Abstract
Ivabradine has recently been demonstrated to have antiarrhythmic properties in atrial fibrillation. The aim of the present study was to assess the electrophysiologic profile of ivabradine in an experimental whole-heart model of long-QT-syndrome. In 12 isolated rabbit hearts long-QT-2-syndrome (LQT2) was simulated by infusion of d,l-sotalol (100 µM). 12 rabbit hearts were treated with veratridine (0.5 µM) to mimic long-QT-3-syndrome (LQT3). Sotalol induced a significant prolongation of QT-interval (+ 40 ms, p < 0.01) and action potential duration (APD, + 20 ms, p < 0.01). Similar results were obtained in veratridine-treated hearts (QT-interval: +52 ms, p < 0.01; APD: + 41 ms, p < 0.01). Of note, both sotalol (+ 26 ms, p < 0.01) and veratridine (+ 42 ms, p < 0.01) significantly increased spatial dispersion of repolarisation. Additional infusion of ivabradine (5 µM) did not change these parameters in sotalol-pretreated hearts but resulted in a further significant increase of QT-interval (+ 26 ms, p < 0.05) and APD (+ 49 ms, p < 0.05) in veratridine-treated hearts. Lowering of potassium concentration in bradycardic AV-blocked hearts resulted in the occurrence of early afterdepolarizations (EAD) or polymorphic ventricular tachycardias (VT) resembling torsade de pointes in 6 of 12 sotalol-treated hearts (56 episodes) and 6 of 12 veratridine-treated hearts (73 episodes). Additional infusion of ivabradine increased occurrence of polymorphic VT. Ivabradine treatment resulted in occurrence of EAD and polymorphic VT in 9 of 12 sotalol-treated hearts (212 episodes), and 8 of 12 veratridine-treated hearts (155 episodes). Treatment with ivabradine in experimental models of LQT2 and LQT3 increases proarrhythmia. A distinct interaction with potassium currents most likely represents a major underlying mechanism. These results imply that ivabradine should be employed with caution in the presence of QT-prolongation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ivabradine is an established inhibitor of the pacemaker current I(f) and was introduced for reduction of heart rate without exertion of negative inotropic effects [1]. It is recommended for treatment of symptomatic chronic heart failure in the presence of beta-blocker therapy for further reduction of heart rate [2]. A prolongation of ventricular repolarization was observed in different experimental models [3, 4]. A reduction of ventricular arrhythmias was observed for digitalis-mediated ventricular arrhythmias [5] as well as in a model of short QT syndrome [6]. In accordance, a significant suppression of atrial fibrillation by ivabradine was described in isolated rabbit hearts [7]. Furthermore, a significant slowing of atrioventricular conduction in the presence of atrial fibrillation was reported for ivabradine [8, 9].
However, it is unclear whether ivabradine may induce a further prolongation of repolarization in the presence of other QT-prolonging agents [10]. Therefore, the aim of the present study was to assess the effects of ivabradine in an experimental of drug-induced QT-prolongation. Acquired long-QT-2 syndrome (LQT2) was mimicked by infusion of the IKr-inhibitor sotalol [11] while long-QT-3 syndrome was simulated by treatment with vetradidine, an inhibitor of INa-inactivation [12].
Methods
All experimental protocols were approved by the local animal care committee and conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (8th edition, revised 2011).
Experimental Protocol
Preparation and isolation of rabbit hearts using the Langendorff setup has previously been described extensively [13]. Rabbits were euthanized by injection of 0.5 g sodium thiopental. Then, hearts were explanted and fixated on the Langendorff-apparatus. The atrioventricular node was mechanically ablated with surgical tweezers to allow standardized recordings of electrophysiological parameters. This resulted in a complete atrioventricular dissociation.
Seven specifically manufactured catheters for recording of monophasic action potentials (MAP) were placed on the epicardium of both ventricles. An eighth catheter was placed in the left ventricle to record MAP from the left ventricular endocardium. Hearts were stimulated at defined cycle lengths (CL, 900–300 ms, one minute per CL) to determine baseline values of action potential duration (APD90) and QT-interval. Thereafter, the extracellular potassium concentration was reduced to 1.5 mM to mimic hypokalemia in order to enhance origination of early afterdepolarizations (EADs) and polymorphic ventricular tachycardia (VT) of the torsade de pointes type in spontaneously beating hearts. Five minutes later, the potassium concentration was returned to 5.8 mM.
In 12 isolated hearts, the class III antiarrhythmic drug d,l-sotalol (100 µM, Sigma Aldrich, St. Louis, USA), a potent inhibitor of IKr was administered for simulation of drug-induced LQT2 [11]. In further 12 hearts veratridine (0.5 µM), an inhibitor of sodium channel inactivation, was infused [12]. Thereafter, ivabradine (5 µM) was administered in both study groups while treatment with sotalol or veratridine was continued.
APD90 was defined as the interval between the fastest upstroke and 90% repolarization. Spatial dispersion of repolarization was determined as the difference between the maximum and the minimum of APD90 from eight endocardial and epicardial catheters. Apico-basal and transmural dispersion were assessed. The greatest values were obtained for transmural dispersion in all experiments. The assessment of temporal dispersion was previously described in detail [14]. In brief, it was done by analyzing consecutive beats using Poincaré plots in which each APD90 is plotted against the preceding APD90. Measurements were performed after 20 min of drug infusion just before starting the pacing protocol. The areas of the plots were determined and their dimensions were calculated. The mean orthogonal distance from the diagonal to the points of the Poincaré plot was determined and referred to as temporal dispersion (STV = |Dn + 1 − Dn|/[30 × 2]), where D represents the duration of APD90.
Statistical Analysis
Statistical analysis was performed using the SPSS Software for Windows, release 24.0.0. (SPSS Inc., Chicago, USA). Drug effects on electrophysiological parameters were assessed using general linear model (GLM) for repeated measures. The χ2-test and the Fisher-test were used to compare the incidences of arrhythmias. Differences are considered significant at p < 0.05.
Results
Drug Effects on Ventricular Repolarization and Dispersion of Repolarization in Sotalol- and Veratridine-Treated Hearts
Treatment with sotalol (100 µM) induced a significant increase of APD90 (+ 14.5%, p < 0.01, Table 1) and QT-interval (+ 17.7%, p < 0.01). In accordance, a significant increase of both APD90 (+ 25.0%, p < 0.01) and QT-interval (+ 20.6%, p < 0.01) was observed in veratridine-treated hearts (0.5 µM). Additional infusion of ivabradine did not significantly alter APD90 (+ 7.0%, p = 0.24) or QT-interval (+ 0.1%, p = 0.93) in sotalol-treated hearts. In veratridine-treated hearts, a further significant increase of APD90 (+ 23.9%, p < 0.05) and QT-interval (+ 8.6%, p < 0.05) was recorded. Detailed cycle-length dependent effects of drug treatment are displayed in Fig. 1.
Drug effects on action potential duration (APD90, top) and QT-interval (bottom): LQT2-group (left): filled circle = baseline, filled triangle = 100 µM sotalol, filled square = 5 µM ivabradine; LQT3-group (right): filled circle = baseline, filled triangle = 0.5 µM veratridine, filled square = 5 µM ivabradine (*p < 0.05 as compared with baseline, #p < 0.05 as compared with sotalol or veratridine infusion)
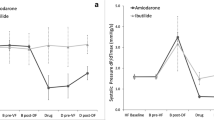
Acute infusion of sotalol significantly augmented spatial dispersion of repolarization (+ 63.4%, p < 0.01). In accordance, an increase of spatial dispersion of repolarization was also observed in veratridine-treated hearts (+ 77.8%, p < 0.01; Fig. 2a). Additional infusion of ivabradine (5 µM) did not significantly alter dispersion of repolarization in sotalol-treated hearts (− 13.4%, p = 0.38) as well as in veratridine-treated hearts (+ 8.3%, p = 0.63). Furthermore, both sotalol and veratridine induced an increase of temporal dispersion of repolarization also known as beat-to-beat variability (Fig. 2b). This effect was also maintained under the influence of ivabradine in both groups.
a Total spatial dispersion of repolarization after infusion of sotalol (top) and veratridine (bottom) and additional infusion of ivabradine (*p < 0.05 vs. baseline). b Representative Poincaré-plots obtained from consecutive action potential duration (APD) intervals at baseline, after infusion of sotalol (top) and consecutive infusion of ivabradine (middle) as well as after infusion of veratridine and subsequent treatment with ivabradine (bottom)
Induction of Torsade de Pointes
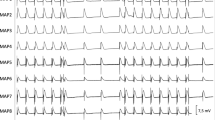
Lowering of potassium concentration to 1.5 mM did not induce EAD and/or torsade de pointes under baseline conditions in both study groups. In hearts treated with sotalol EAD and torsade de pointes were observed in 6 of 12 hearts (Fig. 3, 56 episodes) while also 6 of 12 veratridine-pretreated hearts presented EAD and torsade de pointes (73 episodes). After additional infusion of ivabradine occurrence of torsade de pointes was increased in both study groups. Then, 9 of 12 sotalol-treated hearts were inducible (212 episodes) while 8 of 12 veratridine-treated hearts presented EAD and torsade de pointes after additional treatment with ivabradine (155 episodes, Fig. 4).
Discussion
The results of the present study display for the first time that treatment with ivabradine in the presence of an established therapy with QT-prolonging agents may increase the risk of proarrhythmia. In a sensitive and well-established experimental model of drug-induced QT-prolongation a significant increase of ventricular repolarization that was accompanied by a significant increase of dispersion of repolarization was induced by treatment with sotalol or veratridine. As a result, episodes of torsade de pointes occurred spontaneously and reproducibly. Acute additional infusion treatment with ivabradine did not significantly alter the induced changes of the repolarization period and thereby maintained the highly proarrhythmic milieu. Remarkably, ivabradine increased the occurrence of torsade de pointes resulting in both study groups compared with sole treatment with sotalol or veratridine.
Of note, the occurrence of torsade de pointes was associated with a significant increase of transmural dispersion of repolarization. This observation is congruent with the findings of previous studies in animal models [15]. The close association between an excessive increase of spatial dispersion and the occurrence of proarrhythmia was, for example, described in dogs [16] and isolated, Langendorff-perfused rabbit hearts [13, 14]. In previous studies, a significant reduction of dispersion of repolarization was associated with potent antiarrhythmic properties in the present model. This has been described for poly-unsaturated fatty acids [14], the class-I antiarrhythmic agent mexiletine [12] and the novel antiarrhythmic drug vernakalant [11]. In contrast, ivabradine did not reduce spatial dispersion of repolarization. Of note, in veratridine-pretreated hearts a further significant ascent of QT-interval and APD90 was observed while both parameters remained stable in sotalol-treated hearts. This aspect suggests that ivabradine inhibits potassium currents in the concentration employed in the present study. This could explain the fact that in veratridine-treated hearts where the prolongation of repolarization is due to the preclusion of inactivation of sodium currents a further prolongation of repolarization can be achieved while in sotalol-treated hearts, most likely, potassium currents are already potently inhibited by the IKr-blocker sotalol.
In accordance, both sotalol and veratridine increased temporal dispersion defined as beat-to-beat variability and thereby further supported the proarrhythmic milieu. This effect has been previously identified as a major proarrhythmic factor by our group [14] and in other models [17]. Of note, the additional administration of ivabradine did not alter temporal dispersion but maintained this parameter at elevated levels.
In addition to the described high level of spatial dispersion of repolarization and the increase of temporal dispersion the occurrence of EADs, a major trigger of torsade de pointes, remained stable under the influence of ivabradine during continuous infusion of the above described QT-prolonging agents. Again, this is congruent to previous examinations where the close association of EADs and torsade de pointes was reported [18].
These results are in accordance with previously published work where an inhibition of the hERG1-mediated KCNH2 current and a resulting prolongation of ventricular repolarization were described [4]. A prolongation of ventricular repolarization was also observed in a further study in isolated myocytes [3]. Of note, in this context pharmacovigilance data reported a substantially high number of VT in patients treated with ivabradine was reported and may be attributed to the prolongation of ventricular repolarization as well as to the bradycardic effects of ivabradine that could additionally promote proarrhythmia.
The additive proarrhythmic effect or ivabradine in the presence of QT-prolongation can be explained by a reduced repolarization reserve in sotalol- or veratridine-pretreated hearts. This theoretical concept was introduced as an individual response to a prolonged repolarization period mediated by cardiovascular and non-cardiovascular drugs [19].
Limitations of the Study
In the present study, drug-induced QT-prolongation was induced by sotalol and veratridine. Of note, the electrophysiological characteristic of these drugs differ from the situation in congenital long-QT-syndrome (LQTS). Therefore, the results described above cannot be directly transferred to the situation of inherited LQTS or to QT-prolongation mediated by other agents. During hypokalemia no values for APD, QT-interval and dispersion of repolarization could be obtained as the hearts were not paced to allow spontaneous origination of torsade de pointes. In addition, as the present study was conducted in a whole-heart model direct drug effects on ion currents cannot be visualized. Therefore, the assumption that the effects observed for ivabradine in the present study are due to interference with potassium currents remains speculative.
Conclusions and Implications
The results of the present study confirm the prolonging effects of ivabradine on the ventricular repolarization in an isolated rabbit heart model and its arrhythmogenic potential when vulnerability of the repolarization was already altered by co-administration of sotalol or veratridine. The results are consistent with previous studies where additive proarrhythmic effects of QT-prolonging agents have been reported [20]. These results imply that ivabradine should be administered with caution in the presence of a reduced repolarization reserve [19].
References
El Chemaly, A., Magaud, C., Patri, S., Jayle, C., Guinamard, R., & Bois, P. (2007). The heart rate-lowering agent ivabradine inhibits the pacemaker current I(f) in human atrial myocytes. Journal of Cardiovascular Electrophysiology, 18, 1190–1196.
Ponikowski, P., Voors, A. A., Anker, S. D., Bueno, H., Cleland, J. G., Coats, A. J., et al. (2016). 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. European Heart Journal, 37, 2129–2200.
Melgari, D., Brack, K. E., Zhang, C., Zhang, Y., El Harchi, A., Mitcheson, J. S., et al. (2015). hERG potassium channel blockade by the HCN channel inhibitor bradycardic agent ivabradine. Journal of the American Heart Association, 4(4), e001813.
Lees-Miller, J. P., Guo, J., Wang, Y., Perissinotti, L. L., Noskov, S. Y., & Duff, H. J. (2015). Ivabradine prolongs phase 3 of cardiac repolarization and blocks the hERG1 (KCNH2) current over a concentration-range overlapping with that required to block HCN4. Journal of Molecular and Cellular Cardiology, 85, 71–78.
Frommeyer, G., Weller, J., Ellermann, C., Bogeholz, N., Leitz, P., Dechering, D. G., et al. (2017). Ivabradine reduces digitalis-induced ventricular arrhythmias. Basic & Clinical Pharmacology & Toxicology, 121, 526–530.
Frommeyer, G., Weller, J., Ellermann, C., Kaese, S., Kochhauser, S., Lange, P. S., et al. (2017). Antiarrhythmic properties of ivabradine in an experimental model of Short-QT-Syndrome. Clinical and Experimental Pharmacology & Physiology, 44, 941–945.
Frommeyer, G., Sterneberg, M., Dechering, D. G., Ellermann, C., Bogeholz, N., Kochhauser, S., et al. (2017). Effective suppression of atrial fibrillation by ivabradine: Novel target for an established drug? International Journal of Cardiology, 236, 237–243.
Verrier, R. L., Bonatti, R., Silva, A. F., Batatinha, J. A., Nearing, B. D., Liu, G., et al. (2014). If inhibition in the atrioventricular node by ivabradine causes rate-dependent slowing of conduction and reduces ventricular rate during atrial fibrillation. Heart Rhythm, 11, 2288–2296.
Verrier, R. L., Silva, A. F., Bonatti, R., Batatinha, J. A., Nearing, B. D., Liu, G., et al. (2015). Combined actions of ivabradine and ranolazine reduce ventricular rate during atrial fibrillation. Journal of Cardiovascular Electrophysiology, 26, 329–335.
Mengesha, H. G., Weldearegawi, B., Petrucka, P., Bekele, T., Otieno, M. G., & Hailu, A. (2017). Effect of ivabradine on cardiovascular outcomes in patients with stable angina: Meta-analysis of randomized clinical trials. BMC Cardiovascular Disorders, 17, 105.
Frommeyer, G., Clauss, C., Ellermann, C., Bogossian, H., Dechering, D. G., Kochhauser, S., et al. (2017). Antiarrhythmic effect of vernakalant in an experimental model of Long-QT-syndrome. Europace, 19, 866–873.
Frommeyer, G., Garthmann, J., Ellermann, C., Dechering, D. G., Kochhauser, S., Reinke, F., et al. (2017). Broad antiarrhythmic effect of mexiletine in different arrhythmia models. Europace (in press).
Frommeyer, G., Milberg, P., Witte, P., Stypmann, J., Koopmann, M., Lucke, M., et al. (2011). A new mechanism preventing proarrhythmia in chronic heart failure: Rapid phase-III repolarization explains the low proarrhythmic potential of amiodarone in contrast to sotalol in a model of pacing-induced heart failure. European Journal of Heart Failure, 13, 1060–1069.
Milberg, P., Frommeyer, G., Kleideiter, A., Fischer, A., Osada, N., Breithardt, G., et al. (2011). Antiarrhythmic effects of free polyunsaturated fatty acids in an experimental model of LQT2 and LQT3 due to suppression of early afterdepolarizations and reduction of spatial and temporal dispersion of repolarization. Heart Rhythm, 8, 1492–1500.
Antzelevitch, C. (2007). Ionic, molecular, and cellular bases of QT-interval prolongation and torsade de pointes. Europace, 9(Suppl 4), iv4–i15.
Verduyn, S. C., Vos, M. A., van der Zande, J., Kulcsar, A., & Wellens, H. J. (1997). Further observations to elucidate the role of interventricular dispersion of repolarization and early afterdepolarizations in the genesis of acquired torsade de pointes arrhythmias: A comparison between almokalant and d-sotalol using the dog as its own control. Journal of the American College of Cardiology, 30, 1575–1584.
Thomsen, M. B., Verduyn, S. C., Stengl, M., Beekman, J. D., de Pater, G., van Opstal, J., et al. (2004). Increased short-term variability of repolarization predicts d-sotalol-induced torsades de pointes in dogs. Circulation, 110, 2453–2459.
van Opstal, J. M., Schoenmakers, M., Verduyn, S. C., de Groot, S. H., Leunissen, J. D., van Der Hulst, F. F., et al. (2001). Chronic amiodarone evokes no torsade de pointes arrhythmias despite QT lengthening in an animal model of acquired long-QT syndrome. Circulation, 104, 2722–2727.
Roden, D. M. (1998). Taking the “idio” out of “idiosyncratic”: Predicting torsades de pointes. Pacing and Clinical Electrophysiology, 21, 1029–1034.
Frommeyer, G., Fischer, C., Ellermann, C., Dechering, D. G., Kochhauser, S., Lange, P. S., et al. (2018). Additive proarrhythmic effect of combined treatment with QT-prolonging agents. Cardiovascular Toxicology, 18, 84–90.
Acknowledgements
This study was supported by the German Cardiac Society and the Hans und Gertie-Fischer Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare to have no conflict of interest related to this study.
Additional information
Handling Editor: Bérengère Dumotier.
Rights and permissions
About this article
Cite this article
Frommeyer, G., Weller, J., Ellermann, C. et al. Ivabradine Aggravates the Proarrhythmic Risk in Experimental Models of Long QT Syndrome. Cardiovasc Toxicol 19, 129–135 (2019). https://doi.org/10.1007/s12012-018-9482-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-018-9482-y