Abstract
Accumulated evidences indicate metformin is associated with reduced risk of hepatocellular carcinoma (HCC) in diabetic patients, which inspired researchers to explore its therapeutic potentials in HCC. Since Hepatic stellate cells (HSCs) are believed to be the key contributors to tumor microenvironment in HCC and promotes tumor development, here, we explored the effect of metformin on tumor angiogenesis induced by interplay of HCC and HSCs. Our results showed that conditional medium from co-culture of HCC/HSCs induced VEGF secretions and stimulated human umbilical vein endothelial cells (HUVEC) tube formation. However, 25 µM metformin could inhibit this angiogenesis effect. Furthermore, knockdown AMPK of HSCs, not HCC, could abolish inhibition caused by metformin. Our finding suggested that metformin could inhibit HCC angiogenesis through targeting on HSCs through AMPK pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is one of the serious global challenges and now is the fifth most common malignancy worldwide and the third leading cause of cancer-related deaths [1]. Recently, a significant increase in HCC incidence and mortality rates has been observed in Western world countries [2]. It is known that main risk factors for HCC are hepatitis C virus (HCV) and hepatitis B virus (HBV) infections and chronic alcohol abuse. However, at least 25 % of HCC cases do not have any recognized etiology. Increasing evidences supported that type 2 diabetes mellitus (DM2) is a risk factor for HCC [3–9]. Interestingly, metformin, a first-line drug for DM2, could reduce the risk of cancer with dose-related manner [10]. So far, the underlying molecular mechanism of the protective effect of metformin remains to be explored.
Growing evidences suggest that tumorigenesis is determined not only by malignant cells but also by their microenvironment [11]. In the liver, hepatic stellate cells (HSC), fibroblasts, myofibroblasts as well as immune and endothelial cells represent the main cell types of the hepatocyte microenvironment [12]. Among them, HSCs were recognized as key players in liver tumorigenesis [13]. It has been shown that the exposure of HSC to conditioned media derived from HCC tumor cells resulted in HSC activation, migration, and expression of pro-angiogenic factors such as vascular endothelial growth factor alpha (VEGFA) [14]. Also, crosstalk between hepatoma cells and activated HSC also increased the expression of proinflammatory cytokines and chemokines, and modified the phenotype of hepatoma cells [15–17].
It is unclear whether metformin reduces cancer development by acting directly on cancer cells or by modifying its microenvironment or both. Since angiogenesis is trigged by tumor cell and microenvironment and plays an important role during cancer development, here, we investigated the effects of metformin on tumor angiogenesis which is induced by interplay of HCC and HSCs and tried to find the underlying molecular mechanism.
Materials and Methods
Cell Lines and Cell Culture
HUVEC, HepG2 for Human hepatoma cell line (HCC) and LX-2 for Human hepatic stellate cell line (HSCs) were obtained from ATCC and cultured in DMEM (Invitrogen, Grand Island, USA) supplemented with 10 % fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, MO, USA). All cell lines were subcultured weekly. They were maintained in an atmosphere of humidified air: CO2 (95:5 %) at 37 °C.
Cell Viability Assay
To order to determine any cell number changes during conditional medium preparation, log-phase growth cells were seeded into 96-well plates at a density of 8 × 103 cells/well with the present of culture medium. After overnight growth, culture medium was changed into 1 % FBS in DMEM and cells were incubated for 3 days. At the end of the experimental period, 1 mg/mL of freshly prepared MTT (Sigma, St Louis, MO, USA) was added to each well and incubated for an additional 4 h. A 100-μL aliquot of DMSO was added and allowed to react for 15 min, after which the spectrometric absorbance was measured at 490 nM.
Coculture and Conditional Medium (CM)
HepG2 were directly co-cultured with LX-2 in 1:1 mixture ratio. Once cells reach to 100 % confluence, regular culture medium was changed into 1 % FBS for conditional medium. Conditional medium was collected after 3 days and used for further analysis.
Immunofluorescence Staining
Cells were fixed with 4 % formaldehyde for 15 min at room temperature and permeabilized with 0.01 % Triton X-100. Then the cells were incubated with anti-Heppar-1 antibody (1:80, Dako, USA) or anti-Desmin antibody (1:100, Santa Cruz, USA) at 4 °C overnight followed by FITC or Texas red-conjugated secondary antibody at room temperature for 45 min in the dark. Cell imaging was performed by SPOT imaging system using Nikon Eclipse E800.
Enzyme-Linked Immunosorbent Assay (ELISA)
VEGF protein levels in conditional medium were measured using commercial enzyme-linked immunosorbent assay (ELISA) tests in accordance with the manufacturer’s instructions (R&D, USA). After collecting, conditional medium was then centrifuged at 14,000× g for 5 min to remove cellular debris, and subsequently stored at −80 °C until needed. All standards and samples were assayed in triplicate, and the values were averaged.
Tube Formation
To examine the formation of tube-like structures, HUVECs were seeded onto 96-well plates precoated with matrigel, and tube formation was then examined after 18 h. The extent of tube formation was quantified by measuring the cumulative tube length with ImageJ (National Institutes of Health).
AMPKα Knockdown
For siRNA-based experiments, 80 %n confluent HepG2 or LX-2 in 10-cm plates were transfected by incubating in OPTIMEM containing oligofectAMINE (Invitrogen) and 200 nM siRNA (both control and siRNA AMPK from Qiagen). After 24 h, cells were trypsined and used for co-culture for conditional medium collection.
Western Blot
Fifty micrograms of total proteins were separated by 4–20 % precast gel electrophoresis, transferred to PVDF Immobilon-P membranes (Millipore, Bedford, MA), blocked in 5 % milk, and immunoblotted with anti-AMPKa (Cell signaling, USA) bodies at dilution of 1/250. The blots were developed with ECL chemiluminescence (Pierce, USA).
Statistics
Statistical significance tube formation or concentration of VEGF was determined by ANOVA. Student’s t test was used for two-group comparison. A value of P < 0.05 was considered significant.
Results
In order to study, effect of interaction of cancer cell with their environment, we used co-culture system. Stellate cells were believed to play an important role in hepatocarcinoma. We mixed HepG2 and Lx-2 at 1:1 ratio, and total cell number was equal with HepG2 or LX-2 alone. Conditional medium was collected after days. During conditional medium preparation, cell number was kept unchanged Fig. 1a. To highlight this coculture system, we used HepPAR-1 to stain HepG2 with Green fluoresce and desmin to stain LX-2 with red fluoresce as shown Fig. 1b. HepPAR-1 is a specific marker for HCC; desmin is a specific marker for stellate cells.
Next, we checked the potential of collected conditional medium on angiogenesis. First, we used tube formation assay, one of the most widely used in vitro assays to model the reorganization stage of angiogenesis, to evaluate the potential. As shown in Fig. 2a, conditional medium from coculture generated more tube formation (P < 0.001) compared with HepG2 or LX-2 alone. One of the most potent angiogenic factors identified so far is VEGF. To determine whether VEGF was responsible for the stimulation of conditional medium from coculture system, we used ELISA to analyze the VEGF secretion of different conditional mediums. We found that there was no significant difference of the VEGF level between conditional medium from HepG2 and the one from LX-2. However, the VEGF level had almost 2.5-fold induction in coculture system (P < 0.001).
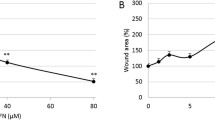
To investigate whether the biguanide metformin, a first-line oral antidiabetic drug, has any effect on interaction of hepatocarcinoma with stellate cells, we used xx metformin to treat coculture system and collected this conditional medium for comparison. Figure 3 indicated that metformin treatment dramatically inhibited tube formation (P < 0.001). Furthermore, this inhibition was associated with lower level of VEGF in metformin-treated conditional medium (P < 0.001) as shown in Fig. 3a, b.
Metformin inhibited angiogenesis induced by interplay of HepG2 with LX-2. a Tube formation assay for conditional medium from coculture system treated with/without metformin (P < 0.001 between two groups). b ELISA analysis of VEGF for conditional medium from coculture system treated with/without metformin (P < 0.001 between two groups)
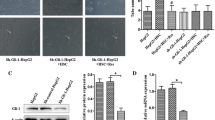
It was not clear whether metformin has this anti-angiogenesis effects by acting on either HepG2 or LX-2, or both. To answer this question, we conducted AMPKα knockdown to observe changes for angiogenic potential of coculture system. The results showed successful knockdown of AMPKα (as shown in Fig. 4a). The efficiency of knockdown was beyond 70%; GADDH was used as a loading control. In the coculture system, AMPK knockdown in HepG2 alone did not alter metformin’s inhibition (P > 0.05, compared with scramble control); however, AMPK knockdown in LX-2 abolished metformin inhibition (P < 0.001, compared with scramble control, but P > 0.05, compared with vehicle control). VEGF secretion assay indicated that AMPK knockdown in LX-2 also restored VEGF level in coculture system under metformin treatment (P > 0.05, compared with vehicle control).
AMPK knockdown in LX-2 abolished metformin inhibition. a Western blot for AMPK knockdown experiment; GAPDH was used as loading control. b Tube formation assay for AMPK knockdown effect on metformin inhibition (*P < 0.001, compared with scramble in metformin-treated coculture system and HepG2 knockdown group. P > 0.05 compared with no treatment group). c ELISA analysis of VEGF in different groups (*P < 0.001, compared with scramble in metformin-treated coculture system and HepG2 knockdown group. P > 0.05 compared with no treatment group)
Discussion
Angiogenesis plays an important role during liver tumor. HCC is a highly angiogenic cancer and displays marked vascular abnormalities [18]. Growing evidences from genetic, genomic, and cell-biology indicate that angiogenesis during tumorigenesis is determined not only by malignant cells but also by their microenvironment [19–23]. In our coculture system, we found that interplay between HCC and HSC could promote angiogenesis (Fig. 2). Moreover, we did not observe angiogenic effect of conditional medium from HepG2 or LX-2 alone. Although other groups reported conditional medium from HCC(24) could contain angiogenic factors (it was also supported by our results but lower level VEGF), our findings basically highlighted that crosstalk between tumor and its environment was more important for its angiogenesis. Following this finding, we wondered whether metformin has any effect this angiogenesis induced by interplay of HCC with HSC. Interestingly, we found that metformin did inhibit angiogenesis induced by crosstalk between HCC and HSC (Fig. 3) and reduced level of angiogenic factor (VEGF).
Metformin is known for AMPK activator and most of its effects through AMPK activation. AMPK is a heterotrimeric complex composed of a catalytic a subunit and regulatory β and γ subunits, each of which is encoded by 2 or 3 distinct genes [24]. AMPK regulates multiple processes inside the cell and can be considered as a potential candidate for key regulator from normal to malignant growth [25, 26]. In order to investigate whether metformin inhibition is through AMPK activation, we used siRNA to knockdown HepG2 and LX-2, respectively, and found that only LX-2 responded AMPK knockdown and abolished metformin inhibition in coculture system.
It was also reported that VEGF expression is frequently detected in well-differentiated HCC rather than in moderately to poorly differentiated HCC [27–29]. In our case, HepG2 displayed less angiogenic potential and less VEGF secretion compared with coculture system though HepG2 is derived from the liver tissue of a 15-year-old Caucasian American male with a well-differentiated HCC. Also LX-2 alone did not showed angiogenic stimulation either. Only crosstalk between both could dramatically promote angiogenesis in vitro. Our data supported the hypothesis that tumor microenvironment plays an important role in tumor development.
Our knockdown experiment implicated that even HCC contributed angiogenesis during crosstalk with HSC, but inhibition of metformin may not directly act on HCC part through classic AMPK pathway. In the future, we are going to explore whether other pathways exit for metformin’s action in HCC. In summary, here, our findings suggested that metformin could inhibit HCC angiogenesis through targeting on HSC.
References
World Health Organization. Mortality database. Available at http://www.who.int/whosis/whostat/EN_WHS08_Part1.pdf. Accessed 4 Dec 2008.
Bosetti, C., Levi, F., Boffetta, P., et al. (2008). Trends in mortality from hepatocellular carcinoma in Europe, 1980–2004. Hepatology, 48, 137–145.
El-Serag, H. B., Tran, T., & Everhart, J. E. (2004). Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology, 126, 460–468.
El-Serag, H. B., Richardson, P. A., & Everhart, J. E. (2001). The role of diabetes in hepatocellular carcinoma: a case–control study among United States veterans. American Journal of Gastroenterology, 96, 2462–2467.
Verlato, G., Bonora, E., Zoppini, G., & Muggeo, M. (2003). Mortality from site-specific malignancies in type 2 diabetic patients from Verona. Diabetes Care, 26, 1047–1051.
Inoue, M., Iwasaki, M., Otani, T., et al. (2006). Diabetes mellitus and the risk of cancer: Results from a large-scale population based cohort study in Japan. Archives of Internal Medicine, 166, 1871–1877.
Lai, M. S., Hsieh, M. S., Chiu, Y. H., & Chen, T. H. H. (2006). Type 2 diabetes and hepatocellular carcinoma: A cohort study in high prevalence area of hepatitis virus infection. Hepatology, 43, 1295–1302.
Chen, C. L., Yang, H. I., Yang, W. S., et al. (2008). Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: A follow-up study in Taiwan. Gastroenterology, 135, 111–121.
Donadon, V., Balbi, M., Casarin, P., Vario, A., & Alberti, A. (2008). Association between hepatocellular carcinoma and type 2 diabetes mellitus in Italy: Potential role of insulin. World Journal of Gastroenterology, 14(37), 5695–5700.
Evans, J. M., Donnelly, L. A., Emslie-Smith, A. M., Alessi, D. R., & Morris, A. D. (2005). Metformin and reduced risk of cancer in diabetic patients. BMJ, 330, 1304–1305.
Hanahan, D., & Coussens, L. M. (2012). Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell, 21, 309–322.
Hernandez-Gea, V., Toffanin, S., Friedman, S. L., & Llovet, J. M. (2013). Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology, 144, 512–527.
Dapito, D. H., Mencin, A., Gwak, G. Y., Pradere, J. P., Jang, M. K., Mederacke, I., et al. (2012). Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell, 21, 504–516.
Friedman, S. L. (2008). Hepatic stellate cells: Protean, multifunctional, and enigmatic cells of the liver. Physiological Reviews, 88, 125–172.
Coulouarn, C., Corlu, A., Glaise, D., Guénon, I., Thorgeirsson, S. S., & Clément, B. (2012). Hepatocyte-stellate cell cross-talk in the liver engenders a permissive inflammatory microenvironment that drives progression in hepatocellular carcinoma. Cancer Research, 72, 2533–2542.
Kang, N., Gores, G. J., & Shah, V. H. (2011). Hepatic stellate cells: Partners in crime for liver metastases? Hepatology, 54, 707–713.
Santamato, A., Fransvea, E., Dituri, F., Caligiuri, A., Quaranta, M., Niimi, T., et al. (2011). Hepatic stellate cells stimulate HCC cell migration via laminin-5 production. Clinical Science, 121, 159–168.
Yang, Z. F., & Poon, R. T. (2008). Vascular changes in hepatocellular carcinoma. The Anatomical Record, 291, 721–734.
Lin, N., Chen, Z., Lu, Y., Li, Y., Hu, K., & Xu, R. (2014). Role of activated hepatic stellate cells in proliferation and metastasis of hepatocellular carcinoma. Hepatology Research,. doi:10.1111/hepr.12356.
Eiró, N., & Vizoso, F. J. (2014). Importance of tumor/stroma interactions in prognosis of hepatocellular carcinoma. Hepatobiliary Surgery and Nutrition, 3(2), 98–101.
Sandhu, D. S., Baichoo, E., & Roberts, L. R. (2014). Fibroblast growth factor signaling in liver carcinogenesis. Hepatology, 59(3), 1166–1173.
Zhang, Z. L., Zhang, J. F., Yuan, Y. F., He, Y. M., Liu, Q. Y., Mao, X. W., et al. (2014). Suppression of angiogenesis and tumor growth in vitro and in vivo using an anti-angiopoietin-2 single-chain antibody. Experimental and Therapeutic Medicine, 7(3), 543–552. Epub 2014 Jan 7.
Zhao, W., Zhang, L., Xu, Y., Zhang, Z., Ren, G., Tang, K., et al. (2014). Hepatic stellate cells promote tumor progression by enhancement of immunosuppressive cells in an orthotopic liver tumor mouse model. Laboratory Investigation, 94(2), 182–191.
Hardie, D. G. (2004). The AMP-activated protein kinase pathway–new players upstream and downstream. Journal of Cell Science, 117, 5479–5487.
Luo, Z., Saha, A. K., Xiang, X., & Ruderman, N. B. (2005). AMPK, the metabolic syndrome and cancer. Trends in Pharmacological Sciences, 26, 69–76.
Shaw, R. J. (2006). Glucose metabolism and cancer. Current Opinion in Cell Biology, 18, 598–608.
Yamaguchi, R., Yano, H., Nakashima, O., Akiba, J., Nishida, N., Kurogi, M., et al. (2006). Expression of vascular endothelial growth factor-C in human hepatocellular carcinoma. Journal of Gastroenterology and Hepatology, 21, 152–160.
Yamaguchi, R., Yano, H., Iemura, A., Ogasawara, S., Haramaki, M., & Kojiro, M. (1998). Expression of vascular endothelial growth factor in human hepatocellular carcinoma. Hepatology, 28, 68–77.
Uematsu, S., Higashi, T., Nouso, K., Kariyama, K., Nakamura, S., Suzuki, M., et al. (2005). Altered expression of vascular endothelial growth factor, fibroblast growth factor-2 and endostatin in patients with hepatocellular carcinoma. Journal of Gastroenterology and Hepatology, 20, 583–588.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qu, H., Yang, X. Metformin Inhibits Angiogenesis Induced by Interaction of Hepatocellular Carcinoma with Hepatic Stellate Cells. Cell Biochem Biophys 71, 931–936 (2015). https://doi.org/10.1007/s12013-014-0287-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-014-0287-8