Abstract
Doxorubicin (DOXO) may cause serious cardiotoxic effects that limit its use as an antineoplastic agent. We aimed to evaluate the protective role of taurine (TAU), a beta amino acid with antioxidant activity, against DOXO-induced cardiotoxicity in a rat model. Thirty-one male Sprague–Dawley rats (300–400 g) were randomized into four groups: control (n = 7, intraperitoneal [ip] saline for 14 days), TAU (n = 8, 150 mg/kg body weight TAU ip for 14 days), DOXO (n = 8, 25 mg/kg body weight DOXO ip on 12th, 13th, and 14th days), and DOXO + TAU (n = 8, TAU for 14 days and DOXO on 12th, 13th, and 14th days). The left ventricular functions were evaluated on 15th day by echocardiography. The heart tissues were then excised for histological evaluation. In DOXO group, left ventricular ejection fraction (LVEF), fractional shortening (FS), and mitral lateral annulus (s') velocity were significantly lower, and the left ventricular end-diastolic and end-systolic diameters (LVEDD, LVESD) were significantly higher than control group (p < 0.05), indicating a significant deterioration in left ventricular functions. However, in comparison to DOXO group, LVESD, LVEDD, LVEF, FS, and s' were significantly improved in DOXO + TAU group (p < 0.05). On histological evaluation, contrary to the normal cellular structure of cardiomyocytes in control and TAU groups, DOXO group showed increased nuclear or cytoplasmic changes and infiltrative cell proliferation (p < 0.001), which were remarkably reduced in DOXO + TAU group (p < 0.001). TAU treatment has a protective effect against DOXO-induced cardiotoxicity on echocardiographical and histological evaluation. For common use of TAU to prevent DOXO-induced cardiotoxicity, our findings should be confirmed by clinical studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Doxorubicin (DOXO) is an anthracycline antineoplastic agent which is effective against several types of malignancies such as leukemia, lymphoma, and breast cancer. However, in addition to its strong antineoplastic action, it has some cardiotoxic effects, which limit its clinical use (Chatterjee et al. 2010). Various agents such as metformin and phenytoin have been suggested to have protective effects against DOXO-induced cardiotoxicity (Argun et al. 2015; Razmaraii et al. 2015). However, none of these drugs have been widely used in clinical practice, and the search for new protective agents against DOXO-induced cardiotoxicity is ongoing.
Taurine (TAU) or aminoethane sulfonic acid is a beta amino acid highly concentrated in heart and muscle. Although TAU has many physiological effects such as modulation of osmotic pressure, cation homeostasis, receptor regulation, cell development, and cell signaling, studies have focused on its antioxidant effects and adverse conditions caused by its depletion (Schaffer et al. 2010). As TAU depletion causes dilated cardiomyopathy, TAU therapy has been shown to have beneficial effects on congestive heart failure in animal models (Azuma et al. 1985). Some biochemical studies have shown that TAU has a cardioprotective effect against DOXO-induced cardiotoxicity (Wang et al. 2015; Ito et al. 2009); however, this protective effect has not yet been confirmed by hemodynamic, echocardiographical, or histological data.
Therefore, in this study, we aimed to evaluate the protective role of TAU against acute DOXO-induced cardiotoxicity in a rat model by echocardiographical and histological evaluation.
Materials and methods
Study animals
Thirty-one male Sprague–Dawley rats (300–400 g) obtained from and housed in Experimental Animals Breeding Unit of Hacettepe University were used for the study. All rats were kept under controlled conditions at 21 ± 2 °C and 30–70% relative humidity with 12 h dark/12 h light illumination sequence (the lights were on between 07.00 and 19.00) with ad libitum access to tap water and standard rat chow.
This study was approved by the Hacettepe University School of Medicine Institutional Ethics Committee for Animal Experiments (date 14/04/2015, no. 2015/34). All the study procedures were performed according to the Guiding Principles for the Care and Use of Laboratory Animals.
Experimental procedure
Rats were allocated randomly into four groups. Control animals (C) (n = 7) received only saline intraperitoneally for a period of 14 days. TAU group (n = 8) was given intraperitoneal TAU (Sigma Chemical Co., St. Louis, MO, USA) at the dose of 150 mg/kg body weight (1 ml/kg per day) for 14 days. DOXO group (n = 8) was intraperitoneally injected with DOXO (Adrimisin 50 mg, Deva, Turkey) at a cumulative dose of 25 mg/kg body weight (1 ml/kg per day) for 3 days; on 12th, 13th, and 14th days. This protocol was adopted from Saad et al. (2001).
DOXO + TAU group (n = 8) was administered same dose of TAU for 14 consecutive days and additionally same dose of DOXO on 12th, 13th, and 14th days. All injections were applied between 9.30 and 10.00 a.m. to minimize the circadian variations.
Echocardiographical examination
On the 15th day, the animals were anesthetized with intraperitoneal ketamine (90 mg/kg) and xylasine (10 mg/kg). The chest of animals was shaved, and they were stabilized in the supine position on the working board. For echocardiographical examination, GE Vivid medical ultrasound machine with a 10-S transducer (GE Medical Systems, Horten, Norway) was used by a blinded, experienced echocardiographer. The left ventricular end-diastolic (LVEDD) and end-systolic diameters (LVESD), interventricular septum (IVS) and posterior wall (PW) thickness were measured with the M-mode on short axis view. Left ventricular ejection fraction (LVEF) and fractional shortening (FS) parameters were calculated by Teichholz method (Fig. 1a). Mitral diastolic E and A waves were measured with pulse Doppler on apical view (Fig. 1b). Mitral lateral annulus e' and s' velocities were measured with tissue Doppler technique on mitral lateral annulus. Echocardiographical examination was performed in the middle of the day to eliminate the effect of circadian changes on diastolic dysfunction.
Histological examination
After the left ventricular functions were evaluated animals were killed using exsanguination; thereafter the heart was dissected, removed quickly, and weighed. They were fixed and sectioned in 10% formalin for light microscopic examination and then embedded in paraffin wax. 5 μm sections were cut and stained with hematoxylin–eosin. At least ten different sections from each heart were captured, and minimum ten cardiomyocytes from each image were evaluated. Myocyte thickness was quantified using latitudinal midsections of the left ventricle. Approximately 100–125 cardiomyocytes were analyzed and averaged for each animal. Infiltrative cell number, nuclear or cytoplasmic cell change such as karyorrhexis and karyolysis were measured from separate three view and averaged for each animal. Photographs were taken and analyzed by Leica DM6000 microscope and the image analyzing system (Istanbul, Turkey).
Statistical analysis
Statistical analysis was carried out using the SPSS software package (Statistical Package for Social Sciences, version 22.0, SPSS Inc., Chicago, Illinois, USA). Results are given as the mean ± standard deviation or median with interquartile range for quantitative data. Normal distribution and the assumptions of equality of variances were checked for all variables by Shapiro–Wilk test. Study groups were compared using one-way analysis of variance (ANOVA) followed by Tukey test for post hoc analysis for parametric results (LVEDD, LVESD, EF, FS, s', infiltrative cell quantity and myocytes with nuclear or cytoplasmic changes) or compared by Krusskal–Wallis analysis for non parametric results (E/e'ratio, cardiac mass, cardiomyocyte cell size). A p value less than 0.05 was accepted as statistically significant.
Results
Echocardiographical findings
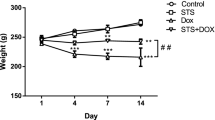
Echocardiography of the control group showed normal systolic and diastolic left ventricular functions. In this group, LVEF was 77.5%, LVEDD was 5.1 mm, and s' was 8.7 mm/s (Table 1, Fig. 2a). Echocardiographical findings of TAU group were within normal limits and similar to control group (Table 1, Fig. 2b). In DOXO group, LVEF, FS, s' were significantly decreased, and LVEDD, LVESD, E/e' ratio were significantly increased as compared with control group (p < 0.05, Table 1, Fig. 2c).
Echocardigraphic results. a Control group showed normal systolic and diastolic left ventricular functions. b TAU group was within normal limits and similar to control group. c In DOXO group, LVEDD, LVESD were significantly increased and LVEF, FS were significantly decreased. d In DOXO + TAU group, LVEDD and LVESD were significantly lower compared to DOXO group. LVEF, FS of DOXO + TAU were also significantly improved compared to DOXO group
TAU significantly reversed DOXO-induced cardiotoxicity. In DOXO + TAU group, LVEDD and LVESD were significantly lower compared to DOXO group (p < 0.05, Table 1, Fig. 2d). LVEF, FS, s' and E/e' ratio of DOXO + TAU were also significantly improved compared to DOXO group (p < 0.05, Table 1).
Histological findings
On histological evaluation, there was no degenerative changes at branched cardiomyocytes that have one or two centrally located nuclei and cross striations in control group. Cells in this group showed intact histological structures (Fig. 3a). In TAU group, histological structure of cardiomyocytes was almost normal. We have not detected any degenerative changes that indicate myofibril loss in cardiomyocytes (Fig. 3b). On the other hand, in DOXO group, cardiomyocytes showed degenerative changes including nuclear damage like karyorrhexis and karyolysis (Fig. 4). Some cardiomyocytes in DOXO group had significant myofibrile loss and vacuolar degeneration. Active fibroblasts with hypertrophic nucleus and infiltrative cell proliferation within the connective tissue between cardiomyocytes were detected. Cardiac mass and cardiomyocyte cell size of the specimens obtained from DOXO group were significantly lower than the control group; besides that infiltrative cell quantification and myocytes with nuclear or cytoplasmic changes were significantly higher than the control group (p < 0.05, Table 2). However, there were no degenerative changes in DOXO + TAU group, indicating the protective effect of TAU on DOXO-induced cardiotoxicity at histologic level (Fig. 5). Myocytes with nuclear or cytoplasmic changes and infiltrative cell proliferation were reduced in DOXO + TAU group compared to DOXO group (p < 0.001, Table 2). Fibroblasts in DOXO + TAU group were observed to be inactive with flattened nuclei. Although not statistically significant, both cardiac mass and cardiomyocyte cell size were higher in DOXO + TAU group than DOXO group.
Histological sections of the hearts obtained from DOXO group. Karyolysis and karyorrhexis (arrow) were observed in degenerative cardiomyocytes, and myofibril loss and vacuolar degeneration (asterisks) were significant in most cardiomyocytes (a). Active fibroblasts (arrow) with hypertrophic nuclei and infiltrative cell (arrowhead) proliferation were also observed (b) (Hematoxylin–eosin, ×400)
Discussion
In this experimental study, both echocardiographical and histological findings showed that TAU had a protective effect against DOXO-induced cardiotoxicity. In echocardiographical examination, DOXO caused left ventricle dilatation and systolic dysfunction, which was reversed by TAU treatment. Histological evaluation showed that DOXO induced degenerative changes at cardiomyocytes and increased infiltrative cell proliferation. TAU also had a protective effect on these degenerative changes and infiltrative cell accumulation.
With the increasing life span of cancer patients, the exposure to chemotherapy along with its side effects increases. Therefore, the side effects of chemotherapeutics play a decisive role in the quality of life, mortality, and morbidity of the cancer patients (Yeh and Bickford 2009).
Antracyclines are highly effective chemotherapeutics that are used for various solid and hematological malignancies such as breast cancer, sarcoma, leukemia, and lymphoma. Although they are effective drugs, they have some hazardous cardiotoxic effects such as left ventricular dilatation and dilated cardiomyopathy (Vejpongsa and Yeh 2014). Since cardiotoxic effects of these agents are generally irreversible, protective approaches against anthracycline-induced cardiotoxicity should be developed (Angsutararux et al. 2015; Cardinale et al. 2015). Some agents including TAU have been suggested to have protective effects against DOXO-induced cardiotoxicity (Argun et al. 2015; Razmaraii et al. 2015; Zhang et al. 2015; Agustini et al. 2015). Wang et al. (2015) studied hemodynamic parameters, levels of liver toxicity markers, and oxidative stress in DOXO-treated rats and found that TAU significantly attenuated the reductions in blood pressure, left ventricular pressure and increases in serum alanine aminotransferase and aspartate aminotransferase activities in serum, and reduces in serum albumin levels induced by DOXO. Ito et al. (2009) reported that TAU suppresses reactive oxygen species generation and regulates gene expression in the DOXO-treated heart in mice. However, protective effect of TAU has not been confirmed by echocardiographical or histological data.
Various mechanisms for the DOXO cardiotoxicity and protective effect of TAU have been hypothesized. First DOXO was suggested to increase intracellular free oxygen radicals leading to degradation of the sarcomere, DNA damage, and mitochondrial damage (Rochette et al. 2015; Narýn et al. 2004). Catalase and superoxide dismutase activity in cardiomyocytes is much weaker than other tissues; therefore cardiomyocytes are more susceptible to oxidative damage (Pai and Nahata 2000). TAU, which is an antioxidant, may have a cardioprotective effect by inhibiting the effects of free oxygen radicals that release during DOXO metabolism (Schaffer et al. 2009; Li et al. 2003; Cantafora et al. 1991). Oxidative stress causes the activation of caspase 3 in mitochondria and thereafter gives rise to apoptosis in the myocardium (Childs et al. 2002). TAU can down regulate caspase 3 activation in dose-dependent manner (Wang et al. 2018). By this means, TAU can protect myocardium from apoptosis and can decrease karyolysis, karyorrhexis.
Second, TAU has also been shown to have cardioprotective effect against various ischemic agents with its antioxidant properties (Shiny et al. 2005). Supporting this hypothesis, we detected less karyolysis, karyorrhexis, vacuolar degeneration on histological examination and better left ventricle systolic performance on echocardiographical examination, which are indicators of ischemic damage, of DOXO + TAU group compared to group receiving only DOXO (Shiny et al. 2005).
Third, DOXO inhibits the sarcolemmal proteins RyR2 and SERCA2A (Hanna et al. 2014). TAU increases SERCA activity and protects against oxidative stress (Schaffer et al. 2010; Rapundalo 1998). TAU deficiency contributes to impaired intracellular calcium homeostasis in cardiotoxicity of DOXO (Harada et al. 1990).
Furthermore, DOXO causes apoptosis through the enhancement of p53, b cell lymphoma 2 like protein 4 (Childs et al. 2002; Guo et al. 2013; Saleme et al. 2019). Taurine can down-regulate Bcl-2 expression and up-regulate p53 expression in dose dependent manner (Wang et al. 2018).
Doxo treatment also results in mitochondrial dysfunction, mitochondrial DNA damage, and alteration in ATP level which all lead to necrosis in cardiomyocite (Renu et al. 2018). TAU also stabilizes mitochondrial membranes, and decreases myocardial ATP consumption. Therefore, TAU can decrease karyolysis, karyorrhexis, vacuolar degeneration on histological examination and can ameliorate left ventricle systolic performance on echocardiographical examination.
Finally, there are many changes in alpha myosin light chain, ventricular myosin light chain isoform-2, and brain natriuretic peptide gene expressions in the case of doxorubicin cardiomyopathy. TAU has been reported to reduce these changes (Ito et al. 2009).
In the present study, 14-day treatment with DOXO induced cardiotoxity on echocardiographical evaluation. In the group treated with DOXO, LVEF, FS, and s' were reduced, LVESD and LVEDD were dilated, and E/e' ratio was increased. Similarly on histological evaluation, DOXO induced cardiotoxicity, which was seen as degenerative changes in cardiomyocytes including nuclear damage as karyorrhexis and karyolysis, myofibrile loss and vacuolar degeneration. These changes could indicate the apoptosis or necrosis of cardiomyocytes induced by DOXO. Active fibroblasts with hypertrophic nucleus could be another sign of DOXO toxicity. In present study, TAU could prevent all of these changes via various pathways just like antioxidant, antiischemic effects or other mechanisms. Cardiac mass and cardiomyocyte cell size of the specimens obtained from DOXO group were also decreased. TAU reversed most of the DOXO-induced cardiotoxicity findings at both echocardiograhic and histologic levels. The study parameters of DOXO–TAU group were comparable to those of control group. However, the improvements obtained by TAU in LVEDD, cardiac mass, and cardiomyocyte cell size were not statistically significant, which may be due to the insufficient duration of TAU medication to remove all of the cardiotoxic effects of DOXO.
The study had some limitations. We could not evaluate the blood levels of brain natriuretic peptide and central venous pressure, which would have supported our findings. We also could not evaluate whether TAU may alter the DOXO’s antineoplastic activity. Nevertheless, this is the first study presenting echocardiographical and histological evidence on protective effect of TAU against DOXO-induced cardiotoxicity.
In conclusion, in this experimental rat model, echocardiographical and histological data showed that TAU has a protective effect against DOXO-induced cardiotoxicity. For common use of TAU to prevent DOXO-induced cardiotoxicity, our findings should be confirmed by further clinical studies.
References
Agustini FD, Arozal W, Louisa M, et al (2015) Cardioprotection mechanism of mangiferin on doxorubicin-induced rats: focus on intracellular calcium regulation. Pharm Biol 1–9
Angsutararux P, Luanpitpong S, Issaragrisil S (2015) Chemotherapy-induced cardiotoxicity: overview of the roles of oxidative stress. Oxid Med Cell Longev 2015:795602
Argun M, Uzum K, Sonmez MF, et al (2015) Cardioprotective effect of metformin against doxorubicin cardiotoxicity in rats. Anatol J Cardiol
Azuma J, Sawamura A, Awata N et al (1985) Therapeutic effect of taurine in congestive heart failure: a double-blind crossover trial. Clin Cardiol 8(5):276–282
Cantafora A, Blotta I, Rossi SS, Hofmann AF, Sturman JA (1991) Dietary taurine content changes liver lipids in cats. J Nutr 121(10):1522–1528
Cardinale D, Colombo A, Bacchiani G et al (2015) Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 131:1981–1988
Chatterjee K, Zhang J, Honbo N, Karliner JS (2010) Doxorubicin cardiomyopathy. Cardiology 115(2):155–162
Childs AC, Phaneuf SL, Dirks AJ, Phillips T, Leeuwenburgh C (2002) Doxorubicin treatment in vivo causes cytochrome C release and cardiomyocyte apoptosis, as well as increased mitochondrial efficiency, superoxide dismutase activity, and Bcl-2: bax ratio. Cancer Res 62:4592–4598
Guo R, Wu K, Chen J et al (2013) Exogenous hydrogen sulfide protects against doxorubicin-induced inflammation and cytotoxicity by inhibiting p38MAPK/NFkappaB pathway in H9c2 cardiac cells. Cell Physiol Biochem 32(6):1668–1680
Hanna AD, Lam A, Tham S, Dulhunty AF, Beard NA (2014) Adverse effects of doxorubicin and its metabolic product on cardiac RyR2 and SERCA2A. Mol Pharmacol 86(4):438–449
Harada H, Cusack BJ, Olson RD et al (1990) Taurine deficiency and doxorubicin: interaction with the cardiac sarcolemmal calcium pump. Biochem Pharmacol 39(4):745–751
Ito T, Muraoka S, Takahashi K, Fujio Y, Schaffer SW, Azuma J (2009) Beneficial effect of taurine treatment against doxorubicin-induced cardiotoxicity in mice. Adv Exp Med Biol 643:65–74
Li YT, Maskos K, Chou CW, Cole RB, Li SC (2003) Presence of an unusual GM2 derivative, taurine-conjugated GM2, in Tay-Sachs brain. J Biol Chem 278(37):35286–35291
Narýn F, Demýr F, Akgün H et al (2004) Doxorubicin-induced experimental cardiotoxicity and effect of pentoxphylline on cardiotoxicity. Turk Kardiyol Dern Ars 32(5):279–287
Pai VB, Nahata MC (2000) Cardiotoxicity of chemotherapeutic agents: incidence, treatment and prevention. Drug Saf 22(4):263–302
Rapundalo ST (1998) Cardiac protein phosphorylation: functional and pathophysiological correlates. Cardiovasc Res 38(3):559–588
Razmaraii N, Babaei H, Nayebi AM, Asadnasab G, Helan JA, Azarmi Y (2015) Cardioprotective effect of phenytoin on doxorubicin-induced cardiac toxicity in a rat model. J Cardiovasc Pharmacol
Renu K, Abilash VG, Tirupathi Pichiah PB, Arunachalam S (2018) Molecular mechanism of doxorubicin-induced cardiomyopathy—an update. Eur J Pharmacol 818:241–253
Rochette L, Guenancia C, Gudjoncik A et al (2015) Anthracyclines/trastuzumab: new aspects of cardiotoxicity and molecular mechanisms. Trends Pharmacol Sci 36(6):326–348
Saad SY, Najjar TA, Al-Rikabi AC (2001) The preventive role of deferoxamine against acute doxorubicin-induced cardiac, renal and hepatic toxicity in rats. Pharmacol Res 43(3):211–218
Saleme B, Gurtu V, Zhang Y et al (2019) Tissue-specific regulation of p53 by PKM2 is redox dependent and provides a therapeutic target for anthracycline-induced cardiotoxicity. Sci Transl Med 11(478)
Schaffer SW, Azuma J, Mozaffari M (2009) Role of antioxidant activity of taurine in diabetes. Can J Physiol Pharmacol 87(2):91–99
Schaffer SW, Jong CJ, Ramila KC, Azuma J (2010) Physiological roles of taurine in heart and muscle. J Biomed Sci 17(Suppl 1):S2
Shiny KS, Kumar SH, Farvin KH, Anandan R, Devadasan K (2005) Protective effect of taurine on myocardial antioxidant status in isoprenaline-induced myocardial infarction in rats. J Pharm Pharmacol 57(10):1313–1317
Vejpongsa P, Yeh ET (2014) Prevention of anthracycline-induced cardiotoxicity: challenges and opportunities. J Am Coll Cardiol 64(9):938–945
Wang Y, Mei X, Yuan J, Lu W, Li B, Xu D (2015) Taurine zinc solid dispersions attenuate doxorubicin-induced hepatotoxicity and cardiotoxicity in rats. Toxicol Appl Pharmacol 289(1):1–11
Wang J, Qi C, Liu L et al (2018) Taurine protects primary neonatal cardiomyocytes against apoptosis induced by hydrogen peroxide. Int Heart J 59(1):190–196
Yeh ET, Bickford CL (2009) Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol 53(24):2231–2247
Zhang S, Meng T, Liu J, Zhang X, Zhang J (2015) Cardiac protective effects of dexrazoxane on animal cardiotoxicity model induced by anthracycline combined with trastuzumab is associated with upregulation of calpain-2. Medicine (Baltimore) 94(4):e445
Acknowledgements
We thank to Ersin Fadıllıoğlu MD, Davut Singer MD for the experiments.
Funding
The study was not funded.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflicts of interest.
Informed consent
This research involves only animal participants; therefore informed consent was not needed.
Additional information
Handling Editor: H. Jakubowski.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Barış, V.Ö., Gedikli, E., Yersal, N. et al. Protective effect of taurine against doxorubicin-induced cardiotoxicity in rats: echocardiographical and histological findings. Amino Acids 51, 1649–1655 (2019). https://doi.org/10.1007/s00726-019-02801-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-019-02801-7