In fact, scaffolds, as more similar to natural ECM in human body, can be more effective in tissue engineering such as skin recovery. One of the important features of ECM to create suitable conditions for cells to interact with its surrounding environment and each other is hydrophilicity and appropriate surface topography. Hydrophilic properties of ECM facilitate the transport of respiratory gases, nutrients and wastes across the cell surface. On the other hand, a three-dimensional topographic feature has a good impact on the induction of pathways involved in cell signaling in phenotype formation and cell fate [1, 25]. Nano-fibers can be produced with appropriate nano-topographic structures to provide an optimal surface topography for successful tissue engineering that may provide a framework for the expected behavior of cells. PCL film has been used in a study conducted by Chung, et al. Based on their report due to lack of three-dimensional structures, PCL film does not have the ability to provide the structure similar to the natural ECM, and as a result, cells would be unable to perform correctly on these scaffolds [26]. In another research, in order to produce three-dimensional PCL nano-fibers a free-form fabrication method was used. Scaffold could not support the cells cultured to behave correctly because of the lack of three-dimensional structure and appropriate surface features [27]. Electrospinning technique is an efficient method to produce scaffolds for tissue engineering by producing nano-fibers with desired nanoscale surface topography, density and two-dimensional structure [28].
Moreover, the use of appropriate production method to generate scaffolds, choosing a suitable material for scaffold formation is one of the important parts of tissue engineering. In tissue engineering, natural and synthetic materials have been used for scaffold formation such as collagen, chitosan, xylene, gelatin, hyaluronic acid, polylactic acid (PLA) and PCL. The main deterrent to the use of natural polymers is their poor mechanical properties [9]. According to the superiority of synthetic polymers and high potentiality in the use of new techniques to improve some inappropriate features, in this study, PCL was chosen as a scaffold material for the production of nano-fibers by electrospinning. One of the characteristics of the polymer used in the preparation of scaffold is its inherent hydrophobicity. This feature has created limitations in the use of this polymer as scaffold in tissue engineering. In different studies, modifications and combination of this polymer with different agents have been used to modify this feature. One of the modifications of the PCL polymer to modify the surface hydrophobicity of PCL films is plasma. In 2010, in order to compare the cell behavior and determine the best case for the oxygen plasma surface modification for PCL scaffolds and fibronectin protein, the PCL scaffolds and its combination with plasma-target protein were used separately. The obtained results showed that plasma improved hydrophilic property significantly and also increased the surface roughness of scaffolds. Initial adhesion of cells on plasma-modified scaffolds was twice higher than on unmodified scaffolds. However, it is noteworthy that the plasma-modified scaffolds have the highest growth rate after combination with plasma–protein [29]. In another study, to investigate the surface modification of PCL scaffolds, dichloromethane and acetone etching solution were used to apply roughness on the scaffold. The results indicated significant differences between the untreated and treated scaffolds in cell proliferation and its suitable morphology [27]. In all the cases mentioned above, adding physical, chemical or biological agents for creating better conditions provided suitable PCL scaffolds. Nevertheless, in all the methods adopted the main focus has been to impose changes in polar groups. Among the most common approaches, surface modifications by plasma can create suitable platform for tissue engineering. In the present study, we created He cold atmospheric plasma through exquisite technique. After that, we used novel plasma and non-thermal O2 plasma for surface modification of PCL scaffolds prepared by electrospinning technique. PCL nano-fibers were used with 40-min spinning time.
Before the application of this substrate for tissue engineering, the morphology of nano-fibers was assessed by SEM test. Based on the obtained images of SEM, the morphology and arrangement of nano-fibers did not show any difference between pristine and surface treatment by cold atmospheric plasma. First, after surface treatment procedure by cold atmospheric and non-thermal plasma the samples were tested for determination of their hydrophilicity by water contact angle. The result of water contact angle showed that when oxygen plasma was applied for surface treatment the contact angle drop reached zero. Oxygen plasma created oxygen functional groups on the surface of the substrate, which improved the hydrophobic property of the substrate. But, when using helium gas for surface treatment by cold atmospheric plasma the contact angle increased to 20° compared to the non-thermal oxygen plasma. The conversion of helium gas to plasma produced CH functional groups on the surface of substrate. Thus, the presence of these functional groups led to an increase in water contact angles than the non-thermal plasma. After that, using ATR technique we found various intensified peaks for different groups on the surface [29, 30]. The results of FTIR are shown in Fig. 4, showing absorbance peaks in 1420 cm−1 for –COO functional groups and the absorbance peaks in 1720 cm−1 for C=O functional groups. It is noteworthy that the absorbance peaks of surface modification by cold atmospheric plasma are more egregious than pristine and non-thermal surface treatment. Existence of these functional groups can improve the intrinsic hydrophobic property of PCL nano-fibers. But, a distinct peak is seen between wave numbers 2852–2940 cm−1 on the surface modified with He cold atmospheric plasma. This peak can be referred to CH group. On the other hand, when the surface was modified by helium gas it intensified the CH groups on nano-fiber surface. To confirm the biocompatibility of nano-fibers, fibroblast cells were cultured at a density of 4 × 104 per well on modified and unmodified nano-fibers sterilized by UV radiation for 24 h. The growth of cells on scaffold was determined by an inverted microscope (Fig. 5). After 24 h of transferring cells onto the PCL scaffolds, based on the images taken by an inverted microscope, the orientation of cells toward the scaffold indicated the biocompatibility of nano-fibers. In order to assess the viability and growth rate of fibroblast cells on the nano-fibers, cell growth was evaluated daily by MTT assay until 72 h. These results are presented in a diagram after statistical analysis (Fig. 6). According to the graph, after 24 h of transferring cells on cold plasma surface and non-thermal plasma-modified scaffolds, the adhesion and proliferation of the cells on defined nano-fibers were significantly higher than on the positive control and unmodified samples which were indicative of the formation of appropriate platform for initial adhesion of the cells. Using thermal and non-thermal plasma to modify hydrophobicity of nano-fibers, there was improvement observed in surface properties of nano-fibers and oxygen plasma induction of several polar groups on the nano-fibers surface. These modifications facilitated more initial attachment of cell surface compared to unmodified nano-fibers. It can be said that using cold and non-thermal plasma may enhance the initial binding of the cells. After 48 h of cell culturing, the rate of cell growth on modified PCL nano-fibers by cold plasma was significantly higher than on the positive control, unmodified nano-fibers and nano-fiber modified by non-thermal plasma. After 72 h of cell transfer onto PCL, as shown in the graphs, growth and proliferation of cells on modified PCL nano-fibers by cold plasma were significantly higher than on the positive control, nano-fiber modified by non-thermal plasma and unmodified samples. This significant difference and drop in growth rate of the fibroblast cells studied here on all types of nano-fibers compared to the modified nano-fiber by cold plasma are due to the lower initial adhesion of the cells. In another study performed by Chung et al., the cell growth rate on defined platforms was significantly lower than on the positive control (cells cultured on the plate). However, the cell initial attachment on unmodified PCL nano-fibers was higher on than positive control [26]. In another study by Yildirim et al. 29, oxygen plasma-modified PCL scaffolds and PCL scaffolds covered by fibronectin were used. Higher initial binding of the osteoblast cells was observed on plasma-modified scaffolds compared to other used methods. These findings are similar to our obtained results, because cold and non-thermal plasma surface modification improves the surface properties of PCL scaffolds and significant increase in the initial attachment of cells [29]. Based on different methods for fabrication of scaffolds, used in previous studies, a three-dimensional environment similar to natural ECM scaffolds was afforded by electrospinning technique. Production of nano-fibers with macropore size resulted in high similarity with the natural ECM.
On the other hand, cold and non-thermal plasma surface modification was used to modify the hydrophobicity feature of PCL polymer. Finally, in order to investigate the morphology of cells grown on nano-fibers a scanning electron microscopy (SEM) was used (Fig. 6). In the present study, cold plasma surface modification allowed accurate control of surface permeability without alteration in surface morphology. Surface modification by cold plasma enabled us to regulate the protein adsorption and initial cell attachment by controlling the surface wettability of the biomaterials. As shown in the figures in this study, cell adhesion and proliferation on PCL nano-fibers modified by cold plasma are significantly higher than on positive control and other types of nano-fiber used in this research.
Conclusion
In the present study, PCL polymer was used as a scaffold in tissue engineering. Scaffolds were prepared by electrospinning technique for 40-min collection. To correct the inherent hydrophobicity property of synthetic PCL biomaterial, cold and non-thermal plasma surface modification was used. Cold atmospheric plasma applied in this study was designed through a novel method. This technique can be used at room temperature, without vacuum system and restricted locally. In fact, induction of plasma on the surface of nano-fibers moderated the conditions for the initial attachment of fibroblast cells. Finally, based on the results of the MTT assay and scanning electron microscopy (SEM) images, PCL nano-fibers with helium cold plasma surface modification have been able to provide better platform to support the adhesion and proliferation of fibroblast cells. At the end, the use of cold atmospheric plasma technique can become a suitable approach for tissue engineering application.
References
Kim, H. N., Jiao, A., Hwang, N. S., Kim, M. S., Kang do, H., Kim, D. H., & Suh, K. Y. (2012). Advanced Drug Delivery Reviews, 65, 389.
Shi, J., Votruba, A. R., Farokhzad, O. C., & Langer, R. (2010). American. Nanotechnology, 10, 3223.
Zhu, X., Cui, W., Li, X., & Jin, Y. (2008). Biomacromolecules, 9, 1795.
Jha, B. S., Colello, R. J., Bowman, J. R., Sell, S. A., Lee, K. D., Bigbee, J. W., et al. (2011). Acta Biomaterialia, 7, 203.
Cunha, C., Panseri, S., & Antonini, S. (2011). Emerging nanotechonolgy approaches in tissue engineering for peripheral nerve regeneration. Nanomedicine, 7, 50–59.
Gloria, A., Russo, T., De Santis, R., & Ambrosio, L. (2009). Journal of Applied Biomaterials and Biomechanics, 7, 141–152.
Santis, R. D., Gloria, A., Russo, T., Amora, U. D., Zeppetelli, S., Dionigi, C., et al. (2011). Journal of Applied Polymer Science, 122, 3599.
Kumbar, S. G., Nukavarapu, S. P., James, R., Nair, L. S., & Laurencin, C. T. (2008). Biomaterials, 29, 4100.
Vance, R. J., Miller, D. C., Thapa, A., Haberstoh, K. M., & Webster, T. J. (2004). Biomaterials, 25, 2095.
Cheng, C., Zhang, L., & Ru-Juan, Z. (2006). Surface & Coatings Technology, 200, 6659.
ŞAŞMAZEL, H.T., MANOLACHE, S., & GÜMÜŞDERELİOĞLU M. (2009). Nanostructured Materials for Advanced Technological Applications, 533–538.
Prabhakaran, M. P., Venugopal, J., Chan, C. K., & Ramakrishna, S. (2008). Nanotechnology,. doi:10.1088/0957-4484/19/45/455102.
Masao, Y., Jianhua, W., Kenichi, M., & Takashi, I. (2009). Engineering and Technology, 58, 171.
Kim, M. C., Song, D. K., Shin, H. S., Baeg, S. H., Kim, G. S., Boo, J. H., et al. (2003). Surface & Coatings Technology, 171, 312.
Domingos, M., Intranuovo, F., Gloria, A., Gristina, R., Ambrosio, L., Bartolo, P. J., & Favis, P. (2013). Acta Biomaterialia, 9, 5997.
Shashurin, A., Keidar, M., Bronnikov, S., Jurjus, R. A., & Stepp, M. A. (2008). Applied Physics Lecture, 93, 181501.
Adams, J. C. (2002). Methods in cell biology: Methods in cell-matrix adhesion. San Diego: Elsevier Science.
Hauser, J., Zietlow, J., Köller, M., Esenwein, S. A., Halfmann, H., Awakowicz, P., & Steinau, H. U. (2009). Materials Science: Materials in Medicine, 20, 2541.
Renata, A. N. P., Fábia, K. A., Alves, C., & Miguel, G. (2010). Carbohydrate Polymers, 82, 692.
Mahmod, G., & Amir Hosein, S. (1386). Plasma physics. Tehran: Islamic Azad University.
Yildirim, E. D., Ayan, H., Vasilets, V. N., Fridman, A., Guceri, S., Friedman, G., & Sun, W. (2008). Polymers, 5, 58.
Keck, M., Lumenta, D., & Kamolz, L. (2013). Skin Tissue Eng (Vol. 13, p. 13). Vienna: Springer.
Supp, D. (2005). Clinic in Dermatology, 4, 203.
Clark, R., Ghosh, K., & Tonnesen, M. (2007). Investigative. Dermatology, 127, 1018.
Barnes, C. P., Sell, S. A., Boland, E. D., Simpson, D. G., & Bowlin, G. L. (2007). Advanced Drug Delivery Reviews, 59, 1413.
Chung, T., Wang, S. S., Wang, Y. Z., Hsieh, C. H., & Fu, E. (2009). Mater Science, 20, 379.
Kumar, G., Waters, M., Farooque, T., Young, M. F., Carl, G., & Simon, J. G. (2012). Biomaterials, 33, 4022.
Pramanik, S., Pingguan-Murphy, B., & Osman, A. A. (2012). Science and Technology of Advanced Materials,. doi:10.1088/1468-6996/13/4/043002.
Yildirim, E. D., Besunder, R., Papas, D., Allen, F., Güceri, S. G., & Sun, W. (2010). Biofabrication, 2, 12. doi:10.1088/1758-5082/2/1/014109.
Lee, H., Sul Jeong, Y., Young jeong, S., Park, S. Y., Bae, J. S., Kim, H. G., & Cho, C. R. (2008). Applied Surface Science, 254, 5700.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Atyabi, S.M., Sharifi, F., Irani, S. et al. Cell Attachment and Viability Study of PCL Nano-fiber Modified by Cold Atmospheric Plasma. Cell Biochem Biophys 74, 181–190 (2016). https://doi.org/10.1007/s12013-015-0718-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-015-0718-1